INTRODUCTION
Plants are an important source of bioactive compounds that have been used either in traditional medicine or in modern pharmaceutical industries. Genus Cistus ( Cistaceae) contains several species of medicinal relevance. There are around 20 species in this genus among which 12 species belong to the Moroccan flora (1). Cistus albidus L . is one of the most common species, which is distributed mainly in the Mediterranean region, particularly in northern Morocco (Rif region) (2).
Studies have revealed that all species of Cistus have already been used in non-conventional medicine for their anti-ulcerogenic, wound healing, anti-viral, anti-inflammatory, antioxidant, and anti-diabetic effects (3–8). The scientific investigation of this genus seems mandatory and attracts the interest of many researchers. So far there are only a few studies investigating C. albidus. For this reason, this research was designed to establish the chemical (flavonoids, proanthocyanidin, and total phenols) and mineral composition of C. albidus extracts, as well as to determine their potential use as an antioxidant, analgesic, and anti-inflammatory agent.
EXPERIMENTAL
Plant material
Leaves and seeds of C. albidus were collected for this study in February 2022 in the Taounate region (Ifrane – Zrizer forest) (N: 34°35′2, W: 4°38′38, altitude: 488 m). A reference specimen (0012023KC1) was added to the herbarium of NAMAP (National Agency for Medicinal and Aromatic Plants) in Taounate, Morocco.
Preparation of plant extracts
To obtain the aqueous and hydroethanolic extracts of the leaves and seeds of C. albidus, a traditional maceration method was used. For this purpose, 100 g of raw material was macerated with 1 L of distilled water or 70 % aq. ethanol, with stirring at 400 rpm for 24 h at laboratory temperature. The preparation was filtered using Whatman paper. The filtered extracts were subsequently frozen at –80 °C and lyophilized. The resulting dry extracts were stored at 4 °C until further use. The yields for aqueous and hydroalcoholic extracts of leaves were 21.1 and 17.8 % ( m/ m), resp., while for the seeds, they were 10.3 and 9.6 % ( m/ m).
Chemical reagents and standards
Standard compounds such as quinic acid, salicylic acid, gentisic acid, gallic acid, ferulic acid, p-coumaric acid, vanillic acid, sinapic acid, resveratrol, pyrocatechol, phloridzin, phloretin, naringenin, quercetin 3- O-glucuronide, kaempferol, epicatechin, dihydroferulic acid, daidzein, chlorogenic acid, caffeic acid, 4-hydroxyphenylacetic acid, 3,4,5-trimethoxycinnamic acid, quercetin, isoquercetin, and avicularin were purchased from Merck KGaA (Germany) and Carl Roth GmbH (Germany), protocatechuic acid, catechin, luteolin, procyanidin B2, apigetrin, taxifolin, cynaroside and aromadendrin from PhytoLab (Germany), rutin, isorhamnetin, apigenin, astragalin and quercitrin from Extrasynthese (France). All chemicals used were of analytical grade.
All solvents used in this study were procured from Sigma Aldrich (USA). Folin-Ciocalteu reagent, ascorbic acid, butylated hydroxytoluene (BHT), 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,2′-diphenyl-1-picrylhydrazyl (DPPH) and carrageenan were from from Sigma-Aldrich. The commercial drugs indomethacin, diclofenac sodium, and acetylsalicylic acid were purchased from Pharma5 (Morocco) in the form of tablets.
Elemental analysis
The mineral composition of leaves and seeds of C. albidus (silver, aluminum, copper, boron, cobalt, chromium, titanium, iron, calcium, potassium, magnesium, manganese, sodium, nickel, phosphorus, palladium, silicon, tin, zinc, and vanadium) was determined by inductively coupled atomic emission spectrometry (ICP-AES, ICPE-9000, Shimadzu, Japan), according to the literature method (9). Briefly, 1 g of C. albidus leaf or seed powder was added to 5 mL of nitric acid (0.1 mol L–1) and after careful heating of this mixture until the residue was dissolved, 10 mL of nitric acid (0.1 mol L–1) and 25 mL of distilled water were added.
Total phenolics content (TPC) assay
The phenolic content of the hydro-ethanolic and aqueous extracts of leaves and seeds of C. albidus was estimated using Folin-Ciocalteu reagent (10). Briefly, 25 μL of the sample was mixed with 250 μL of Folin-Ciocalteu reagent and 200 μL of sodium carbonate. After 2 h of incubation in the dark at room temperature, absorbance was read at 760 nm. Gallic acid was used as the standard and the results are expressed as mg gallic acid equivalent g–1 dm of extract (GAE).
Total flavonoid content (TFC) assay
The total flavonoid content of the hydro-ethanolic and aqueous extracts was determined using the known method (11) with some modifications. Briefly, to 25 μL of each extract 150 μL of AlCl3 (2 % in ethanol) and 150 μL of NaNO2 (1 %) were added. After 1 h of incubation in the dark at room temperature, the absorbance was measured at 510 nm. Quercetin (in 96 % ethanol) was used as a standard to construct the calibration curve. The results were expressed as mg of quercetin equivalent g–1 dm of extract (QE).
Assay of proanthocyanidin content (PC)
The proanthocyanidin content of the hydro-ethanolic and aqueous extracts was determined using the reported method (1). Fifty μL of the extract was mixed with 1500 μL of 4 % vanillin solution (in ethanol) and then 750 μL of conc. HCl was added. The well-mixed solution was incubated at room temperature in the dark for 20 min, then the absorbance was measured at 500 nm. Catechin (100–1000 μg mL–1) was used as a standard and results are expressed as mg of catechin equivalent g–1 dm of extract (CE).
Quantification of phenolic constituents by UPLC-ESI-MS/MS
The aqueous extract of the leaves of C. albidus was analyzed using an Acquity liquid chromatography UPLC system coupled to a Waters Xevo TQ-S triple quadrupole system (Waters, USA) as described by Zuoyi et al. (12). Briefly, separation was conducted on a Waters Acquity UPLC BEH C18 column (130 Å, 1.7 μm, 2.1 × 150 mm) at 40 °C. The flow rate was 0.196 mL min–1. HPLC-grade solvents, 0.1 % formic acid in water (A), and 0.1 % formic acid in acetonitrile (B) were used. The elution gradient for solvent B was as follows: 1 % B from 0.0 to 9.91 min, 26 % B from 9.91 to 18.51 min, 65 % B from 18.51 to 18.76 min, maintained at 99 % B from 18.76 to 20.76 min, 1 % B from 20.88 to 23 min. The injection volume was 5 μL. The detection was done via an electrospray ionization (ESI) interface in multiple reaction monitoring (MRM) and negative ionization mode (Table III). The identification of the molecules was confirmed by comparing the typical fragments identified for each standard and for the aqueous extract of C. albidus. The needle capillary voltage was set at 3.5 kV. The source operated at 300 °C.
All ESI and mass spectrometer conditions were optimized individually for each target compound. Data acquisitions and quantifications were performed using Waters® Mass-Lynx software. The chromatograms of the target compounds are presented in the supplementary file.
Antioxidant activity
DPPH assay. – The known method (13) was used to test the ability of the extracts to trap the DPPH radical (DPPH•) , (2.2-diphenyl-1-picrylhydrazyl). DPPH solution (0.2 mmol L–1) in ethanol (96 %) served to obtain an absorbance of 0.7 at 515 nm. Then, 25 μL of hydroethanolic and aqueous extracts of leaves and seeds of different concentrations (0–1044 μg mL–1) were mixed with 825 μL of DPPH reagent. The reaction mixture was vortexed and then left in the dark at laboratory temperature for 30 min. The discoloration produced was measured at 517 nm using a spectrophotometer (UV-1700APC, Macylab, China). The results obtained are compared with the BHT standard. The 50 % inhibitory concentration was calculated using various concentrations, where the percent inhibition ranged between 20 and 80 %. The results were reported in µg mL–1.
ABTS assay. – The described procedure (14) was used to measure the activity of hydro-ethanolic and aqueous extracts of leaves and seeds of C. albidus in blocking the cationic radical (ABTS•+) . An aqueous solution of ABTS (7 mmol L–1) was combined with 2.5 mmol L–1 potassium persulfate to regenerate ABTS•+. The solution was left at room temperature and in the dark for 16 hours. Using a spectrophotometer, the mixture was adjusted with ethanol (96 %) to give an absorbance of 0.70 at 734 nm. Then, 25 μL of the extract at different concentrations was mixed with 825 μL of ABTS•+ reagent and the absorbance was measured after 6 min. The standard was ascorbic acid. The ABTS radical scavenging activity was expressed in µg mL–1.
Reducing power assay (RP). – The reducing potential of the extracts was determined according to the known method (15) with some adaptations. Briefly, 1 mL of the extract at different concentrations (0–1000 μg mL–1) was mixed with 2.5 mL of 0.2 mol L–1 phosphate buffer (pH 6.6) and 2.5 mL of 1 % potassium ferricyanide solution. The mixture was incubated in a water bath at 50 °C for 20 minutes. Following incubation, 2.5 mL of 10 % trichloroacetic acid was added to stop the reaction, and the tubes were centrifuged at 3000 rpm for 10 min. An aliquot (2.5 mL) of the supernatant was then combined with 2.5 mL of distilled water and 0.5 mL of 0.1 % aqueous FeCl3 solution. The absorbance was measured at 700 nm and compared with an established standard, ascorbic acid.
Total antioxidant capacity (TAC). – The method used for the phosphomolybdate (PPM) test was as follows (11). Phosphomolybdate reagent was prepared from H2SO4 (0.6 mol L–1), NaH2PO4 (28 mmol L–1), and ammonium molybdate (4 mmol L–1). Briefly, 25 μL of C. albidus extracts (hydro-ethanolic and aqueous of leaves and seeds) were mixed with 1 mL of phosphomolybdate reagent. The absorbance was read at 700 nm after 90 min of incubation at 96 °C. The results are given in mg of ascorbic acid equivalents g–1 dm of extract (AAE) from a calibration graph.
Experimental animal protocols
In this study, a total of 84 adult male and female Wistar albino rats (3 months of age) with a body mass of 180–200 g were used. The rats were bred in the NAMAP animal facility (Morocco). All experimental rats were housed under standard environmental conditions (5 % humidity, 22 ± 3 °C, and 12 h light/dark cycles) and had easy access to water. Experimental rats were offered 16 h of fasting before testing. The study has received approval from the Institutional Ethics Committee for the Care (16) and Use of Laboratory Animals at the Faculty of Sciences, Meknes, University Moulay Ismail, Morocco (reference number 04/2019/LBEAS).
Anti-inflammatory activity
Potential anti-inflammatory activity was assessed using the carrageenan-induced rat paw edema test (17). Carrageenan (1 %, m/ V) prepared in (NaCl 0.9 %) was used to induce paw edema. A total of 18 rats were divided into three groups ( n = 6 per group) and injected with carrageenan to induce edema. Group 1 was treated with aqueous extract of the leaves (500 mg kg–1 bm) given orally 30 minutes before carrageenan injection. Group 2 was treated with distilled water (5 mL kg–1 bm) as a negative control, and group 3 was treated with indomethacin (10 mg kg–1 bm) as a positive control. Paw volume was measured before and 1, 2, 3, 4, 5, and 6 hours after carrageenan injection using a LE 7500 plethysmometer (Panlab, Spain).
Inhibition of albumin denaturation
To investigate the possible in vitro anti-inflammatory efficacy of C. albidus leaves aqueous extract, we used the albumin denaturation assay according to the literature (18) with slight modifications. Briefly, 0.5 mL of bovine serum albumin (BSA) (0.2 %) prepared in Tris buffer (pH 6.8) was mixed with 0.5 mL of aqueous extract of leaves at different concentrations or with the standard (diclofenac sodium). The samples were first incubated for 15 min at 37 °C and then immediately shifted to 72 °C for 5 min. When the tubes were cooled, the absorbance was read at 660 nm.
Potential analgesic activity
Writhing test. – Contortion provoked by acetic acid was performed as previously reported (19). A total of 18 rats were randomized into three groups of 6 rats each. Group 1 (control) was treated with 0.9 % saline, group 2 was pre-treated with standard drug acetylsalicylic acid (150 mg kg–1 bm), and group 3 received aqueous extract of leaves (500 mg kg–1 bm).
Thirty minutes after the treatments, 3.75 mL kg–1 bm of intraperitoneal acetic acid solution (3 %) was injected to cause contortions. The rats were housed individually in transparent cages, and the contortions were counted during a 10-min observational period beginning immediately after the injection of acetic acid.
Tail flick test. – This test was conducted as previously detailed (20). Rats were separated into 4 groups. Each group consisted of 6 rats. The first group was treated with 0.9 % saline, the second group was pre-treated with the standard drug acetylsalicylic acid (150 mg kg–1 bm). The third and fourth groups were administered orally aqueous extracts of C. albidus at a dose of 250 or 500 mg kg–1 bm. Rats were subjected to an analgesiometer (ANALGESY- METER LE 7106, Panlab), in which the terminal part of the tail was positioned. The tail reaction was measured as a sensation of radiant heat from the spine; a cutoff time of 20 s was preserved. The tail test was performed in each batch before treatment and 30, 60, 90, and 120 min after drug administration.
Plantar test. – To determine the nociceptive response to thermal stimuli, we performed a plantar test (UGO BASILE model 37370, Italy) following the method previously reported (21). Rats were divided into four groups of six animals each, as outlined: group 1: administered 0.9 % saline, group 2: pre-treated with acetylsalicylic acid (150 mg kg–1 bm), group 3: given the aqueous extract of the leaves (250 mg kg–1 bm), group 4: received the aqueous extract of the leaves (500 mg kg–1 bm).
The rats were placed within a transparent plastic chamber featuring a glass floor. Before the test, the animals were allowed to acclimate to their surroundings for five minutes. The right hind paw was examined with a moving infrared heat light. The time-to-paw withdrawal response was automatically measured at 30-minute intervals to prevent thermal sensitization and minimize behavioral disturbances.
The findings are reported as the mean ± standard error. Graph Pad Prism 8.02 was used to conduct all statistical analyses. Analysis of variance (ANOVA) and multiple comparisons (Tukey's test) on the different activities of C. albidus extracts were conducted; differences are deemed significant when p ≤ 0.05.
RESULTS AND DISCUSSION
Mineral composition
As documented in Table I, the seeds of C. albidus had higher contents of K+ (1120 mg kg–1) and phosphorus (64.0 mg kg–1) than the leaves, However, C. albidus leaves contain higher amounts of Ca2+ (346 mg kg–1) and Mg2+ (93.9 mg kg–1) than the seeds, whereas the contents of silver, cobalt, lead, tin, titanium, and vanadium ions were detected in small amounts (less than 28 mg kg–1), in both seeds and leaves.
This study showed that the seeds and leaves of C. albidus can be important sources of potassium, calcium, magnesium, and phosphorus. Similar results have been documented previously (1) for C. monspeliensis and C. salviifolius.
Table I. Elemental analysis
a The seeds and leaves’ powder of C. albidus.
Total phenolics, proanthocyanidins and flavonoids
The total amounts of phenolic compounds, proanthocyanidins, and flavonoids in hydro-ethanolic and aqueous extracts of C. albidus seeds and leaves are shown in Table II. Hydroethanolic extract of the leaves contained the highest concentration of total polyphenols and flavonoids (343.7 mg GAE g–1 dm and 38.3 mg QE g–1 dm, resp.). However, the highest level of proanthocyanidins was determined in seeds’ aqueous extract (165.0 mg CE g–1 dm). In a study by Sayah et al. (1), the leaves of C. salviifolius were analyzed, and the total content of phenolics, flavonoids, and proanthocyanidins in the water extract was estimated to be 408.4 mg GAE g–1 dm, 140 mg of rutin equivalents (RE) g–1 dm and 154.1 mg CE g–1 dm, resp.
The content of phenolic constituents in the aqueous extract of C. albidus is closely linked to its botanical origin, the collection season, and the climatic conditions (22).
Table II. Total phenolics, proanthocyanidins and flavonoids
TPC – total phenolic compounds, TPC – total phenolic compounds, PC – proanthocyanidin content, GAE – gallic acid equivalent, QE – quercetin equivalent, CE – catechin equivalent
Mean ± SEM, n = 3.
Quantification of phenolic compounds
The results obtained by UPLC-ESI-MS/MS are presented in Tables III and IV. In the aqueous extract of leaves of C. albidus, thirty-four phenolic compounds were quantified. The most abundant are catechin (1627.6 mg kg–1), quercitrin (1235.8 mg kg–1), and gallic acid (628.2 mg kg–1). Other components detected were salicylic acid, 4-hydroxyphenylacetic acid, p-coumaric acid, ferulic acid, dihydroferulic acid, sinapic acid, resveratrol, 3,4,5-trimethoxycinnamic acid, apigenin, vanillic acid, naringenin, phloretin, kaempferol, luteolin, aromadendrin, epicatechin, quercetin, taxifolin, isorhamnetin, chlorogenic acid, avicularin, gentisic acid, isoquercetin, quinic acid, astragalin, rutin, protocatechuic acid, caffeic acid, phloridzin, quercetin-3- O-glucuronid (miquelianin) and procyanidin B2. The identification of these compounds was based on their MS/MS profiles (precursor and product ions) and retention times, which were compared with data from reference standards.
Antioxidant activity
Comparison of the antioxidant power of all the extracts studied by DPPH, ABTS, and RP tests with those of BHT and ascorbic acid are reported in Table V.
In the DPPH assay, data documented that the tested C. albidus extracts have a dose-dependent radical scavenging effect. Indeed, the hydroethanolic extract of the leaf showed slightly higher anti-DPPH radical activity (50 % inhibitory concentration = 3.2 μg mL–1) than that of the seeds extract (50 % inhibitory concentration = 4.8 μg mL–1.
Regarding the ABTS assay, there were no marked differences between the examined extracts.
In addition, the RP method gave 50 % inhibitory concentration values of 2.3 and 2.1 μg mL–1 for the aqueous and hydroethanolic extracts of C. albidus leaves, resp.
The total antioxidant activity (TAC) of the studied extracts ranged from 47.7 to 106.0 mg AAE g–1 dm. C. albidus leaf extracts showed the highest result for molybdenum reduction in the hydroethanolic medium (106 mg AAE g–1 dm), followed by the aqueous extract (95.2 mg AAE g–1 dm), while the lowest result was found for the hydroethanolic extract of seeds (47.7 mg AAE g–1 dm) (Table V).
Our results are consistent with the results of Sayah et al. (1) who used the same tests but on other Cistus species. In addition, there is another study that was focused on the leaves of C. creticus (23) using two methods, FRAP and DPPH: the IC50 values of the crude extract were shown to be 100 and 26.6 μg mL–1, resp. Similarly, Haida et al. (24) reported that the water-acetone extract of C. monspeliensis was the most active against DPPH and ABTS radicals and showed IC50 values of 79 and 10.2 μg mL–1, resp.
Potential anti-inflammatory activity
The results for the anti-inflammatory potential of the aqueous extract of leaves of C. albidus by carrageenan-induced rat paw edema are presented in Fig. 1. Relative to the control group, the aqueous extract showed significant inhibition of the paw edema from one hour to six hours after carrageenan injection. The extract (500 mg kg–1 bm) exhibited an inhibitory action of 76.1 % and indomethacin (10 mg kg–1 bm) of 75.1 %, after 6 hours of treatment.
Table III. Retention times and optimized mass spectrometry parameters of 34 phenolic compounds detected using multiple reaction monitoring (MRM) transitionsa
ES-: negative electrospray
a See Table IV and the Supplementary file.
Table IV. Quantification of phenolic components in aqueous extract of leaves of C. albidusa
nd – not detected
Mean ± SEM, n = 3.
a Quantification is achieved through a calibration range established by a series of dilutions using standard stock solutions, coefficient of determination R2 > 0.991.
Table V. Antioxidant activity of C. albidus extracts
AAE – ascorbic acid equivalents
Mean ± SEM, n = 3.
The presented data go hand in hand with those documented by Sayah et al. (19) who reported that the aqueous extracts of the leaves of C. monspeliensis and C. salviifolius possess 85.7 and 91.5 % inhibitory activity 6 h after the carrageenan injection, resp. Another similar result from El Hamsas et al. (21) revealed that the aqueous extract of C. ladaniferus leaves showed a strong anti-inflammatory action in the carrageenan-induced paw edema model.
Carrageenan-induced inflammation follows a three-step process, involving the initial secretion of serotonin and histamine, followed by bradykinin-induced inflammation, and finally the presence of prostaglandins (25, 26). Our aqueous extract may exert its effects by inhibiting the secretion of vasoactive substances and/or reducing prostaglandin production.
Indeed, studies have demonstrated that quercetin and kaempferol present in our aqueous extract of the leaves exhibit anti-inflammatory activity by inhibiting the COX-2 and iNOS enzymes (27).
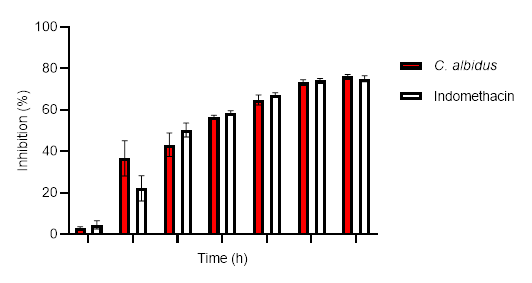
Fig. 1. Anti-inflammatory effects of aqueous extract (500 mg kg-1 bm) of C. albidus (mean ± SEM, n = 3).
Inhibition of albumin denaturation
Table VI presents the 50 % inhibitory concentration values of the in vitro anti-inflammatory effect. It is indicated that the aqueous extract of C. albidus leaves (191.0 μg mL–1) inhibited denaturation of bovine serum albumin in a dose-dependent manner.
Table VI. Inhibition of albumin denaturation
| 50 % inhibitory concentration | |
|---|---|
| C. albidus leaves aqueous extract (μg mL– 1 ) | 191.07 ± 1.73 |
|
Diclofenac sodium (μg mL– 1 ) Diclofenac sodium (μmol L– 1 ) |
181.63 ± 1.04 570.9 ± 3.28 |
Mean ± SEM, n = 3.
This result is probably due to the high content of polyphenols and flavonoid compounds present in Cistus species, which would promote immunomodulatory and anti-inflammatory actions (28). On the other hand, Ali and coworkers (29) revealed that phenolic compounds (caffeic acid, coumaric acid, and gallic acid) increase the thermal stability of bovine serum albumin (BSA); also, ferulic acid has been documented to have the same effect (30).
Analgesic effects
To study the analgesic and central antinociceptive activity of the aqueous extract of C. albidus leaves two tests, namely the tail flick and the plantar test, were performed.
The acetic acid-induced contortion model is primarily employed to evaluate peripherally acting analgesic activity. In this test, the effect of the aqueous extract of C. albidus leaves on the rat is presented in Table VII. The number of twists (in 30 minutes) was highest in the control group (46) and lowest in the aqueous extract (23 at 500 mg kg–1) and acetylsalicylic acid (26 at 150 mg kg–1) groups.
Table VII. Effect of C. albidus leaves on acetic acid-induced writhing in rats
| Treatment | Dose (mg kg – 1 bm) | Number of writhes | Inhibition (%) |
|---|---|---|---|
| Negative control | – | 46.33 ± 1.52 | – |
| Acetylsalicylic acid | 150 | 26 ± 1.72 | 43.9 |
| C. albidus leaves extract | 500 | 22.66 ± 1.87* | 51.1 |
Mean ± SEM, n = 3.
Statistically significant difference versus negative control:* p < 0.0001.
The tail analgesia method showed dose-dependent antinociceptive activity by oral administration of the aqueous extract of C. albidus leaves (Table VIII). Analgesia started at 30 minutes and remained for 120 minutes relative to the control. The aqueous extract of leaves showed significant inhibition ( p < 0.01) at both doses (200 and 500 mg kg–1 bm). The group treated with 500 mg kg–1 bm showed a reaction time of 13.03 s at 120 min, while the group treated with saline had a reaction time of 4.03 s and the group treated with acetylsalicylic acid had the longest reaction time of 15.13 s.
Table VIII. Tail-flick latency with C. albidus aqueous extract of leaves in the tail-flick model of pain
Mean ± SEM, n = 3.
Statistically significant difference vs. negative control: *p < 0.05,** p < 0.01; vs. C. albidus (200 mg kg–1 bm): # p < 0.05;## p < 0.01.
In the plantar test, the aqueous extract of C. albidus leaves (200 and 500 mg kg–1 bm) showed potent analgesic effects after 90 min ( p < 0.05) which maintained constant throughout the observation time (20 min) (Fig. 2); acetylsalicylic acid significantly increased the pain tolerance time ( p < 0.01). Similar results were reported in the studies of Andrés et al. (31) and Ark et al. (32), in which aqueous and chloroform extracts of the leaves of C. populifolius and C. laurifolius revealed important central analgesic activities. Similarly, Küpeli and Yesilada (33) isolated molecules of 3,7- O-dimethyl kaempferol, 3- O-methyl quercetin and 3,7- O-dimethyl quercetin from the leaves of C. laurifolius that exhibited analgesic and anti-inflammatory activity. According to Sayah et al. (19), aqueous extracts of the Moroccan plants ( C. monspeliensis and C. salviifolius) significantly improved reaction times to thermal stimuli while reducing the abdomen contortion. Characterization of the chloroform extract of C. albidus by Tahiri et al. (34) showed that kaempferol and quercetin were the main constituents. Furthermore, Barrajõn-Catalán et al. (35) isolated in the aqueous extract of C. albidus from Spain the following molecules: quercetin-3- O-2-cumaroyl-rutinoside, quercitrin, and myricitrin. Previous studies have shown that flavonoid compounds, particularly flavonols, have anti-nociceptive and anti-inflammatory properties. Myricetin-3- O-α-rhamnoside (myricitrin) could totally reduce TNF-α-induced nociception in mice (36), and showed strong anti-inflammatory activity in vivo in carrageenan-induced paw edema according to Meotti and collaborators (37).
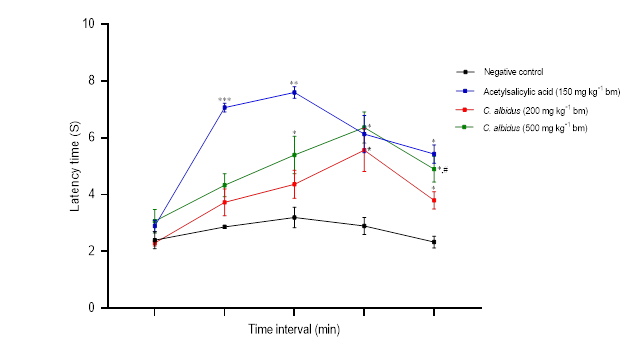
Fig. 2. Effect of C. albidus leaves aqueous extract on plantar test nociceptive responses (mean ± SEM, n = 3). Statistically significant difference vs. negative control: * p < 0.05, ** p < 0.01, *** p < 0.001; vs. C. albidus (200 mg kg–1 bm):# p < 0.05.
Cistus albidus L.‘s aqueous extract of the leaves demonstrates moderate and dose-dependent analgesic effects in the three utilized pain models. These effects are possibly attributable to the involvement of both peripheral and central inhibitory mechanisms.
CONCLUSIONS
Based on the results of our study, aqueous and hydro-ethanolic extracts of leaves and seeds of C. albidus might be recommended as antioxidant agents. Furthermore, the aqueous extract of leaves of C. albidus had shown anti-inflammatory and analgesic activity, which could be induced by peripheral and central processes. It is interesting to note that the mineral content of this plant could add to these health-beneficial properties. Our findings support the traditional medical practice of treating many forms of pain using C. albidus leaves and revealed that the content of phenolic compounds, flavonoids such as catechin, quercetin, gallic acid, isoquercetin, astragalin, quinic acid, rutin, in our extracts may be responsible for the antioxidant, anti-inflammatory and analgesic activities.
However, further investigations should be addressed to validate these activities with the determination of the mechanisms of their action. In addition, toxicological experiments are needed to validate the safety of C. albidus extracts at different doses and administration methods.
Supplementary materials available upon request.
Acknowledgements. – The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the project number (RSP2022R457), and are grateful to Professor Amina Bari, Faculty of Sciences, Dhar El Mahraz, University of Sidi Mohamed Ben Abdellah, Fez, for the identification of plant species.
Conflict of interest. – The author declares that there are no conflicts of interest.
Authors contributions. – Conceptualization, A.Z. and N.E.M.; methodology, Y.E.; software, T.B; validation, H.L.; formal analysis, M.C., H.A; investigation, A.E; resources, A.M.S.; data curation, A.Z., H.N.; writing, original draft preparation, A.Z, B.S., A.E.; writing, review and editing, A.Z, M.B.; visualization, L.H.; supervision, L.H.; project administration, L.H., Y.B.J.; funding acquisition.; Y.B.J. All authors have read and agreed to the published version of the manuscript.
