INTRODUCTION
Multiple myeloma is one of the frequently reported hematological neoplastic disorders which is characterized by the malignant proliferation of plasma cells within the bone marrow tissue (1). Often associated with a number of physiological and clinically heterogenous facets, this disease is characterized by a very low prognosis of survival, accounting for approximately ten percent of blood cancer cases and ranked as the second most common blood cancer (2). The overall five-year survival rate of multiple myeloma is around 40 percent (3). Despite the recent advancements in the therapeutic procedures applied against human multiple myeloma, satisfactory clinical success is far from being achieved against this malignancy and the survival rates are still not very promising owing to higher rates of metastasis and drug resistance (4). It is thus inevitable to look for new therapeutic options, for example, chemical molecules with better healing action against multiple myeloma. For this purpose, the natural compounds are being screened and their anticancer therapeutic activities are continuously revealed. The compounds obtained from natural sources are particularly preferred due to their potential to affect multiple targets in multiple myeloma with less pronounced side effects (5). There are reports that natural compounds belonging to the flavonoid class of plant secondary metabolites like quercetin exhibit antiproliferative and pro-apoptotic effects against the multiple myeloma cells, in vitro (6). Also belonging to flavonoids, deguelin is described as a rotenoid type of flavonoid molecule isolated from the African plant Mundulea sericea belonging to Fabaceae. This compound besides possessing high antioxidant and ant-inflammatory potential is valued for its anticarcinogenic effects against a number of human cancer cell lines (7). This compound was shown to exhibit antitumorigenic activity against human esophageal cancer cells (8). Interestingly, deguelin was shown to sensitize the pancreatic cancer cells to doxorubicin treatment (9). Previous studies have shown that deguelin had a pro-apoptotic effect on multiple myeloma cells, induced cell cycle arrest, and sensitized multiple myeloma cells to radiation therapy (10–12). With the aim to elucidate potential mechanisms of action, the anticancer effects of deguelin were further elaborated in the present study against multiple myeloma cells. As a result, the cancer cells showed significantly lower proliferation, migration, and invasion when treated with deguelin. When deguelin was applied, the cells entered the process of apoptosis and G2/M phase cell cycle arrest by modulating the expression of cell cycle regulatory mRNAs. Deguelin caused multiple myeloma cells to undergo apoptosis and this was mediated by the inhibition of Akt and an increase in the activation of the p38 MAPK and p53 pathways. The study, therefore, brings insight into the antiproliferative and antimetastatic effects of deguelin against multiple myeloma cells suggesting its potential for further development as a small molecule-therapeutic agent.
EXPERIMENTAL
Chemicals
U266 transformed cancer cells and normal plasma cells (PCs) were purchased from the Cell Bank of the Chinese Academy of Sciences (China). RPMI-1640, DMEM, and penicillin/streptomycin antibiotics were obtained from (Gibco, USA). Fetal bovine serum (FBS) was purchased from Hyclone. MTT reagent was obtained from Sigma-Aldrich. The GSH assay kit (GSH-400) was obtained from Oxis International (USA). Annexin V-FITC was purchased from Thermo Fisher Scientific (USA). Propidium iodide and RevertAid First strand cDNA synthesis kit were purchased from Thermo Fisher Scientific. SYBR Green RT-PCR kit was obtained from Takara Shuzo Co. Ltd. (Japan).
Cell line growth and maintenance
The propagation of the multiple myeloma cell line (U266) and PCs was carried out at 37 °C with 5 % CO2 in a controlled incubator. The cells were cultured in RPMI-1640 medium supplemented with 10 % FBS and 1 % penicillin/streptomycin to provide essential nutrients and support their growth and viability. These optimal culture conditions ensured the proper maintenance and proliferation of both U266 cells and PCs for experimental and research purposes.
The cells were cultured in T-75 flasks until they reached 80 % confluence. Routine trypsinization (using 0.05 % trypsin/EDTA) was performed to detach the cells from the flask. The cell count was determined using an automated cell counter (NucleoCounter, New Brunswick Scientific). This process allowed for efficient and accurate measurement of cell numbers during routine cell culture and experimental procedures.
MTT assay
U266 cancer cells and normal plasma cells were incubated with varying deguelin concentrations (0.78, 1.56, 3.12, 6.25, 12.5, 25, 50, and 100 µmol L–1) for 24 h at 37oC in 96-well plates. 15 µL MTT reagent was added and their re-incubation was performed at 37 °C for 2.5 h. After harvesting, DMSO was used for re-suspending the cell pellets. In the end, the OD570 value was recorded for each sample using a spectrophotometer.
Phase contrast microscopy
The cell morphological parameters were analyzed using a phase contrast microscope. The U266 cancer cells without or with deguelin treatment (6, 12, or 18 µmol L–1) for 24 h at 37 °C were harvested through centrifugation and then PBS washed. The cells were finally observed under the phase contrast inverted microscope (Leica DMI 3000B, Germany) for the assessment of the morphometric study.
Clonogenic assay
Using a hemocytometer, cell suspension containing roughly 5000 U266 cancer cells without or with deguelin treatment (6, 12, or 18 µmol L–1) was added to each well of a 6-well plate and grown for 2 weeks to form colonies at 37oC with 5 % CO2. The colonies were then fixed using methanol and subsequently stained with crystal violet stain. The pictures were taken and colonies were counted and a comparison of colonies was done in terms of percent values.
GSH assay
A commercial colorimetric assay kit was used for the detection of GSH levels. U266 cancer cells were grown in a 12-well plate (2 mL medium/well) for 24 h. Cells were treated with different concentrations of deguelin (6, 12, or 18 µmol L–1) for 24 h at 37 °C. After treatment, cells were harvested and homogenized in a metaphosphoric working solution. After centrifugation, 50 μL of R1 solution (a chromogenic reagent in HCl) was added to 900 μL of the supernatant, and the mixture was gently vortexed. Subsequently, 50 μL of R2 solution (30 % NaOH) was added, and the mixtures were incubated at 26 °C for 10 minutes. After another round of centrifugation, the clear supernatant's absorbance was measured at 400 nm to determine the intracellular GSH level.
SOD assay
To measure superoxide dismutase (SOD) activity, U266 cancer cells were plated in a 12-well plate (2 mL medium/well) for 24 h. Cells were treated with different concentrations of deguelin (6, 12, or 18 µmol L–1) for 24 h at 37 °C. Subsequently, the cells were washed with cold PBS, scraped, and suspended in 10 mmol L–1 phosphate buffer (pH 7.5). The cell lysates were obtained by sonicating the suspension twice for 15 seconds on ice. The addition of Triton X-100 (1 %) followed by a 10-minute incubation on ice facilitated the lysis of the cells. The lysates were then clarified by centrifugation at 5000 × g for 10 minutes at 4 °C to remove cellular debris, and the protein content of the resulting supernatant was determined.
The assessment of SOD activity was based on the inhibition of the auto-oxidation of epinephrine. Specifically, 50 μg of protein was combined with 50 mmol L–1 phosphate buffer (pH 10.2) containing 0.1 mmol L–1 EDTA and 0.4 mmol L–1 epinephrine. Epinephrine undergoes rapid auto-oxidation at pH 10, leading to the formation of adrenochrome, a pink-colored product that can be measured at 480 nm using a UV/VIS spectrophotometer in kinetic mode. SOD hinders the auto-oxidation of epinephrine, thereby reducing the formation of adrenochrome. The rate of inhibition was continuously monitored at 480 nm.
AO/EB and Annexin V-FITC apoptosis methods
The U266 multiple myeloma cells were seeded into a 96-well plate with a cellular density of 5 × 105 cells per well. The cells were incubated with different concentrations of deguelin (0, 6, 12, or 18 µmol L–1) for 24 h at 37 °C. The cells were then collected by centrifugation, cleaned with PBS, and fixed with 70 % ethanol for one hour at room temperature. The cells were subsequently given another PBS wash and 0.2 % Triton X-100 treatment. The cancer cells were then stained for 20 minutes at room temperature using the AO/EB reagent (Solarbio Biotechnology, China). Cells were then given a PBS wash before being viewed under a fluorescent microscope.
To more thoroughly examine the apoptosis of deguelin-treated U266 cancer cells (0, 6, 12 or 18 mol L–1), Annexin V-FITC/PI staining was used. Following their administration with varied deguelin treatments for 24 h, the cancer cells were harvested and washed with PBS twice. 4 % paraformaldehyde was then used for fixing the cells for 1 h at room temperature. Afterward, the cells were again washed with PBS and then stained with Annexin V-FITC and propidium iodide solutions. Finally, the apoptosis of U266 cancer cells was studied with the help of FACSCanto II (BD Biosciences, USA) Flow Cytometer system.
Flow cytometry
For cell cycle analysis, 250 µL cell suspension with 103 U266 cancer cells without or with deguelin treatment (6, 12, or 18 µmol L–1) for 24 h at 37 °C was added with 15 μL propidium iodide (PI) solution. This was followed by 15 min dark incubation at room temperature. The stained cells were then analyzed with a FACSort Flow Cytometer (BD, San Jose, USA).
RNA isolation and qRT-PCR
Total RNA was isolated from cell lines using Trizol reagent (Invitrogen) as per the standard protocol. Synthesis of complementary DNA (cDNA) was performed using the RevertAid First strand cDNA synthesis kit. For evaluating the relative transcript levels of miRNA-340-5p and XIAP, the quantitative RT-PCR was performed with the help of a SYBR Green RT-PCR kit. The thermo-cycling was performed on the Quant Studio 5.0 Real-Time PCR system (Applied Biosystems, USA). The quantitative assessment of miRNA and mRNA expression levels was made using the 2−ΔΔCt method. Human actin, GAPDH, and snRNA U6 were used as endogenous expression controls. The sequence of the RT-PCR primers used were: -Cdc25C F: 5’-GAACAGGCCAAGACTGAAGC-3’; R: 5’-GCCCCTGGTTAGAATCTTCC-3’, Cyclin B1 F: 5′-CGGGAAGTCACTGGAAACAT-3′; R: 5′-AAACATGGCAGTGACACCAA-3’ and CDC2; F: 5′-TGGGGTCAGCTCGTTACTCA-3’; R: 5′-CACTTCTGGCCACACTTCATTTA-3’; Actin, F: 5′- CACCATTGGCAATGAGCGGTTC-3 and R: 5′- AGGTCTTTGCGGATGTCCACGT-3.
Wound healing assay
To determine the effects of deguelin, 1.5 × 105 U266 cancer cells/well were cultured in six-well plates to about 90 % confluence. A sterile 200 μL pipette tip was used to make a scratch on the plates and photographed. Subsequently, the scratched cells were added to the serum-free medium and the photographs were again taken at 24 h AxioCam single-channel camera (Carl Zeiss, AG).
Invasion assay
Transwell chambers having polycarbonate members (8 µm pores) were placed in 6-well plates. Coating of the lower compartments was done by using I-type collagen (10 µg mL–1). In the upper chamber, 200 mL of U266 cancer cells (1.4 × 105 cells/mL) and in the lower chamber 800 µL media supplemented with FBS (20 %) was placed and subsequently incubated for 24 h at 37 °C. Thereafter, cells that invaded lower chambers via the membranes were subjected to staining by crystal violet, examined under an inverted microscope, and photographed.
Western blotting
Cell lysis was performed using RIPA buffer, supplemented with phenylmethylsulfonyl fluoride (PMSF) at a concentration of 0.1 mmol L–1, sodium orthovanadate at 1 mmol L–1, and aprotinin and leupeptin at 2 μg mL–1. Following lysis, the mixture was centrifuged at 12000 × g for 20 minutes at 4 °C, and the resulting supernatant was collected. The protein concentration in the supernatant was determined using a Bio-Rad protein assay kit. Subsequently, the cell lysate protein samples were boiled for 10 minutes in the presence of 2-mercaptoethanol and then separated on either a 10 or 15 % sodium dodecyl sulfate-polyacrylamide gel (SDS gel). The separated proteins were then transferred onto pre-equilibrated PVDF membranes.
After blocking with skimmed milk, the membranes were individually incubated with primary antibodies against phosphorylated Akt (Ser473), p38 MAPK, p53 or GAPDH at a dilution of 1:1000. Detection of the bound antibodies was achieved using horseradish peroxidase-labeled sheep anti-mouse IgG or horseradish peroxidase-labeled donkey anti-rabbit IgG (GE Health Care Corp, USA). Finally, the immunoblots were developed using enhanced chemiluminescence reagents, following the manufacturer's recommendations.
Statistical analysis
Mean ± S.D. terms were used for the final presentation of the quantitative data obtained from at least 3 independent experimental replicates. Student's t-test or ANOVA (one-way) was used for the analysis of statistical differences which were considered to be significant at the value of p < 0.05.
RESULTS AND DISCUSSION
Deguelin inhibited multiple myeloma cell growth, selectively
The multiple myeloma cells (U266), as well as normal plasma cells (PCs), were incubated with variable treatment concentrations of deguelin (Fig. 1a) for 24 h at 37 °C to analyze the effect of changing deguelin concentration on the respective cell proliferation. Deguelin administration though minimized the proliferation of cancer as well as the normal cells but the U266 cancer cells were shown to be affected more prominently as compared to the normal plasma cells (Fig. 1b). The IC50 of deguelin was 6 µmol L–1 against U266 cancer cells. On the other hand, it was more than 8 times higher for PCs, i.e., 50 µmol L–1 which signified the potential selective growth inhibitory action of deguelin against the multiple myeloma cells.
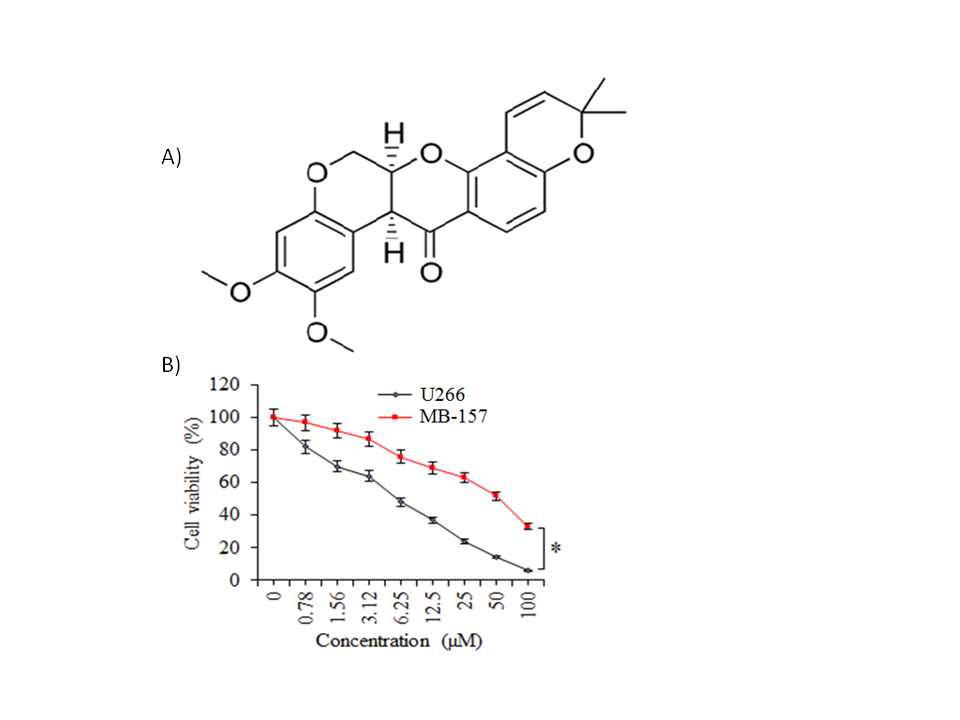
Fig. 1. Selective inhibition of human multiple myeloma cell growth by deguelin. a) Chemical structure of deguelin; b) MTT assay for assessment of percent viability of U266 cancer cells and normal plasma cells (PCs) treated without or with varying doses of deguelin. The experiments were performed in triplicate and expressed as mean ± SD (* p < 0.05).
Deguelin declined the multiple myeloma viability and colony formation
To further look into the growth-declining action of deguelin against the multiple myeloma cells, U266 cancer cells were incubated at 37 °C with (6, 12, or 18 µmol L–1) or without deguelin. Cells were then examined under a phase contrast microscope and it was found that the cellular deformity was noticed to be increasing with increasing deguelin concentrations (Fig. 2a). Furthermore, the cancer cells administered with the same deguelin concentration treatments were examined for clone-forming potential by clonogenic assay. The clone formation decreased proportionally with increasing treatment (Fig. 2b). Thus, multiple myeloma cells show lower viability and clone-forming potential under in vitro deguelin treatment.
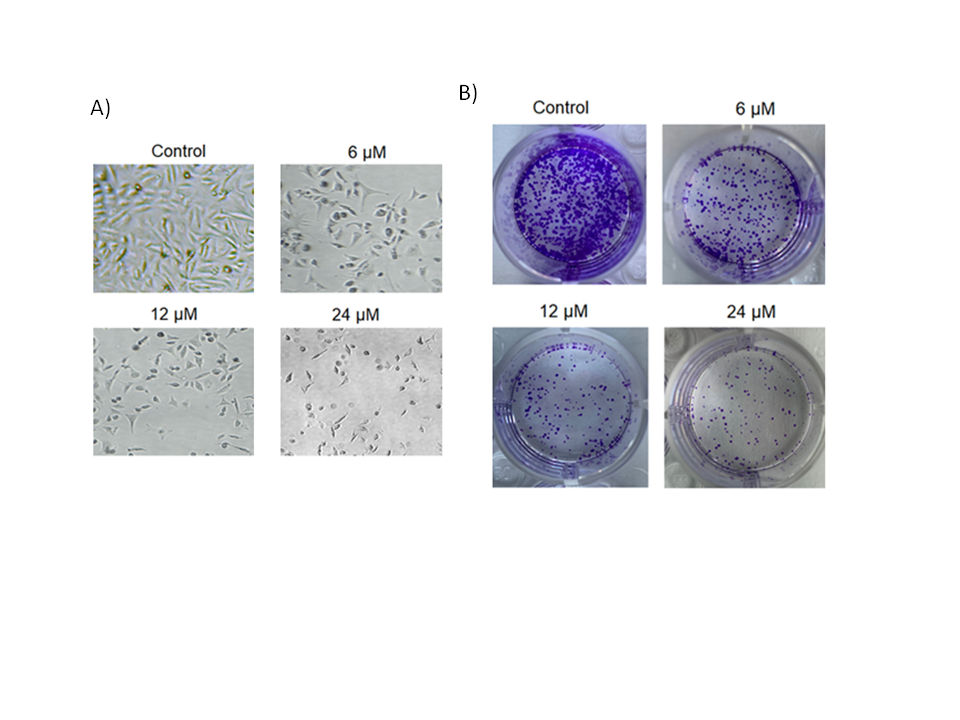
Fig. 2. Decline in cell viability and colony forming potential of human multiple myeloma cells by deguelin. a) Analysis of cell viability potential of U266 cancer cells treated with 0, 6, 12 or 24 µmol L–1 deguelin; b) analysis of colony forming ability of U266 cancer cells treated with 0, 6, 12 or 24 µmol L–1 deguelin. The experiments were performed in triplicate.
Deguelin induces superoxide generation and increases glutathione levels in multiple myeloma cells
The evaluation of deguelin's impact on antioxidant enzyme activity demonstrated a concentration-dependent increase in SOD (Table I). Moreover, treatment with 18 µmol L–1 deguelin for 24 hours resulted in elevated protein levels of SOD. These findings suggest that deguelin enhances the enzymatic activity of important antioxidant enzymes, which could contribute to its potential protective effects against oxidative stress.
GSH, as one of the most abundant intracellular antioxidants, plays a crucial role in protecting cells from oxidative stress (13). Monitoring changes in GSH levels offers valuable insights into cellular oxidative status. In this study, the cellular GSH level was found to be significantly higher in deguelin-treated cells compared to the control cells. This indicates that deguelin treatment may enhance the cellular antioxidant defense mechanism by increasing GSH levels, potentially helping to counteract oxidative stress and maintain cellular health.
In certain colorectal carcinomas, reduced activity and expression of SOD lead to increased superoxide radical production (14). Superoxide accumulation stimulates cell growth by affecting the redox status of transcriptional factors and cell cycle regulators. Induced overexpression of SOD has been shown to suppress malignant phenotypes in experimental in vitro models, making SOD a considered tumor suppressor that acts indirectly through ROS. In this study, the expression of SOD during deguelin-induced apoptosis was examined. The precise mechanism by which SOD suppresses cancer development remains unknown, but its expression may play a significant role in maintaining cellular redox status. SOD's tumor suppressor effects are partly mediated through the modulation of specific oncogenes. Recent reports highlight the relationship between SOD expression, DNA-binding activity modulation, and the transcriptional activation of redox-sensitive oncoproteins and tumor suppressor proteins. The data from this study revealed that deguelin treatment increased GSH levels in multiple myeloma cells.
Table I. Deguelin induces SOD generation and increases GSH levels in multiple myeloma cells
Deguelin treatment induced apoptosis in multiple myeloma cells
To specifically figure out the underlying mechanism of the antiproliferative action of deguelin against the multiple myeloma cells, the U266 multiple myeloma cells were administered with 0, 6, 12, or 18 µmol L–1 for 24 h. Next, the cells were incubated with an AO/EB dual staining mix and notably, the relative percentage of multiple myeloma cells stained with acridine orange (AO) was shown to increase with increased deguelin concentrations (Fig. 3a). Moreover, the Annexin V-FITC/PI staining combined with flow cytometry showed that U266 multiple myeloma cells were induced with apoptotic cell death by deguelin treatments (Fig. 3b). The relative abundance of apoptotic multiple myeloma cells was shown to be increasing with increasing concentrations of deguelin (0–18 µmol L–1). The results were therefore suggestive of the pro-apoptotic activity of deguelin against multiple myeloma cells, evidenced by a decline in cell proliferation and viability.
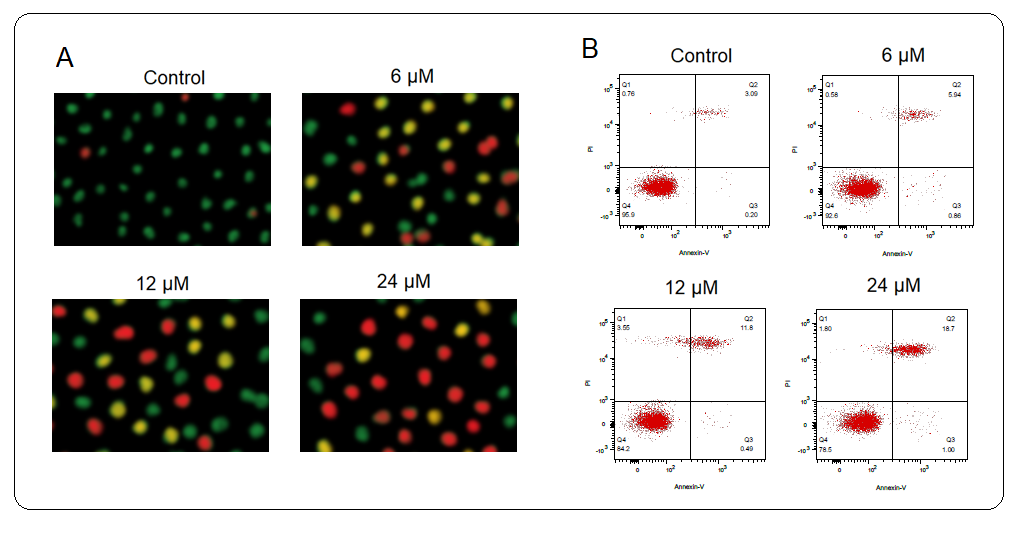
Fig. 3. Induction of apoptosis U266 cells by deguelin. a) Showing relative percentage of multiple myeloma cells stained with acridine orange (AO); b) flow cytometric examination of apoptosis of U266 cells treated with 0, 6, 12, and 24 µmol L–1 deguelin. The experiments were performed in triplicate.
Many researchers investigating the therapeutic benefits of natural chemicals have suggested that these compounds could become important players in the fight against a number of lethal malignancies and other human disorders. (15). Natural substances are being favoured more and more in place of synthetic ones because of their comparatively mild negative effects on healthy human cells (16). In a similar type of observation, deguelin was shown to significantly reduce the growth of multiple myeloma cells in vitro on one side and on the other side; it had little effect on the viability of the normal breast epithelial cells. Such results of selective growth inhibition have been also reported by previous workers for deguelin against multiple myeloma cells (17). The antiproliferative action of deguelin has been credited to its pro-apoptotic effects on cancer cells together with the induction of arrest of cancer cell mitosis (18, 19). In coherence with such findings, the administration of deguelin exhibited marked levels of apoptosis induction as confirmed by AO/EB and Annexin V-FITC/PI staining procedures.
Deguelin-induced G2/M cell cycle arrest in multiple myeloma cells
Whether deguelin also affected the cell cycle progression of multiple myeloma cells to exert its ant-proliferative effect, the cell cycle of untreated and deguelin (6, 12, or 18 µmol L–1) treated U266 cancer cells was inspected with the help of a flow cytometer. It was observed that the proportion of cancer cells at the G2 phase increased greatly and in a linear fashion with increasing deguelin concentration (Fig. 4). The results are suggestive of cell cycle arrest in deguelin-treated multiple myeloma cells at the G2/M check-point. To further validate the results, the mRNA expression levels of cell cycle regulatory proteins; cdc25C, Cyclin B1, and cdc2 were analyzed. The expression of all these mRNAs was shown to be decreasing significantly and in a dose-dependent way under deguelin treatment (Fig. 5). Therefore, deguelin-induced G2/M phase mitotic arrest in multiple myeloma cells by modulating the expression of mRNAs of regulatory proteins to decline their proliferation, in vitro.
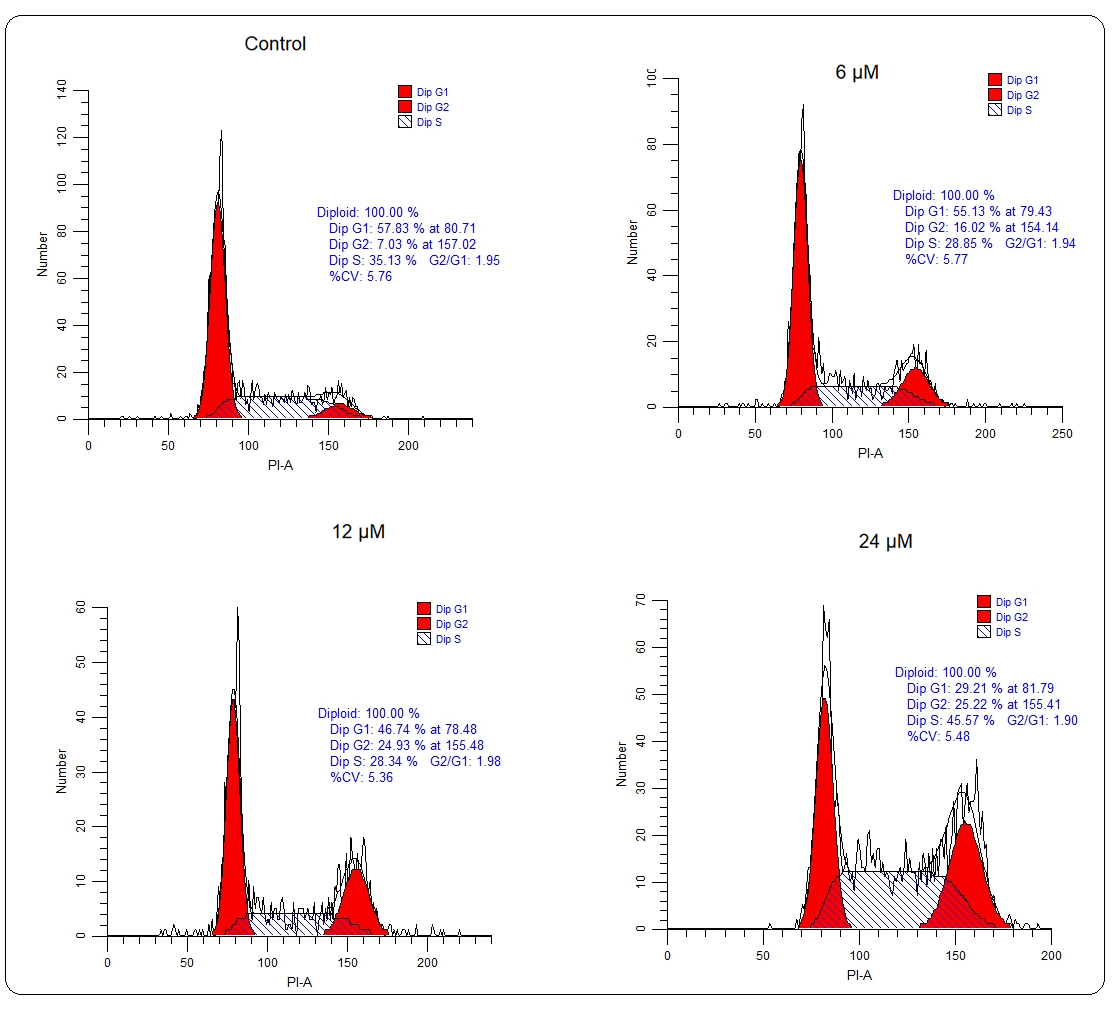
Fig. 4. Cell cycle analysis: Flow cytometry analysis showed that U266 cells treated with 6, 12, and 24 µmol L–1 deguelin enhanced abundance at G2-phase than negative control.
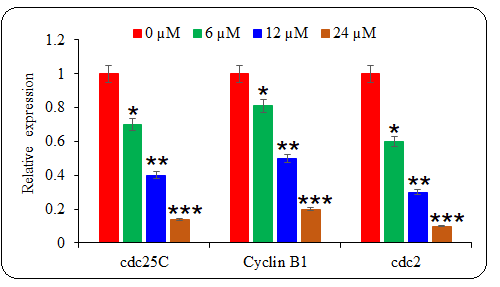
Fig. 5. The effect of deguelin treatment was analyzed against the mRNA expression levels of cell cycle regulatory proteins. The qRT-PCR representative graph indicates the statistical differences. The experiments were performed in triplicate and results are presented as mean ± SE values; the p-values are represented as follows: * p < 0.05, ** p < 0.01, *** p < 0.001.
Besides, the results of the current study indicated that deguelin modulated the expression of cell cycle regulatory mRNAs cdc25C, Cyclin B1, and cdc2 in multiple myeloma cells. Cyclin B1/CDK1 complex is vital for G2/M transition which is activated by cdc25C (20). Targeting of cdc25C is thus seen with great therapeutic importance in treating human cancer. The mitotic entry of eukaryotic cells is also crucially regulated by cdc2 phosphatase (21). The repression of expression levels of cdc25C, Cyclin B1, and cdc2 mRNAs by deguelin thus resulted in cell cycle arrest at the G2/M phase. Previously, the cell cycle progression of esophageal cancer cells was also shown to be restricted through G2/M phase mitotic halt (22).
Deguelin minimized the migration and invasion of multiple myeloma cells
The effect of the in vitro administration of deguelin on the migration and invasion of multiple myeloma cells was also assessed. The migration of untreated U266 cancer cells and those treated with 6 µmol L–1 deguelin was analyzed by wound-healing assay. The wound-healing potential was significantly higher for untreated cells while it was invariably lower for the deguelin-treated cells (Fig. 6a). The cancer cells were also shown to march into the media-filled chamber with significantly higher invasiveness than under the deguelin administration (Fig. 6b). The results indicate that the migration and invasion of multiple myeloma cells are reduced by deguelin, considerably.
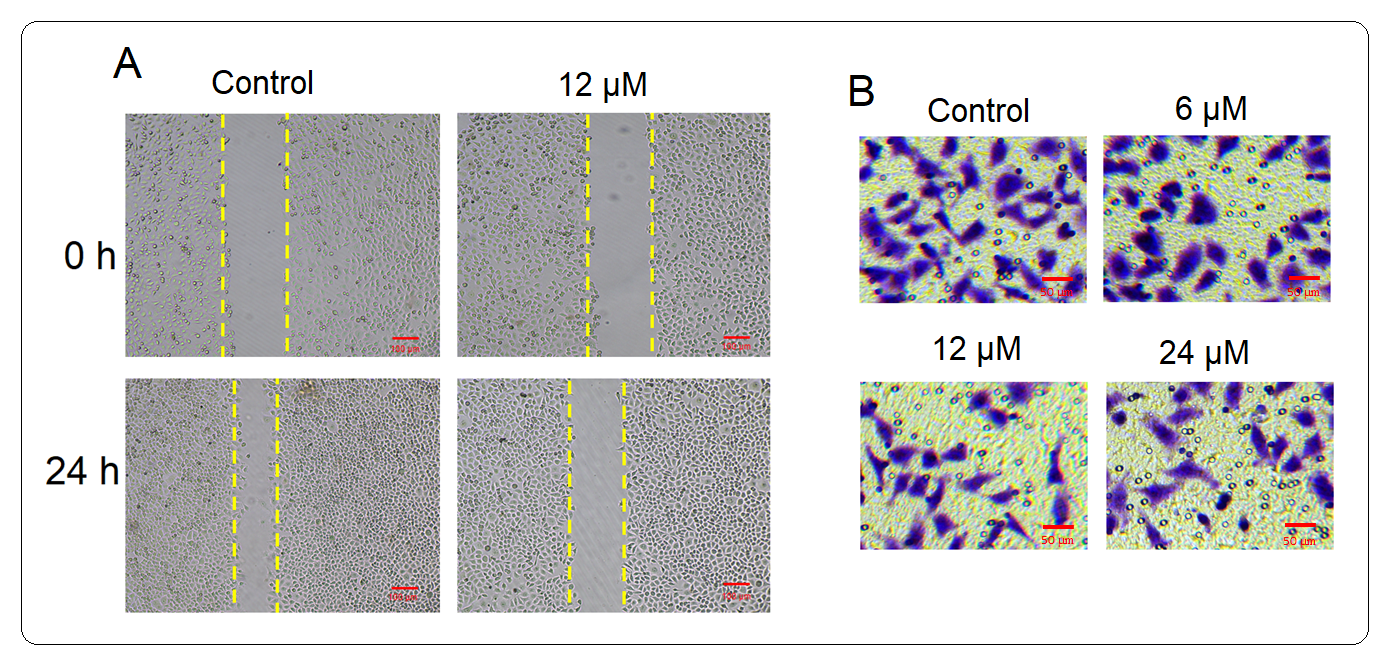
Fig. 6. Restriction of migration of U266 cells by deguelin treatment. a) Wound-healing assay for the analysis of migration of U266 cells treated with deguelin (12 µmol L–1) for 24 h compared to untreated cells; b) transwell chamber invasion assay of U266 cells treated with different concentrations of deguelin compared to untreated cells. Each experiment was conducted using three independent biological replicates.
The current study also showed that multiple myeloma cells migrated with much lowered potential when treated with deguelin and exhibited significantly lower invasiveness. The results thus suggest that deguelin exhibits an antimetastatic effect against the multiple myeloma cells in vitro. The attenuation of metastasis of cancer cells by deguelin has already been established for non-small lung carcinoma cells (SLCC) wherein the effects were exerted through the CtsZ/FAK signaling pathway (23). Moreover, the inhibition of metastasis by restriction of epithelial to mesenchymal transition under deguelin administration has been reported for pancreatic cancer (24). In sum, the current study explored the in vitro antiproliferative and antimetastatic effects of deguelin against multiple myeloma cells and thus highlights its effectiveness to be used in multiple myeloma treatment. However, the proposition might only be enforced if deguelin exhibits similar in vivo effects in the future.
Deguelin inhibits Akt and enhances p38 MAPK activation
To assess the impact of deguelin on Akt activity, the phosphorylation status of Akt was examined. Phosphorylation of Akt indicates its activation, and the results revealed an increase in Akt phosphorylation in multiple myeloma cells. However, when treated with different concentrations of deguelin (6, 12 18 µmol L–1) for 24 h, the phosphorylation of Akt was noticeably inhibited, suggesting that deguelin interferes with Akt activation (Fig. 7a,b).
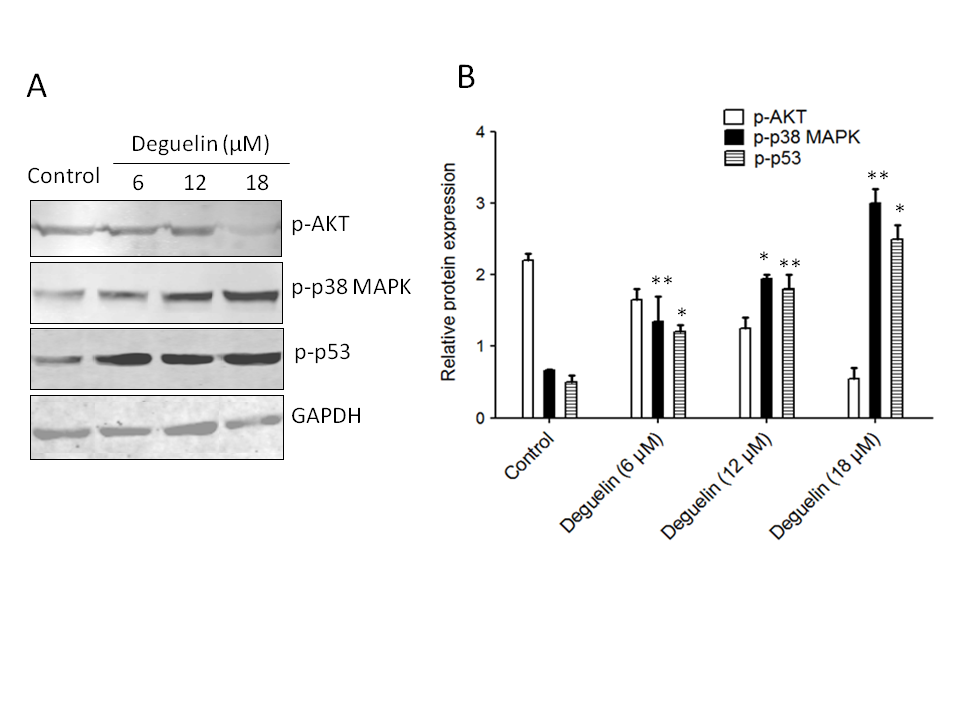
Fig. 7. Effects of deguelin on Akt, p38 MAPK, and p53 phosphorylation in multiple myeloma cells. a) Western blotting: the effect of deguelin treatment was analyzed against the protein expression levels of phosphorylated forms of Akt p38 MAPK and p53; b) densitometry analysis of expressed proteins.
Furthermore, the study explored the effect of deguelin on p38 MAPK and p53, which are critical regulators of cell responses to stress and apoptosis. Following exposure to different concentrations of deguelin (6, 12, or 18 µmol L–1) for 24 h, a noticeable increase in the phosphorylation of p38 MAPK and p53. This indicates that deguelin enhances the activation of these signaling molecules, potentially contributing to its anticancer effects.
The PI3-kinase/Akt pathway plays a significant role in tumor formation as it enhances the antiapoptotic actions of Akt, a key signaling molecule (25). Akt exerts its antiapoptotic effects by phosphorylating proteins like Bad, GSK3, and caspase-9, and by activating transcriptional factors such as Forkhead (FOXO1) and NFκB (26). These processes collectively promote cell survival and inhibit programmed cell death (apoptosis), contributing to cancer progression. In the context of this study, we investigated the effect of deguelin on Akt activity in human multiple myeloma cells. An increase in Akt phosphorylation was observed, which is a well-established indicator of Akt activation. However, when treated with deguelin, the phosphorylation of Akt was notably inhibited. This finding indicates that deguelin interferes with the activation of Akt, an essential mechanism by which it may combat multiple myeloma cells. The inhibition of Akt phosphorylation by deguelin represents a crucial mode of action in these cancer cells.
Indeed, the suppression of Akt activation can have a profound impact on the activation of p53, leading to the activation of pro-apoptotic signaling pathways (27). In the context of the current study, we observed an increase in the phosphorylation of p53 in multiple myeloma cells treated with deguelin. This increase in p53 phosphorylation is likely influenced by the PI3K-Akt pathway, which is known to regulate p53 activity. When Akt is inhibited, it can no longer exert its inhibitory effects on p53. As a result, p53 is freed from Akt-mediated repression and can become activated. Activated p53, in turn, triggers the activation of pro-apoptotic signaling pathways. These pro-apoptotic pathways promote cell death and play a significant role in combating cancer cells.
The findings of this study suggest that deguelin's ability to suppress Akt activation in multiple myeloma cells may contribute to the observed increase in p53 phosphorylation. This activation of p53 can potentially enhance the pro-apoptotic responses in multiple myeloma cells. Understanding the crosstalk between the PI3K-Akt pathway and p53 in multiple myeloma cells is of great importance. It sheds light on potential therapeutic targets and highlights the significance of deguelin as a modulator of these pathways to enhance the treatment outcomes in multiple myeloma. Further research in this area could lead to the development of novel therapeutic approaches to tackle human multiple myeloma and improve patient outcomes.
CONCLUSIONS
In agreement with the previously established activity of deguelin, the deguelin exhibited marked growth inhibitory action against the multiple myeloma cells with minimal effects on the normal cells. The decline in cell proliferation resulted as a consequence of the induction of apoptosis and G2/M phase cell cycle arrest. The migration and invasion of multiple myeloma cells were also reduced significantly by deguelin. By targeting the Akt and p38 MAPK pathways, deguelin disrupts the balance of signals that support the survival and proliferation of multiple myeloma cells, leading to the observed inhibitory effects on cell growth. The results are thus conclusive of the potential antiproliferative and antimetastatic activities of deguelin against the multiple myeloma cells which can be further explored by applying semi-synthetic medicinal chemistry approaches.
Funding. – This study was supported by the Key Research and Development program of Ningxia Hui Autonomous Region (No: 2021BEG03045)and Ningxia Natural Science Foundation of Ningxia Province (No: 2020AAC03395).
