Introduction
Today, different types of yoghurts are produced. Although yoghurt is a product of good microbiological stability, acid-resistant fungi (yeasts and moulds) can contaminate it at all stages of production until supply (Delavenne et al., 2013). Fungi can ferment lactose and sucrose, use lactate, hydrolyse lipids and proteins and grow even at refrigerator temperature. Food spoilage fungi can produce unsuitable gas, alcohol, and aromatic compounds. Even certain moulds can produce a wide range of harmful mycotoxins for consumers. Various food preservatives such as organic acids, sodium benzoate, potassium sorbate, potassium benzoate, and pimaricin are used to prevent fungal spoilage. Increasing fungal resistance to chemical preservatives, consumer demand for natural and healthy products, and the development of monitoring systems have led the food industry to find new ways to increase food shelf life. The use of bio-preservatives is one of the proposed solutions (Farag et al., 2021). Bio-preservatives refer to the use of natural or controlled microbial populations or their metabolic products to inhibit or eliminate undesirable microorganisms in food, which increase the shelf life and promote the safety of food products. Because LAB are naturally abundant in food systems and have a long history of safe use in fermented foods, therefore, natural safe bacteria are generally recognized as safe (GRAS) and so have a high potential for use as natural bio-preservatives (Ananou et al., 2007; Nath et al., 2014; Felix et al., 2017). Bacteriocins, organic acids and hydrogen peroxide are the main constituents produced by LAB (Singh, 2018). Bacteriocins are natural compounds that can affect the safety and quality of food (Settanni and Coesetti, 2008). Bio-preservatives cultures are an alternative to chemical preservatives or complementary tools to hurdle's technologies to delay or prevent fungal spoilage in dairy products (Leyva Salas et al., 2018). Competitive exclusion, like competition for a limited resource by various microorganisms, has been reported as an important mechanism for lactobacilli against fungal spoilage in fermented dairy products (Siedler et al., 2020).
In addition to their preservative properties, LAB are also important in promoting health and probiotic properties (Saito, 2004). One of the most important mechanisms proposed for the probiotic effect is to regulate the immune system response and the production of antimicrobial compounds to eliminate pathogenic microbes (Cai et al., 2014). LAB can inhibit the growth of other microorganisms by various mechanisms. Most of the preservative effects of microorganisms in fermented foods are due to the production of acids such as lactic acid and acetic acid. There are a wide range of small inhibitory molecules such as hydrogen peroxide, diacetyl, hypothiocyanate, rutarin, bacteriocins and bacteriocin-like inhibitors that sometimes have high antimicrobial activity against pathogenic and spoilage microbes. Some Lactobacillus species isolated from traditional dairy products and affect gastric cancer cell line (Marhamatizadeh et al., 2019). One of the strains of L. rhamnosus is able to create a creamy taste without negatively affecting the rheology of dairy products and is used in the production of yoghurt and low-fat cheese with high diacetyl content. Also, the effect of preventive chemotherapy related to L. rhamnosus IMC501 in the gastrointestinal tract has been reported (Bocci et al., 2015). L. rhamnosus is a highly resistant intestinal bacterium that has many health effects including reducing the duration of viral diarrhoea, strengthening the immune system, and improving colon disease, treatment and prevention of allergies, etc. (Kabak et al., 2009). In recent years, the use of LAB and their metabolites to increase the shelf life of food products has received much attention (Ben Said et al., 2019). The antifungal properties of LAB have been studied or proven by some researchers over different periods of time (Obadina et al., 2006; Kim, 2005; Lind et al., 2005; Khanafari et al., 2007; Gerez et al., 2009; Voulgari et al., 2010; Schwenninger et al., 2008; Gerbaldo et al., 2012; Mohammaed and Ijah, 2013; Delavenne et al., 2013, 2015; Siroli et al., 2016; Yepez et al., 2017; Leyva Salas et al., 2018; Ben Said et al., 2019; Ouiddir et al., 2019; Luz et al., 2020).
Today, due to the increasing incidence of dangerous diseases such as cancer, consumer demand for natural and healthy products and the acceptance of products that do not contain preservatives and chemicals has increased. Therefore, in recent years, extensive research has been conducted to discover alternative solutions to prevent food spoilage. So, the aim of this study was to use the lactic acid bacterium L. rhamnosus as a food bio-preservative in yoghurt and to investigate some properties of the produced yoghurt.
Materials and methods
Materials
Milk from Pazhan Company (Tehran, Iran), Kluyveromyces lactis (PTCC: 5185), Penicillium expansum (PTCC: 89046), Yarrowia (ATCC: 18942), Saccharomyces cerevisiae (PTCC: 5052), Aspergillus niger (PTCC:5145) from Persian type Culture collection (Iranian Research Organization for Science & Technology (IROST) (Tehran, Iran), Lactobacillus rhamnosus from Pasteur Institute (Tehran, Iran) and starter LBB-R2 from Chr. Hansen (Denmark). All chemicals required for the tests were purchased from Merck (Germany).
Selection of fungus strain
Initially, 10 6 cfu/mL was prepared from L. rhamnosus by half McFarland method. Then, L. rhamnosus was cultured linearly on MRS agar plate and incubated in an anaerobic jar at 30 °C for 48 hours. A pre-prepared potato dextrose agar medium was inoculated with 1 cc of various fungal solutions ( Yarrowia, P. expansum, S. cerevisiae, A. niger, K. lactis); separately, 10 cc of each fungal solution was poured onto plates containing grown L. rhamnosus and allowed to thaw. The plates were incubated at 28 °C for 48 h and ocular inhibition of fungal growth by L. rhamnosus was observed (growth of Yarrowia fully, growth of P. expansum more than 50 % and growth of A. niger about 50 % inhibited) (Hasani et al., 2017; Sulieman et al., 2012). It should be noted that the growth of S. cerevisiae and K. lactis was not inhibited by L. rhamnosus and both fungi were fully grown. Then, through factory research in Iran (6 factories), including Kaleh, Domino, Pegah Tehran, pasteurized milk of Pegah Golpayegan, Asil Golpayegan factory, Pagen Company) it was found that in yoghurt, spoilage through P. expansum is more common than two another fungus ( Yarrowia and A. niger); Therefore, P. expansum was selected.
Production of yoghurt samples
After standardization of fat (3.5 %) and dry matter (9.5-10 %), milk was pasteurized (78 °C/15 seconds) and then, to reach the desired temperature for inoculation, it was tempered to 45 °C in a water bath. The contents of the LBB-R2 yoghurt starter package were dissolved in 100 mL of sterile water and 1 mL of this solution was added to the sanitized and cooled milk (10 L). After inoculating the yoghurt-starter culture bacteria into milk and packing them in 200 mL-polystyrene containers, incubation was performed at 43 °C for 4 hours. After incubation, the samples were transferred to the refrigerator at 4 °C.
It should be noted that L. rhamnosus (it was co-inoculation with yoghurt-starter culture bacteria) and P. expansum (it was inoculated into yoghurt after 24 hours of refrigeration) were inoculated according to
Table 1. The presence of T2 is only to investigate the bioprotective effect of L. rhamnosus on P. expansum (T3) and also to investigate the shelf life of T3
Physicochemical analysis
pH was measured at room temperature using a Mettler Toledo pH meter equipped with MA235 electrode (Switzerland) and the viscosity of yoghurt samples produced using a Brookfield viscometer (RV-DVII, USA) (Jozve-Zargharabadi et al., 2020). The syneresis of yoghurt samples produced by refrigerated centrifuge (Sigma model, made in Germany) was measured at 1220 g for 10 minutes at 4 °C (Hassan et al., 1996).
Microbial analysis
L. bulgaricus and S. thermophilus were enumerated as described by Jozve-Zargharabadi et al. (2020). For enumeration of P. expansum, 10 g of the sample was first poured into an Erlenmeyer flask containing 90 mL of physiological saline solution and was shaken slightly to make the sample uniform in physiological saline solution; and then, 1 mL was removed using a sterile pipette and transferred to a tube containing 9 mL of saline. This sequence continued until tube number 7 and the so-called dilution 7. Then, 0.1 mL was removed from tubes 4, 5, 6 and 7; the agar was poured onto plates containing potato dextrose medium and spread evenly with an L-shaped tube. The plates were placed directly in the incubator for 25 h at 25 °C (Sulieman et al., 2012).
Sensory evaluation
The samples were given to the panellists one day after storage at 4 °C. Panellists were asked to drink water after eating each sample so that the previous sample had no effect on their evaluation of the new sample. The sensory characteristics of the samples (including color, texture, odour, and overall acceptability) were assessed by 10 trained evaluators. Samples were provided to each panellist separately so that the panellists’ opinions did not affect each other. The evaluation was performed in the context of a 5-point hedonic test. Then parametric data were converted to non-parametric data; in this way, in a very unfavourable to very favourable expression, a score of 1 to 5 was given, respectively. It should be noted that since the final indicator of evaluation is overall acceptability, so in this study, only the results of overall acceptability during storage have been reported.
Statistical analysis
In order to evaluate the quantitative characteristics of the data due to the existence of 6 treatments and 3 replications, one-way analysis of variance was used and also, to compare the mean data, Duncan test was used at a significance level of 5 % to evaluate the significance of the results. Statistical analysis was performed by SPSS 16 software and Kruskal-Wallis nonparametric test was used to analyse the data obtained from sensory tests.
Results and discussion
pH
pH is an important factor in preparing a probiotic product; lowering the pH during the shelf life of the product is associated with increased acid production by bacteria and the predominant acid produced is lactic acid. If the amount of this acid is too much, it affects the taste of the product and creates unfavourable conditions for the product (Bueno et al., 2007). On the other hand, pH is one of the most important factors affecting the survival of probiotic bacteria (Tripathi and Giri, 2014). In general, the viability of probiotics in fermented products is relatively low due to their low pH (Varsha et al., 2014; Kitazawa et al., 2001). Lactobacilli are often resistant to pH changes (4.3-3.3) (Tripathi and Giri, 2014).
During storage (Table 2), the pH of yoghurt samples containing L. rhamnosus decreased (p<0.05). In general, in yoghurt, fermentation of lactose to lactic acid by the activity of starter bacteria increases the acidity, which has been reported in many studies (Cho et al., 2020; Shahbazi and Shavisi, 2019; Amadou et al., 2018; Jeong et al., 2018; Dabija et al., 2018; Shokery et al ., 2017; Joung et al., 2016; Lee and Chen, 2005; Tarakçi, 2010; Bakirci and Kavaz, 2008; Walstra et al., 2005). Many studies have confirmed the decrease in yoghurt pH during storage (Jozve-Zargharabadi et al., 2020; Shahbazi and Shavisi, 2019; Amadou et al., 2018; Dabija et al., 2018; Shokery et al., 2017; Joung et al., 2016; Lee and Chen, 2005; Falade et al., 2015; Zamberlin et al., 2011; Bano et al., 2011; Papastoyiannidis et al., 2006; Salwa et al., 2004).
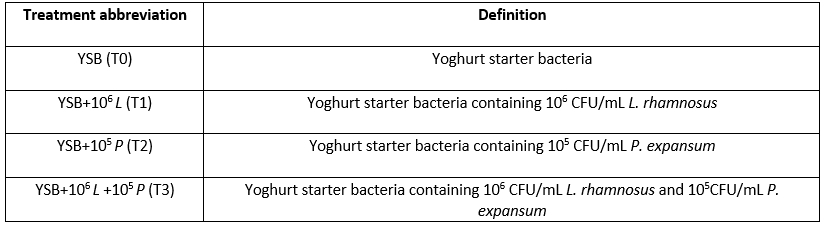
Table 2. pH of yoghurt samples containing L. rhamnosus during storage at 4 °C
*Means with different subscripts differ significantly (p<0.05).
In all the studied days, the lowest pH value belonged to T1 and then to T3; the reason for this can be attributed to the presence of probiotic bacteria L. rhamnosus along with yoghurt starter bacteria in the mentioned samples. The low pH of the sample containing L. rhamnosus compared to other treatments is due to the production of more lactic acid (Farnworth et al., 2007). Osaili et al. (2013) also found that acidity and its changes depended on the bacterial composition inoculated into yoghurt. Although L. rhamnosus and S. thermophilus were present in all samples, different pH changes could be related to inactivation or reduction in the number of living cells of these bacteria in different samples. Sharaf et al. (2015), Hussain and Atkinson, (2009) and Farzaneh et al. (2021) also reported that while decreasing the pH during storage, the highest pH decrease was related to probiotic samples compared to the control sample (without probiotic bacteria). Leyva Salas et al. (2018) using a dual combination of lactic acid bacteria (A1 = L. plantarum + L. harbinensis and A3 = L. plantarum + L. rhamnosus) concluded that antifungal cultures had no effect on pH in semi-hard cheese and sour cream. However, post acidification was reported in sour cream at the highest inoculated concentration (2 x 10 7 cfu/mL).
Syneresis
One of the major disadvantages of yoghurt is syneresis, which is actually the appearance of serum or whey on the surface of the yoghurt. Yoghurt syneresis occurs due to the shrinkage of the three-dimensional structure of the protein network, which leads to a decrease in the ability to bind whey proteins with water. In general, the structure of yoghurt can be explained as a three-dimensional network of casein micelle chains and clusters that have retained their spherical shape (Lucey, 2004; Malone et al., 2003; Supavititpatana et al., 2010). In general, the weakening of the hydrogen and electrostatic bonds of the gel and the increment of ion repulsion increase the hydration.
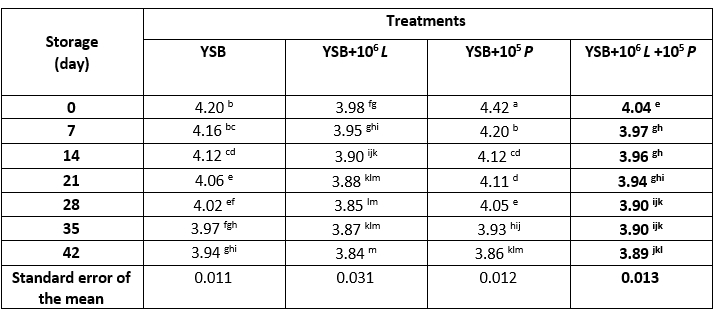
Table 3. Syneresis of yoghurt samples containing L. rhamnosus during storage at 4 °C
*Means with different subscripts differ significantly (p<0.05)
During the storage period (Table 3), no statistically significant difference was observed in the syneresis of treatments (p>0.05). Syneresis of all samples during 42 days showed an increasing trend so that the highest rate of syneresis was observed at days 35 and 42. No statistically significant difference was observed in the syneresis of T1 (p>0.05). In T3, the amount of syneresis at days 0 and 7 was significantly lower than other days (p<0.05), and no statistically significant difference was observed on other days (p>0.05). In general, the increase in syneresis of yoghurt samples over time can be attributed to the increase in acidity and decrease in pH of the product, as well as the contraction of the gel network due to cooling (Tamime and Robinson, 2007). On the other hand, due to the hydrolysis and digestion of product proteins, the amount of hydration increases with increasing storage time; because the proteins that cause the desired texture lose their properties and their bond with the water is broken; pH changes are also involved; because they denature the structure of the protein. Due to the denaturation of the protein, the attached water was released and consequently syneresis increased (Tarakci and Kucukoner, 2003; Vahedi et al., 2008; Jozve-Zargharabadi et al., 2020). In the present study, the syneresis rate in probiotic yoghurt samples was higher than in the control sample, which can be attributed to the higher acidity (lower pH) of these samples. Some studies have also reported an increase in yoghurt syneresis during storage (Tamime et al., 2005; Vahedi et al., 2008; Supavititpatana et al., 2010; Osaili et al., 2013; Jozve-Zargharabadi et al., 2020).
Viscosity
One of the important factors affecting the quality of the product is the apparent viscosity, which depends on factors such as the composition and acidity of milk and its dry matter, heating temperature, type of starter used, additives, and storage conditions (Lee and Lucey, 2003; Tamime and Robinson, 2007). Denaturation of whey proteins before fermentation has a great effect on increasing the viscosity of yoghurt, which can be due to the increased capacity of proteins to bond together (Capela et al., 2006).
During storage, the viscosity of sample T0 (Table 4) on day 42 was higher than other treatments (p<0.05). In all samples, the viscosity on day zero was significantly higher than the other days and decreased over time (p<0.05).
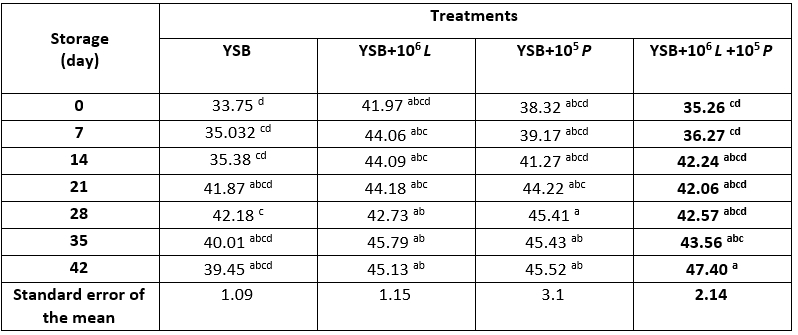
Table 4. Viscosity (cp) of yoghurt samples containing Lacticaseibacillus rhamnosus during storage at 4 °C
*Means with different subscripts differ significantly (p<0.05)
During storage, the viscosity of the samples decreased; the cause can be attributed to the hydrolysis and digestion of the casein micelle matrix by proteases activity (Dabija et al., 2018) and the rupture of their bond with water and the loosening of the gel network by lowering the pH. In fact, decrease in viscosity is due to the reduced water holding capacity of the product as a result of increasing the acidity of the product during storage (Jozve-Zargharabadi et al., 2020). In reducing the viscosity, Li et al. (2013) in investigating the inhibitory effects of L. casei AST18 as a natural control agent on fungal spoilage of yoghurt stated that this strain did not have a significant effect on viscosity. Also, other researchers have concluded that the viscosity of yoghurt samples decreased during storage (Lee and Lucey, 2003; Jozve-Zargharabadi et al., 2020; Cho et al., 2020).
Microbial characteristics
Survival of yoghurt starter bacteria
During the storage, the survival of Streptococcus thermophilus (Table 5) in T0 and T2 samples was significantly reduced (p<0.05) so that the lowest survival of S. thermophilus was observed at days 35 and 42 (p<0.05). In sample T1, the survival of S. thermophilus decreased although it was not statistically significant (p>0.05) and on days 0 and 7, no statistically significant difference was observed in the survival of S. thermophilus (p>0.05). On days 14 and 21, the highest survival of S. thermophilus was present in samples T1 and T3 (p<0.05).
Table 5. Population of Streptococcus thermophilus, Lactobacillus bulgaricus (log CFU/mL), yoghurt samples containing Lacticaseibacillus rhamnosus during storage at 4 °C
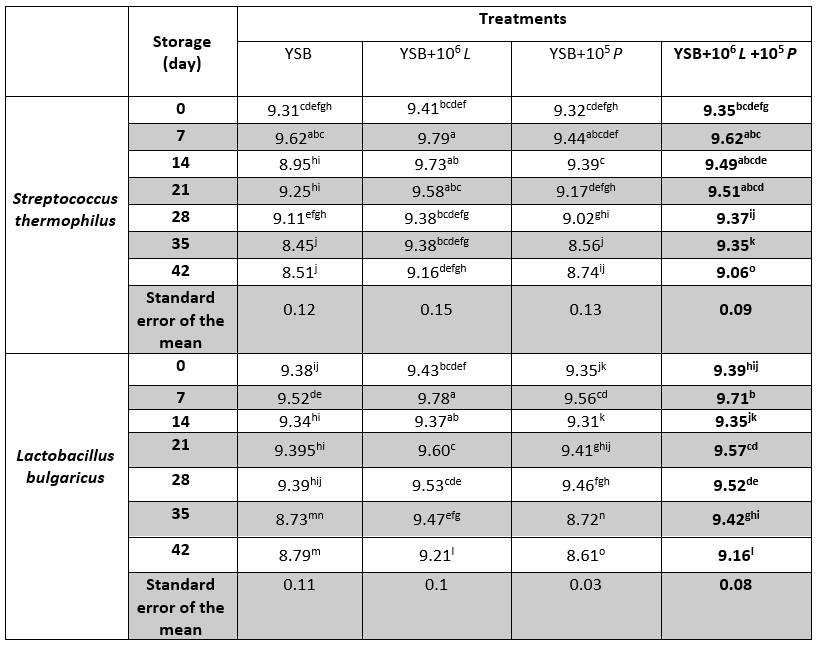
*Means with different subscripts differ significantly (p<0.05)
During storage, the lowest survival of Lactobacillus bulgaricus (Table 5) was observed on days 35 and 42. On days 0, 7 and 14, the highest survival of L. bulgaricus belonged to sample T1; on other days, it was assigned to T1 and T3 samples (p<0.05).
It is also important to evaluate the microbial behaviour of fermented milk products containing active and sensitive microorganisms. Researchers have reported that the major growth of S. thermophilus occurs in the early hours of fermentation, if at first, Lactobacillus bulgaricus grows slowly. When the pH of the samples reaches 4.9, the growth of L. bulgaricus is enhanced, it inhibits the growth of S. thermophilus (Ozer and Rabinson, 1999). Thus, initially, the microbial population of Streptococcus is greater than that of the initiating Lactobacilli, but during the storage, the microbial population of S. thermophilus decreases. Streptococci are more sensitive to lactic acid accumulation and die faster (Schnürer and Magnusson, 2005). This may be the reason for the greater reduction of streptococci than lactobacilli in this study. The reduced viability of S. thermophilus during storage can be attributed to the accumulation of organic acids as well as products produced by bacterial activity such as hydrogen peroxide (Shori, 2013).
Overall, the reduction in bacterial viability over time can be attributed to the production of acid, the decrease in pH, and the consumption of nutrients used by the bacteria. Co-culture of probiotic bacteria with yoghurt starter bacteria creates a kind of one-way cooperative biological relationship (Nighswonger et al., 1996), gradually, however, they limit their growth or death. A clear example of such event can be observed in the biological relationship of probiotics with L. bulgaricus. The bacterium initially accelerates the growth of probiotics by casein proteolysis and providing the available non-protein nitrogen. However, further fermentation, with rapid growth, intense acidification, lowering of pH and production of hydrogen peroxide and bacteriocin during fermentation and storage period, adversely affects the growth and survival of these bacteria. (Rybka and Kailasapathy, 1997; Hull et al., 1984). The presence of more protein compounds intensifies the growth of lactobacilli (Schnürer and Magnusson, 2005).
The problem with fermented products such as yoghurt is that the amount of acid increases during the storage period of the product and the survival of lactic acid bacteria is affected by the increase of hydrogen ions compared to lactate ions (Shah, 2000). The adverse pH decline is mainly due to the uncontrolled growth of L. bulgaricus species in high acidity during refrigeration (Shiby and Mishra, 2013). The reason for the different treatments can be attributed to the type of starters used and the conditions required for their survival (such as pH and nutrient compounds used, etc.).
Decreased survival of L. bulgaricus in probiotic yoghurt samples may be due to the inhibitory effect of probiotic bacteria or a decrease in yoghurt moisture during storage; increasing the osmotic pressure by decreasing the humidity can affect this. As a result, bacterial antagonists, which may be related to production metabolites such as antimicrobial compounds including bacteriocins, are likely to have a negative effect on sensitive strains in yoghurt starters and reduce their viability (Vinderola et al., 2002; Tamime et al., 2005). Wang et al. (2002) reported that, in some cases, decreased starter survival was associated with the production of organic acids. Thus, the decrease in starter bacteria in probiotic yoghurt during storage can be attributed to the antagonistic effect of starters and probiotic bacteria on each other (Lacroix, 2010). The results indicate a change in the ratio of cocci bacteria, which ultimately leads to an increase in yoghurt acidity (Walstra et al., 2005).
Some researchers have also reported reduced survival of starter bacteria during storage of probiotic yoghurt (Kneifel et al., 1992; Tamime et al., 2005; Damin et al., 2008; Lacroix, 2010; Vinderola et al., 2002). Leyva Salas et al. (2018) using a dual combination of lactic acid bacteria as: A1 = L. plantarum + L. harbinensis and A3 = L. plantarum + L. rhamnosus concluded that antifungal cultures have no effect on the growth of starter cultures and pH in semi-hard cheese and sour cream.
Survival of P. expansum
Use of microorganisms with antagonistic effects and GRAS to reduce the growth of moulds is one of the most important methods of biological storage of foods (Silva et al., 2015). Due to the health effects of LAB as probiotic bacteria, these bacteria are highly regarded (Dalie et al., 2010). LAB have the ability to produce antimicrobial compounds such as organic acids, diacetyl, acetone, hydrogen peroxide, antifungal peptides and bacteriocins that affect a wide range of pathogenic and spoilage micro-organisms (Galvez et al., 2007).
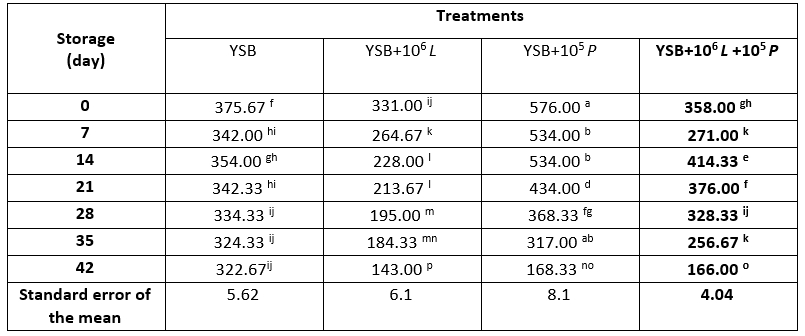
During storage (Table 6), on all days except day zero, the population of P. expansum in T2 was higher than T3 (p<0.05). The reason for this difference can be attributed to the antifungal role of L. rhamnosus; however, L. rhamnosus was not able to completely prevent the growth of mould.
Table 6. Population of Penicillium expansum (log CFU/mL) in yoghurt samples containing Lacticaseibacillus rhamnosus during storage at 4 °C
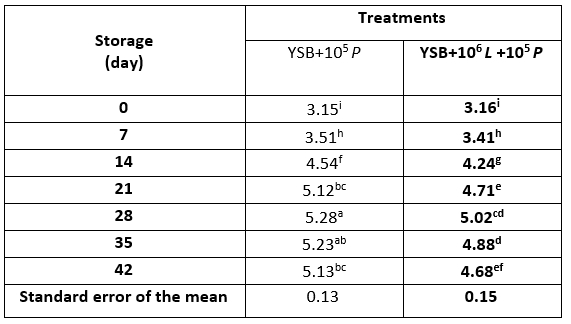
*Means with different subscripts differ significantly (p<0.05)
Li et al. (2013) reported that adding 2 % L. casei AST18 to yoghurt completely inhibited mould growth during storage. Delavenne et al. (2015) examined the antifungal effect of L. harbinensis and L. rhamnosus strains in yoghurt and found that L. harbinensis strain had the highest antifungal activity and the maximum inhibition of yeast growth was observed when it was 2.5 × 10 6 cfu/g. Gourama and Bullerman (1997) by examining the inhibitory effect of Penicillium mould growth by different strains of Lactobacillus ( L. casei, L. rhamnosus, L. fermentum, L. acidophilus) concluded that all the strains studied had the antifungal effect which was attributed to the production of 3-phenyllactic acid (PLA). Lavermicocca et al. (2000) showed that lactic isolates obtained from sourdough can significantly inhibit the growth of Aspergillus, Penicillium and Fusarium by producing phenyl lactic acid and 4-hydroxyphenyl lactic acid. Tropcheva et al. (2014) also reported that different subspecies of L. brevis isolated from Bulgarian fermented yoghurt could completely inhibit the growth of Aspergillus awamori and P. claviform, as well as the growth of Aspergillus flavus and the production of aflatoxin B1 significantly reduced. Stiles (1996) reported that sodium acetate and L. rhamnosus had a synergistic effect on inhibiting most of the moulds studied, including Penicillium. Florianowicz (2001) reported that most of the studied lactobacilli (such as L. casei, L. rhamnosus, L. delbrueckii, L. bulgaricus) had antifungal effect against P. expansum. Bian et al. (2016) by examining probiotic soy milk and contaminating the samples with Penicillium mould after fermentation showed that mould was able to grow in samples containing L. holoticus only until day one and then the growth was inhibited. On the seventh day, mould was not observed and counted in the samples containing probiotics, but in the control sample, its number increased from 2.04 cfu/mL on day zero to 6.44 cfu/mL on day 28. Leyva Salas et al. (2018) using a dual combination of LAB as: A1 = L. plantarum + L. harbinensis and A3 = L. plantarum + L. rhamnosus concluded that A1 delayed growth of Penicillium commune, Mucor racemosus, Rhodotorula mucilaginosa in sour cream for 2-24 days depending on inoculation of antifungal culture. Also, A1 and A3 delayed the growth of P. commune in semi-hard cheese by 1-6 days and one day, respectively.
Overall acceptability
During storage, the overall acceptability score (Table 7) decreased in all treatments; so that the score in the control on days 0, 7 and 14 was significantly higher and on day 42 was significantly lower than the other days. At T1, T2 and T3, the lowest overall acceptability score was observed on day 42 (p<0.05). On all storage days, the score in T3 was lower than the control sample (p<0.05).
Table 7. Overall acceptability of yoghurt samples containing Lacticaseibacillus rhamnosus during storage at 4 °C
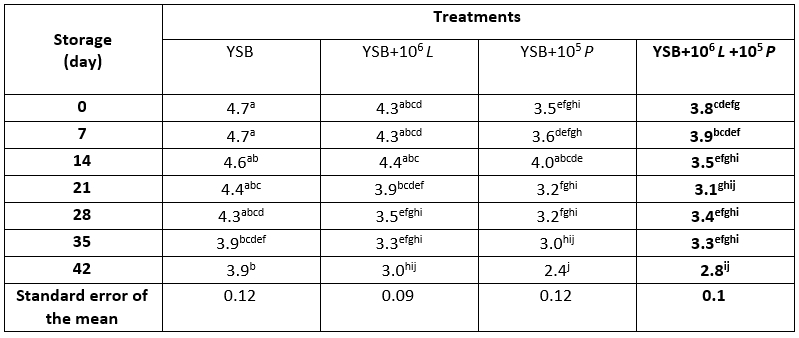
*Means with different subscripts differ significantly (p<0.05)
In general, the taste and odour scores were higher in samples containing L. rhamnosus (unreported data), which can be attributed to the flavour-producing compounds such as diacetyl and acetone by L. rhamnosus. According to the researchers, diacetyl and its related compounds such as acetone are responsible for creating the desired taste and odour in many fermented products produced by LAB such as L. rhamnosus (Lo et al., 2018). It should be noted that the texture score in samples containing probiotic bacteria decreased (unreported data) and since the texture of products such as yoghurt is very effective in the overall acceptability score of the product; therefore, low scores were recorded by the panellists for these samples.
Hekmat and Reid (2006) and Li et al. (2013) reported that the control yoghurt sample was superior to the probiotic yoghurt sample. Delavenne et al. (2015) concluded that L. harbinensis and L. rhamnosus had high antifungal activity and the least effect on the overall acceptability score of yoghurt samples was observed. Leyva Salas et al. (2018) using a dual combination of lactic acid bacteria: A1 = L. plantarum + L. harbinensis and A3 = L. plantarum + L. rhamnosus concluded that A1 in low inoculation (10 6 cfu/mL) did not affect sensory characteristics of sour cream.
Conclusion
Investigation of the inhibitory effect of L. rhamnosus as a natural controlling agent on yoghurt spoilage showed that inoculation of L. rhamnosus (10 6 CFU/mL) reduced the count of P. expansum during yoghurt storage time. Overall, the results indicated that Lactobacillus rhamnosus improved the growth of S. thermophilus and L. bulgaricus. However, due to the fact that in this sample, the overall acceptability score and viscosity decreased, the syneresis increased and the growth of mould did not stop completely, it can be concluded that L. rhamnosus (10 6 CFU/mL) cannot be used alone as a natural preservative in yoghurt to prevent fungal growth and increase shelf life; therefore, the use of L. rhamnosus with natural antifungal compounds such as essential oils or plant extracts in yoghurt is recommended.
Acknowledgment
The authors of the paper express their gratitude to Pazhan milk factory in Tehran for providing the necessary facilities for this research.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Karakterizacija jogurta s dodatkom Lacticaseibacillus rhamnosus kao protektivne kulture protiv kvasaca i plijesni
Sažetak
Bakterije mliječne kiseline (BMK) igraju vrlo važnu ulogu kao prirodni biokonzervansi u hrani. Prema tome, istražen je inhibicijski učinak Lactobacillus rhamnosus ( L. rhamnosus) prema 4 vrste Yarrowia kvasaca i plijesni: Penicillium expansum ( P. expansum), Saccharomyces cerevisiae ( S. cerevisiae), Aspergillus niger ( A. niger), Kluyveromyces lactis ( K. lactis). Zatim je P. expansum odabran kao najvažniji kontaminant iz roda plijesni u jogurtu kako bi se istražila upotreba L. rhamnosus za selektivno smanjenje kvarenja jogurta uzrokovanog navedenim sojem plijesni. Također je određivan učinak ove biozaštitne kulture na broj starter bakterija i neke karakteristike jogurta. L. rhamnosus (10 6 cfu/mL) inokuliran je u mlijeko zajedno s jogurtnom starter kulturom pri čemu su proizvedena četiri uzorka T 0 ( L. rhamnosus = 0, P. expansum = 0), T 1 ( L. rhamnosus = 10 6 cfu/mL, P. expansum = 0), T 2 ( L. rhamnosus = 0, P. expansum = 10 5 cfu). /mL); T 3 ( L. rhamnosus = 10 6 cfu/mL, P. expansum = 10 5 cfu/mL). U svim uzorcima je određivana pH vrijednost, viskoznost, sinereza, mikrobiološki parametri tj. preživljavanje Streptococcus thermophilus, Lactobacillus bulgaricus i P. expansum, te ukupna prihvatljivost. L. rhamnosus je inhibiraio preživljavanje P. expansum (p<0,05), ali ga nije mogao u potpunosti kontrolirati. L. rhamnosus je u uzorku T 3 pokazao jak inhibicijski učinak od prvog dana do kraja skladištenja. Nadalje, tijekom skladištenja preživljavanje sojeva jogurtne starter kulture u uzorcima probiotičkog jogurta bilo je veće od kontrolnog (p<0,05). T 3 je imao niži pH, viskoznost i ukupni rezultat prihvatljivosti u usporedbi s kontrolnim uzorkom, a sinereza mu je bila viša (p<0,05). Općenito, dodavanje 10 6 cfu/mL L. rhamnosus nije u potpunosti inhibiralo rast soja P. expansum (10 5 CFU/mL) tijekom perioda skladištenja jogurta.
Ključneriječi: protektivna kultura ; L. rhamnosus; jogurt; P. expansum
