INTRODUCTION
Emerging knowledge acquired in recent decades regarding the complex composition and functions of skin has led to a greater recognition of its active role in human health (1, 2). The principal function of skin is to form an effective physical, biochemical, immunological, and microbial barrier between the human body and its environment. The protective function is mainly provided by the stratum corneum (SC), which represents the superficial part of the epidermis (3). The SC consists of up to about 25 layers of flattened, metabolically inactive keratinocytes called corneocytes. In contrast to metabolically active cells, which contain a relatively large amount of water, corneocytes are densely packed with rigid keratin matrix formed in the cross-linking process of keratin fibers with filaggrin. Keratin and filaggrin are thus acknowledged as the key structural proteins of skin that provide the physical integrity of the SC. Moreover, filaggrin’s degradation products, collectively referred to as natural moisturizing factors (NMFs), are responsible for maintaining SC hydration. Protein-lipid envelope of corneocytes instead of a phospholipid membrane is another unique feature of corneocytes contributing strongly to the physical resilience of the SC. On the exterior, corneocytes are embedded in intercellular lipids such as ceramides, cholesterol, and free fatty acids, which are organized into distinctive lamellar phase structures. Due to this characteristic structural organization SC acts as a barrier to xenobiotic penetration and excessive transepidermal water loss (TEWL) (4, 5). In addition, various cytokines, antimicrobial peptides, and enzymes are found in the SC, which provide the skin’s immunological and microbial defense (6, 7).
Numerous experimental, genetic, and clinical studies have shown that impaired integrity of the SC interferes with skin barrier function and represents a precondition for the development of certain skin disorders and diseases (8–10). In this regard, atopic dermatitis (AD) is the most common inflammatory skin disease with a lifetime prevalence of 20 %, characterized by skin barrier dysfunction. AD most often affects children and young people. Namely, AD typically starts in infancy or early childhood and often gradually declines with age, although the onset of the disease is not limited only to early life, as it may persist or even develop in adulthood (11, 12). Clinical signs and symptoms of AD include recurring episodes of dry, scaly, and red eczematous lesions along with severe pruritus that significantly affect patients' quality of life (13).
The etiology of AD is complex and involves a combination of genetic and environmental factors. Genetic predisposition due to mutations and/or polymorphisms in various genes has been shown to play an important role in the development of AD. Notably, genetic abnormalities related to structural protein filaggrin contribute to dry atopic skin with impaired barrier integrity (14). Polymorphisms in genes encoding immunological responses are also relevant, as CD4+ T helper Th2-predominant chronic inflammation is typical for the skin of AD patients. Increased levels of inflammatory mediators lead to the development of characteristic clinical signs of inflammation, such as redness, swelling, and itching (15). Impaired skin barrier function is also a reflection of qualitative and quantitative changes in the composition of the SC’s intercellular lipids (5). The pathology of AD is accompanied by an imbalance in skin microbiota as well, which causes decreased antimicrobial defense of the skin (16). Regarding environmental aspects, chronic exposure to external triggers of oxidative stress such as air pollution and UV radiation is particularly noteworthy, as they generate reactive oxygen and nitrogen species that impair the skin's barrier function. In addition, aggressive (cosmetic) products, and certain dietary and inhalation allergens also weaken skin’s barrier function or trigger excessive immune response and thus cause exacerbation of AD (17).
According to the current guidelines, topical treatments remain the mainstay of therapy for mild to moderate AD that represents the most common form of the disease. The treatment involves consistent application of emollients together with intermittent use of topical corticosteroids during the flare-ups (18, 19). In this regard, conventional dosage forms like creams, lotions, and ointments are typically used. However, considering the poor drug stability and permeation, as well as the limited skin barrier-strengthening effect often associated with conventional formulations, there is a growing interest in novel dermal drug delivery systems (20–22).
Innovative lamellar lyotropic liquid crystals (LCCs), based on hempseed or flaxseed oil and loaded with betamethasone dipropionate drug for potential AD treatment were developed by our research group (23–25). The composition of the novel LCCs was primarily driven by the objective of formulating an advanced delivery system for dermal application with skin-compliant and barrier-strengthening characteristics. The promising results obtained within in vitro biocompatibility studies encouraged us to evaluate their impact on skin barrier function in humans.
Therefore, in the study presented here, eight novel LCCs were investigated for the first time in a double-blind, interventional, before-after, single-group trial with healthy adult subjects. The effects on TEWL, SC hydration, erythema index, melanin index, and tolerability were evaluated before (baseline), after 8 hours (short-term effect), and at the end of the 14-day (long-term effect) study period with the twice-daily application. The influence of the LCCs on skin barrier function was linked with their composition and microstructure.
EXPERIMENTAL
The study protocol as well as other essential documents were approved by the National Medical Ethics Committee of the Republic of Slovenia (approval number: 0120-479/2022/8). The study was performed according to the general principles of the Declaration of Helsinki with all its amendments. The study was conducted on healthy adult subjects at the University of Ljubljana, Faculty of Pharmacy. Subjects who completed the eligibility requirements and submitted written informed consent were included in the study. Subjects were informed of the study procedure before the beginning of the study.
The study was a double-blind, interventional, before-after, single-group study. Eight LCCs were prepared as previously described (23). Due to our interest in the evaluation of the LCCs’ influence on skin barrier function, only unloaded LCCs were tested. The composition of the LCCs studied is presented in Table I.
Table I. Composition of the novel LCCsa,b
a percent (m/m);b L – lecithin, T – Tween 80, M – Montanov 68, Ho – hempseed oil, Fo – flaxseed oil, the number indicates the bidistilled water content.
The study design is schematically presented in Fig. 1. Instructions were given to subjects regarding even application of LCCs over the assigned volar forearms. Subjects were requested to apply a thin layer of each LCC on the test areas twice a day for 14 days. All subjects had two test areas marked on each volar forearm, each with a test area size of 16 cm2. Given that we had eight LCCs to test, the study procedure was structured in two cycles. More specifically, four LCCs were first assessed, followed by a formulation-free interval of 14 days, after which the assessment of the remaining four LCCs was undertaken. All the assessments were performed before (baseline), 8 hours (short-term effect), and 14 days (long-term effect) after the first application of LCCs.
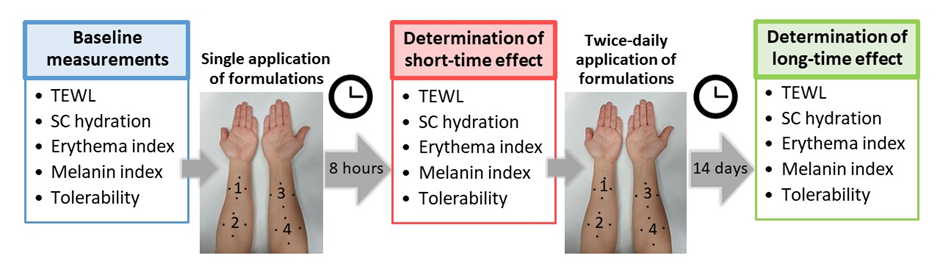
Fig. 1. The assessments were performed before (baseline), 8 hours (short-term effect), and 14 days (long-term effect) after the first application of LCCs. All subjects had two test areas marked on each volar forearm (four test areas per subject in total). Given that we had eight LCCs to test, the study protocol was structured in two cycles. Hence, four LCCs were first assessed, followed by a formulation-free interval of 14 days, after which the assessment of the remaining four LCCs was undertaken.
Healthy female subjects aged between 20 and 30 years were eligible for the study enrolment. Only females were recruited in the study to minimize the heterogeneity of the study group. In total, 12 subjects were enrolled, and all completed the study. Pregnancy or lactation were the initial exclusion criteria. Further exclusion factors were dermal or systemic disorders or diseases, interfering with the interpretation of the results; usage of drugs (antihistamines, corticosteroids, or other immunosuppressive drugs) prior to and during the study that would impend the results; the presence of tattoos, marks, scratches, or wounds on the volar forearms; any documented allergies to LCCs’ ingredients; and nonavailability and poor study compliance.
Subjects were not permitted to undergo intensive exposure of the test areas to artificial tanning, UV therapy, or sun 1 month prior to and during the study. They were also not allowed to use any cosmetic products (except shower gel) 1 week prior to and during the study. In addition, subjects were requested not to change their lifestyle habits over the entire study course. On the days of study visits, subjects were requested not to consume any caffeinated beverages or to smoke within 2 hours before the instrumental measurements.
Subjects were requested to spend at least 30 minutes in the environmentally controlled room (21.5 ± 1.5 °C, 40.0 ± 5.0 % relative humidity) to acclimatize before the instrumental measurements. Monitoring of possible adverse effects occurring during the study took place over the entire study period.
TEWL was assessed by Tewameter® TM 300 (Couraga + Khazaka electronic GmbH, Germany) before, 8 hours, and 14 days after the first application of LCCs. In each test area, 1 measurement was performed per assessment time point. Individual measurement lasted for 60 seconds, with 1 reading collected per second. The average of 10 consecutive readings with the lowest SD represented the TEWL value used for further analysis. The results were expressed in g h–1m–2.
SC hydration measurements were performed by Corneometer® CM 825 (Couraga + Khazaka electronic GmbH) according to the same assessment time schedule as described above. For each assessment time point 6 measurements, which were averaged to obtain the final value for further analysis, were performed per test area. The results were expressed in arbitrary units (a.u.).
Erythema level and melanin content were determined by Mexameter® MX 18 (Couraga + Khazaka electronic GmbH) at the same time points as described above with both values gained within a single measurement in the allocated test areas per assessment time. For erythema measurement, skin reflection was measured at the wavelengths of 568 nm and 660 nm, which correspond to the absorption peaks of haemoglobin and avoid other colour influences ( e.g., bilirubin). For melanin evaluation, the wavelengths of 660 nm and 880 nm were used, which correspond to different absorption rates of the pigments. The results were expressed in a.u.
The skin tolerability of the novel LCCs was assessed by subjective and objective dermatological evaluations conducted at all assessment time points.
The mean basal value and mean change from baseline at each post-application assessment time were calculated, and results are expressed as mean ± standard deviation (SD). Data of the mean change from baseline were statistically analyzed using a paired sample t-test. GraphPad Prism version 9 for Windows was used for statistical analyses. The significance level was set to 0.05.
RESULTS AND DISCUSSION
Skin barrier dysfunction, a major feature of AD, is associated with increased TEWL values, while a decrease in levels signifies an improvement of skin barrier function. Assessment of TEWL with the corresponding probe is performed indirectly by determining the flux of water vapor density at the skin surface relative to the environment in which the measurement is carried out. The measurement quantifies the extent of water diffusion across the SC and thus provides valuable insight into the functionality of the skin's barrier. Therefore, TEWL assessment was utilized in this study to evaluate the impact of the novel LCCs on the barrier integrity of skin.
The results of the TEWL assessment obtained over the entire study course are summarized in Table II with mean absolute values and mean changes from baseline presented due to comparable TEWL levels among the study participants. The single application as well as the subsequent twice-daily treatment within 14 days caused a decrease in TEWL values in the case of all the LCCs studied. These results indicate a positive impact of the LCCs studied on the skin barrier function. However, variations in TEWL values were observed among the formulations with statistically significant differences observed only for some of the LCCs tested. Namely, 8 hours following the first application, a significant difference in mean TEWL values compared with mean basal values was observed in the case of (L/T)Ho30 (–1.98 g h–1m–2; p = 0.03) and (L/T)Fo30 (–1.93 g h–1m–2; p = 0.04). Furthermore, at the end of the treatment period (14 days) with twice-daily application, a statistically significant change in mean TEWL levels from mean baseline was again proven for (L/T)Ho30 (–2.39 g h–1m–2; p = 0.01) and (L/T)Fo30 (–2.68 g h–1m–2; p = 0.01) plus for (L/M)Ho60 (–1.64 g h–1m–2; p = 0.03) and (L/M)Fo60 (–1.69 g h–1m–2; p = 0.03).
It should be noted that among all the LCCs studied, (L/T)Ho30 and (L/T)Fo30 particularly stood out as the only two formulations that showed a 2-fold reduction in TEWL levels in terms of both short-term and long-term effects. It can be assumed that their composition in conjunction with microstructure are the key factors here. Namely, these two LCCs contain the largest proportion of lipids, i.e., 28 % ( m/m), with a high content of essential fatty acids known for their nourishing, immunoregulatory, and anti-inflammatory effects (26–28). Moreover, topical application of lipids may also be beneficial in terms of repairing the deficient lipid composition of atopic skin. In addition, structural characterization using polarized light microscopy and small-angle light scattering showed the predominant presence of tightly packed lamellae in these LCCs (24, 25). Notably, the lamellar microstructure of formulations for dermal application is extremely beneficial due to its great similarity with the SC lipids. Taken together, it appears that both selected lipids, i.e., hempseed or flaxseed oil, along with the lamellar microstructure of the LCCs can efficiently strengthen the skin barrier integrity. Therefore, due to the strengthened barrier function of the skin, excessive water loss from the skin surface is reduced, resulting in decreased TEWL values.
Table II. Absolute transepidermal water loss (TEWL) values and changes from baseline 8 hours after a single application of the novel LCCs (short-term effect) followed by a twice-daily application for 14 days (long-term effect)
Data are given in g h–1m–2.
N = 12. All data are shown as mean ± SD.
p-value* – change from baseline after 8 hours, paired t-test.
p-value** – change from baseline after 14 days, paired t-test.
Dry skin is another important characteristic of AD related to impaired barrier function. Therefore, the moisturizing potential of the novel LCCs was determined by SC hydration assessment as it provides quantitative information on the skin surface hydration levels. The methodology relies on the well-known principle that water exhibits a significantly higher dielectric constant in comparison to most other substances. Hence, the corneometer determines the electrical capacitance of the SC, and the resulting values reflect its hydration levels. Low levels of electrical capacitance correspond to dry skin, while higher levels correlate with well-hydrated skin. Monitoring of the SC hydration is thus useful in assessing the moisturizing efficacy of formulations.
Table III presents the results of the SC hydration measurements over the entire study period. Given similar electrical capacitance values among the subjects, mean absolute values and mean changes from baseline are shown. At 8 hours after the first application, no significant difference in mean skin surface capacitance from mean basal value was observed for any of the LCCs studied. However, after repeated applications twice a day within 14 days, a long-term moisturizing effect of some of the LCCs studied was demonstrated. Namely, statistical comparison revealed a significant difference between baseline and day 14 in case of (L/M)Ho60 (11.78 a.u.; p = 0.00), (L/M)Fo60 (11.11 a.u.; p = 0.00), (L/M)Ho80 (13.95 a.u.; p = 0.00), and (L/M)Fo80 (11.68 a.u.; p = 0.00). This observation can be attributed to the high proportion of water in these formulations, 60 and 80 % ( m/m), respectively), which enhances the skin’s water content and thus restores hydration of the skin surface. In addition, it can be observed that in the case of these four LCCs, the skin-hydrating effect is in positive correlation with the proportion of water in the formulation.
However, it is worth mentioning that the moisturizing effect of dermal formulations for AD is indeed very important, but we should also keep in mind that impaired barrier function is the root cause of dehydrated atopic skin. Therefore, although the other LCCs studied did not show a statistically significant moisturizing effect, the exceptional skin barrier-strengthening characteristics observed in the TEWL assessment for (L/T)Ho30 and (L/T)Fo30 are of utmost importance.
Table III. Absolute skin capacitance values and changes from baseline 8 hours after a single application of the novel LCCs (short-term effect) followed by a twice-daily application for 14 days (long-term effect)
Data are given in Corneometer® CM 825 arbitrary units (a.u.).
N = 12. All data are shown as mean ± SD.
p-value* – change from baseline after 8 hours, paired t-test.
p-value** – change from baseline after 14 days, paired t-test.
Further, other homeostasis skin parameters related to skin barrier function, such as erythema index and melanin index, were also assessed in this study. Regarding AD, a decrease in erythema levels is particularly important as it indicates a decrease in skin inflammation, suggesting a positive response to treatment. However, as a part of the comprehensive assessment of skin barrier function, melanin levels were also determined. The mexameter probe measures the skin colors erythema and melanin simultaneously, based on the absorption/reflection principle. During measurement, the probe emits three specific wavelengths of light, and the light reflected by the skin indicates the erythema and melanin levels.
The results of skin erythema levels obtained throughout the course of the study are shown in Fig. 2, while Fig. 3 displays the results of melanin content measurements. As erythema and melanin index reflect individual-related characteristics of skin (thickness, pigmentation, etc.), the differences in baseline as well as absolute values between subjects are relatively large. For this reason, we presented the results obtained for each individual separately.
The values of the erythema index obtained within the study indicate preserved or decreased erythema for all the LCCs studied with the greatest decrease observed for (L/T)Ho30 and (L/T)Fo30 at both postbaseline time points ( i.e., short-term and long-term exposure). This is in agreement with improved skin barrier function following the application of these two LCCs as noted by decreased TEWL values. The effect observed is also in accordance with literature data on the regenerative effect of hempseed and flaxseed oils (26–28). As none of the LLCs’ components interfere with melanogenesis, the results of the melanin index follow the trend of erythema which was in line with our expectations linked to the measurement’s specificity. Namely, high correspondence between erythema and melanin index relates to the fact that melanin absorbs light in a broad range of wavelengths, including those that contribute to erythema (29, 30). Further, regarding tolerability evaluations, applications of all the novel LCCs were associated with good skin tolerability. The subjective and objective dermatological evaluations of treated skin areas, which were performed at all assessment time points, revealed no difference between the LCCs studied. No subject reported a local or systemic adverse effect to be related to the formulations tested. To conclude it is important to emphasize that the results obtained confirm a low irritating potential of the novel LCCs.
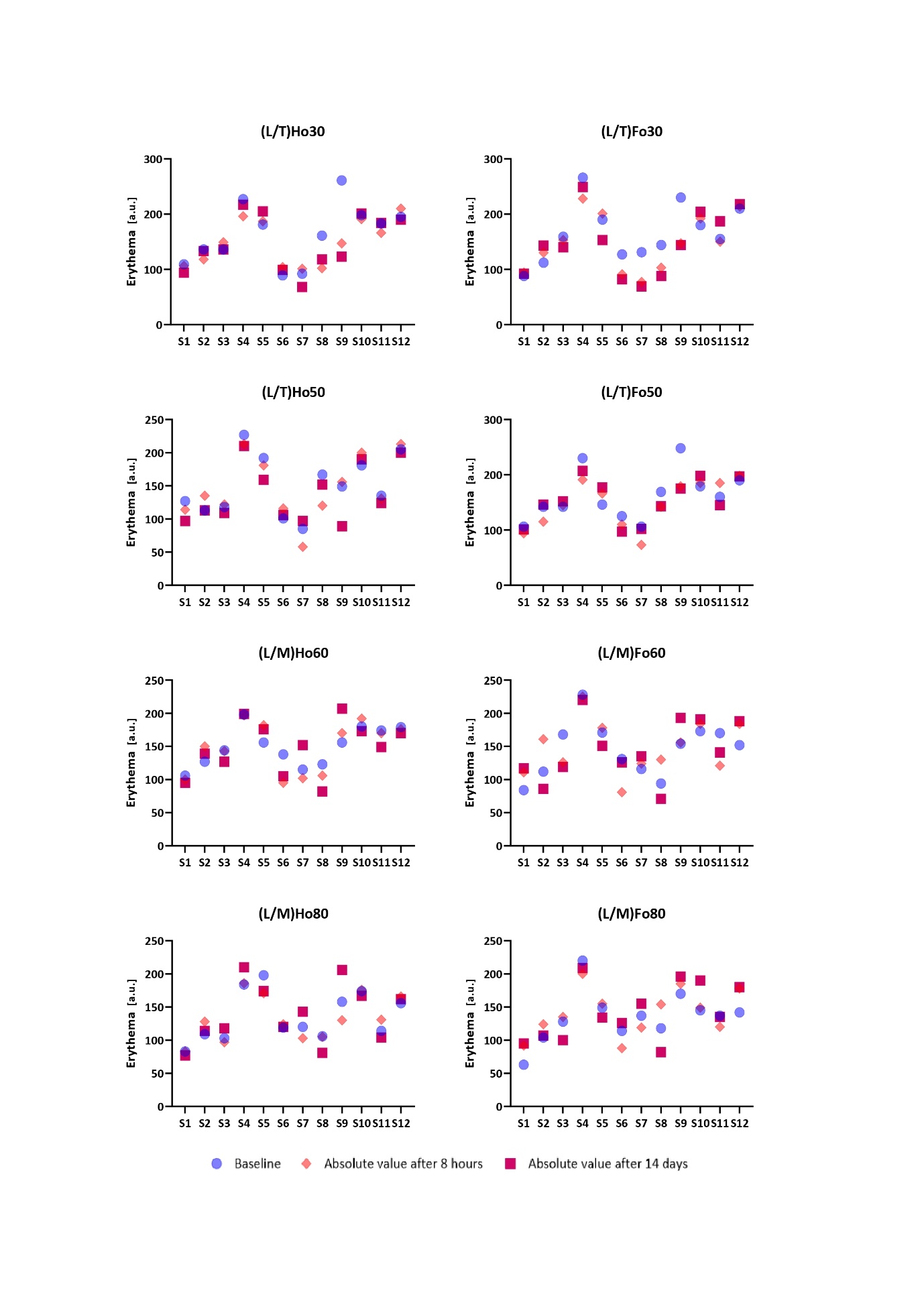
Fig. 2. Absolute erythema values and changes from baseline 8 hours after a single application of the novel LCCs (short-term effect) followed by a twice-daily application for 14 days (long-term effect). The results are presented for each individual separately. The letter “S” indicates the subject, and the number indicates its order number.
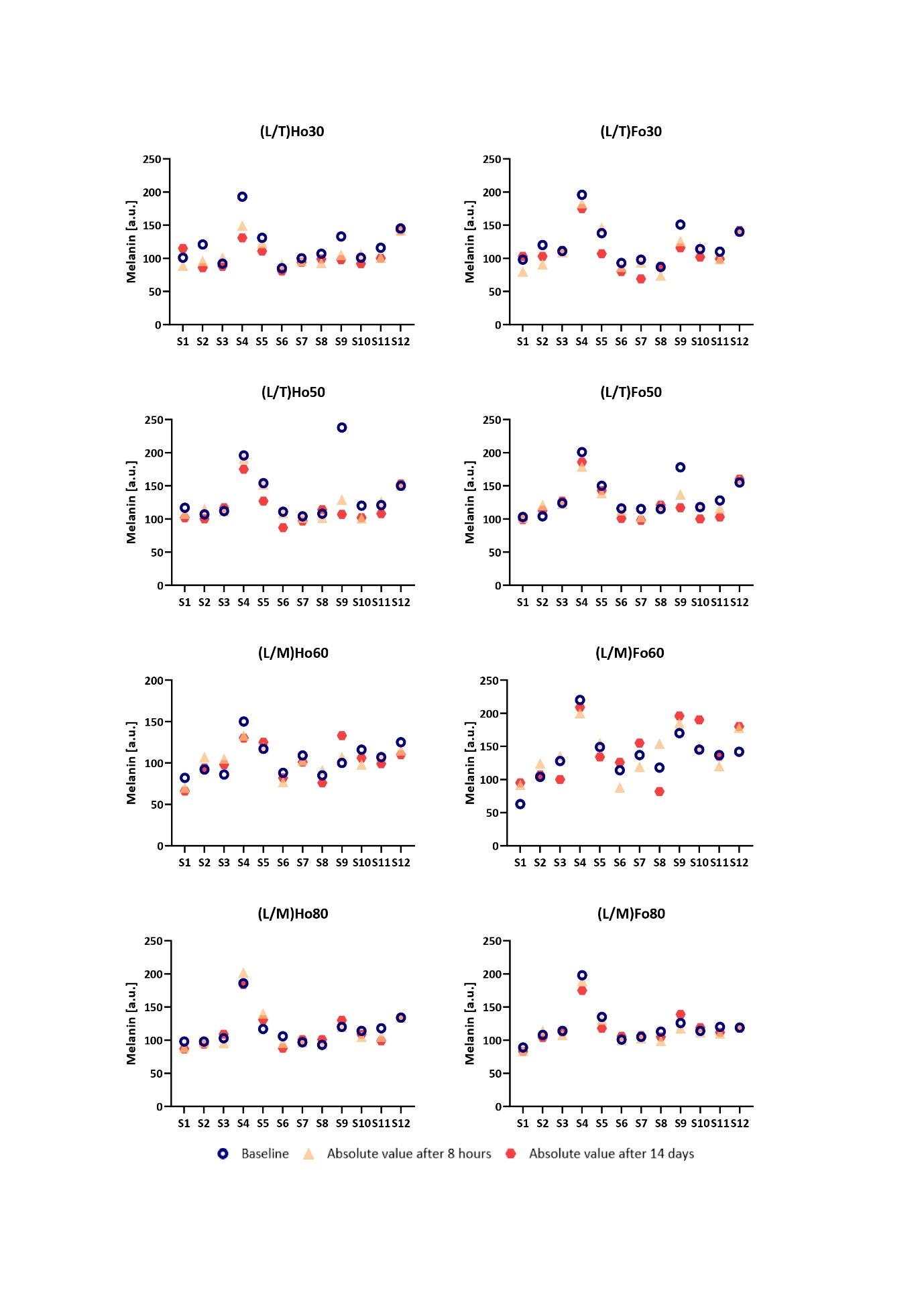
Fig. 3. Absolute melanin values and changes from baseline 8 hours after a single application of the novel LCCs (short-term effect) followed by a twice-daily application for 14 days (long-term effect). The results are presented for each individual separately. The letter “S” indicates the subject, and the number indicates its order number.
CONCLUSIONS
In the present study, eight novel LCCs were evaluated for the first time on healthy adult subjects. The short- and long-term effects of the LCCs on TEWL, SC hydration, erythema index, melanin index, and tolerability were evaluated and compared with basal values. The study regimen included the twice-daily application of the LCCs studied. Limitations of this trial are the small population ( n = 12) of study participants and the lack of a control group. Nevertheless, the applied biometric study represents a valuable tool for the evaluation of skin features in humans at a sub-visible level, especially by measuring TEWL which enables early detection of changes in skin barrier function.
Among all the LCCs studied, (L/T)Ho30 and (L/T)Fo30 stood out as the formulations that provided a remarkable 2-fold reduction in TEWL values in both the short- and long-term exposure, indicating their exceptional skin barrier-strengthening characteristics. In addition, they also demonstrated the most distinctive short- and long-term decrease in skin erythema levels. With the effects observed on healthy subjects, even more beneficial outcomes on AD patients with impaired skin barrier function can be expected. (L/T)Ho30 and (L/T)Fo30 therefore show great potential for clinical use as novel delivery systems for AD treatment, capable of repairing skin barrier function.
Availability of data and materials. – The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments. – The authors thank Tamara Ribać for her contribution to the laboratory work.
Conflict of interest. – M. Vitek and M. Gosenca Matjaž declare that they have no potential conflict of interest.
Funding. – This research was funded by the Slovenian Research and Innovation Agency under grant number P1-0189.
Authors contributions. – Conceptualization, M.V. and M.G.M.; methodology, M.G.M.; formal analysis, M.V.; investigation, M.V. and M.G.M.; resources, M.G.M.; original draft preparation, M.V.; review and editing, M.G.M.; visualization, M.V.; supervision, M.G.M. All authors have read and agreed to the published version of the manuscript.
