Introduction
The term "production diseases" traditionally referred to those diseases caused by management practices, e.g. metabolic diseases. Dairy cows most often suffer from production diseases, that is, diseases related to poor feeding or handling. Nir (2003) states that the term "production disease" has been expanded to include other disorders, such as infertility, and diseases such as mastitis and laminitis that may involve infectious agents but are exacerbated by nutritional or management factors. Although epidemic infectious diseases can result in large economic losses during outbreaks and generate much more publicity, production diseases are economically more important for the overall efficiency of animal production (Hogeveen et al., 2019). The most widespread production disease of dairy cattle is mastitis (Seegers et al., 2003). Mastitis is the inflammation of the parenchyma of the mammary gland, and the most common cause is the intramammary infection (IMI) when an infectious pathogen is present. It is characterized by physical, chemical, and usually bacteriological changes in the milk and pathological changes in the gland tissue. This inflammation can be in an acute or chronic stage. According to the signs of inflammation, mastitis in dairy cows is divided into clinical (where there are visible changes in the milk, mammary gland, or even at the level of the entire organism) and subclinical (without visible signs in the milk, mammary gland or organism). According to severity, (Adkins and Middleton, 2018), clinical mastitis is divided into mild; only changes in the milk - usually manifested by clots, scales, and/or changes in the colour and consistency of the milk secretion; moderate: changes in (the) milk and mammary gland - manifested by the inflammatory changes in the tissue such as redness, heat, pain, and swelling; and severe: changes in the milk, mammary gland and signs of systemic disease - manifested by changes in body temperature, rumination rate, appetite, hydration status, and behaviour. Regarding the frequency of the manifestation of clinical cases of mastitis according to severity: the largest number of cases are mild, followed by moderate, and to a lesser extent severe (Narváez-Semanate et al., 2022). The diagnosis of mastitis is generally based on the clinical observations or direct or indirect measurements of the inflammatory response to infection, while the diagnosis of IMI is based on the identification of the causative agent of the infection. Furthermore, detecting subclinical mastitis in dairy cattle is a challenging task, and it poses a significant risk as it can cause more harm than clinical mastitis. Even minor changes in productivity can result in long-term losses in production. Unfortunately, measures are only taken when the milk yield has already dropped significantly, leading to costly treatment procedures. Subclinical mastitis is significantly more frequent than clinical mastitis, and it can account for up to 80 % of milk production losses (Romero et al., 2018). Furthermore, besides a substantial decrease in milk production, affected udder quarters can dry out entirely, resulting in a higher rate of culling from the herd and even death (Hur et al., 2013). According to Halasa et al. (2007), mastitis, no matter the type, can have negative consequences for the dairy industry. It can result in both decreased milk production and quality. Moreover, Özkan Gülzari et al. (2018) illuminated the environmental implications of mastitis and how its prevalence can contribute to greenhouse gas emissions. By preventing and managing subclinical mastitis, farmers can reduce the amount of emissions per kilogram of milk produced, ultimately increasing profits. This can be accomplished by minimizing milk losses, optimizing culling rates, and reducing variable costs. The advantage of early detection of mastitis is that it allows for early treatment that minimizes or potentially eliminates the need for antibiotics and in turn maintains production continuity. Early diagnosis of mastitis is vital because changes in the udder tissue occur before they become visible (Argaw, 2016). The benefit for the sick cow lies in the shorter duration of the infection, which in most cases can lead to less damage to the udder. On the other hand, if the duration of mastitis infection, mainly contagious mastitis, is prolonged, the possibility of infection of other cows in the herd increases. Early diagnosis of mastitis also has economic implications as it allows to reduce losses in milk production and increases the chances of recovery (Kamal et al., 2014). The somatic cell count (SCC) is a vital aspect of milk recording information as it can reveal intramammary infections and facilitate the monitoring of milk quality at the individual, herd, and population levels (Schukken et al., 2003). The tracking of somatic cell counts for each animal in the herd provides insight into the health of their udders. Elevated somatic cell counts tend to signify a more severe infection, making this metric a valuable tool for assessing udder health.
Somatic cells in milk are usually found as discarded udder epithelial cells and blood-derived cells that play a role in defence against infection. They are present as part of the innate immune system of the udder. It mainly consists of leukocytes (neutrophil granulocytes - PMN, macrophages, and lymphocytes) and a smaller number of discarded epithelial cells (Sordillo et al., 1997). In the total number of somatic cells, the share of leukocytes is from 75 to 85 %, and the share of epithelial cells is 15 to 25 % (Barrett, 2002). The composition of the somatic cells of milk depends on the stage of lactation, the degree of exposure to pathogenic organisms from the environment, and the general state of health of the individual. The number of somatic cells (SCC) is related to inflammatory processes, so it can be used as a diagnostic method in the assessment of udder health (Ivanov et al., 2016). Pathogen invasion of the mammary gland tissue promotes the trafficking of various immune cells to the site of inflammation increasing the number of somatic cells in the secreted milk (Alhussien and Dang, 2018). It was found that the increase in SCC during successive lactations was only associated with an increase in the number of polymorphonuclear leukocytes (PMN), while an increase in SCC during any lactation was associated with an increase in both PMN and other milk somatic cells (Blackburn, 1966). The udder is considered uninfected if the count is less than 100,000 cells/mL milk (Smith et al., 2001), i.e. healthy milk contains from 20,000 to 100,000 SCC/mL (Mikó et al., 2016). Loss in milk production due to an increased number of somatic cells results in economic losses for dairy farmers (Hadrich et al., 2018), and total milk losses in the herd depend on the distribution of SCC at the cow level and parity within the herd (Chen et al., 2021). Some studies have shown that the level of SCC, as an indicator of the presence of different groups of pathogens, had a negative impact on the accuracy of the results at the thresholds of 150,000 SCC/ml in a common sample and 200,000 SCC/mL in samples from individual udder quarters due to low specificity (Petzer at al., 2017). Others have proven a significant drop in milk yield (Mikó et al., 2016) which is directly related to an increase in SCC. The loss of milk production was significantly related to the level of SCC, so cows with an SCC in the interval of 50,000 to 100,000 cells/mL showed a loss greater than 8 %, while cows with an average SCC between 100,000 - 250,000 cells/mL had reduced milk production by more than 15 %, and even up to 18 % (Pfützner and Ózsvári, 2016). The main factor affecting SCC at the herd and cow levels is the presence of intramammary infections, while other effects, genetic and environmental, such as the order and stage of lactation, season, fitness, presence of heat stress, and daily variation will have a lesser effect on somatic cell variability (Harmon, 1994). The presence of intramammary infection (IMI) and the type of microorganisms involved were the main factors responsible for the variation in SCC, but udder quarter (posterior vs. anterior), age, and parity were significantly associated with variation in SCC, regardless of IMI (Sumon et al., 2020). The differences in somatic cell count (SCC) in an environment characterized by heat stress were found in Holstein cows, with variability regarding the daily production level, breed, and parity (Gantner et al., 2011; 2017). As mastitis is a common issue at Holstein cattle farms, this study aimed to investigate the impact of different milk recording seasons on the prevalence of mastitis and subsequent milk production that is, on the cows' recovery potential regarding the season.
Materials and methods
Database
For the statistical analysis, the database of milk recording of cows under selection in Croatia, taken from the Croatian Agency for Agriculture and Food (HAPIH), was used. Milk recording in Croatia is carried out following the alternative AT4 / BT4 method by the control assistant of the HAPIH/breeder. Milk recording using an alternative method involves measuring the amount of milk and sampling milk from each lactating cow during morning/evening milking every four weeks. Milk samples are analysed in the Central Laboratory for Milk Quality Control (SLKM) of HAPIH. The procedure for taking milk samples during milk recording as well as laboratory testing of samples is defined by the International Committee for Animal Recording (ICAR, 2017). Milk samples are tested for chemical composition (content of milk fat, protein, lactose, dry matter, dry matter without fat, urea, and freezing point, and additionally for the content of casein, free fatty acids, pH value of milk and content of ketone bodies in milk) and somatic cell count following accredited laboratory methods (infrared spectrophotometry for the proportion of milk fat, proteins, lactose, and urea, and the fluoro-opto-electronic method for counting somatic cells). The chemical quality of milk is tested by MilkoScan analyzers, while Fossomatic analyzers are used for determining the number of somatic cells.
The database of milk recording, before logical data control, contained a total of 5,691,083 test-day records for the Holstein breed. Test-day records with the following values of individual traits: daily milk yield < 3 kg, > 100 kg; daily milk fat content < 1.5 %, > 9 %; daily protein content < 1 %, > 7 %; and daily lactose content < 3 %, > 6 % were deleted from the database. Furthermore, test-day records with missing or illogical values for the lactation stage (< 5 days, > 400 days), parity (< 1, > 10), age at first calving (< 21, > 36 months), calving date, milk recording date, and herd code were deleted from the database. After logical data control, the database contained a total of 3,953,637 test-day records of Holstein cows referring to the milk recording period from 1/1/2005 to 31/12/2022.
Considering the parity, the cows were grouped into four different classes: 1., 2., 3., ≥ 4. Depending on the herd size, six classes were formed: I. (<5 cows); II. (5-10 cows); III. (10-50 cows); IV. (50-200 cows); V. (200-500 cows); and VI. (>500 cows). Furthermore, considering the month of milk recording, the test-day records were grouped into four seasons (December, January, and February - winter; March, April, May - spring; June, July, August - summer; and September, October, November - autumn).
The prevalence of mastitis was evaluated by analysing the daily somatic cell count (SCC) of cows. An SCC below 200,000/mL indicates healthy cows, an SCC in the interval from 200,000/mL to 400,000/mL indicates cows at risk of mastitis, while an SCC exceeding 400,000/mL signifies the presence of mastitis in cows.
Statistical analysis
The prevalence of mastitis in the population of Holstein cows was defined as the share (%) of cows in a particular class of mastitis (according to the number of somatic cells per day) of the total number of animals. Furthermore, the prevalence was calculated separately by milk recording season. The analysis of the effect of the mastitis prevalence on production indicators (daily milk, fat, and protein yield) during successive milk recordings included only cows with a determined mastitis (SCC > 400,000/mL). The daily milk yield measured on the milk recording date when the mastitis prevalence was determined was defined as a reference value. Furthermore, the mastitis index was defined with regard to the number of days after the mastitis determination as follows: D-0 = test-day record when the mastitis prevalence was determined, A-1 = within 35 days, A-2 = between 36 and 70 days, A-3 = between 71 and 105 days, and A-4 = more than 105 days. The effect of the mastitis index on the daily milk production (milk, fat, and protein yield) was analysed separately by milk recording season using the following statistical model (1):
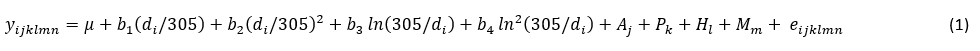
where:
Yijklmn = estimated milk production trait (daily milk, fat and protein yield);
µ = intercept;
b1, b2, b3, b4 = regression coefficients;
di = stage of lactation (i = 6 to 400 day);
Aj = fixed effect of age at first calving (j = 21 to 36 month) * only for firs parity;
Pk = fixed effect of parity k (k=1, 2, 3, ≥4);
Hl = fixed effect of herd size (l = I, II, III, IV, V, VI);
Mm = fixed effect of mastitis index m (m = D–0, A–1, A–2, A–3, A–4);
eijklm = residual.
The significance of the differences between the estimated LSMeans was tested by Scheffe's method of multiple comparisons using the MIXED procedure of SAS (SAS Institute Inc., 2019). Estimated differences in daily yields (milk, fat, and protein) between the analysed milk recordings (D-0, A-1, A-2, A-3, A-4) was presented separately by each milk recording season (spring, summer, autumn, winter).
The total difference in milk yield in the analysed period of four successive milk recordings (from D-0 to A-4) after mastitis determination was estimated using the following equation:
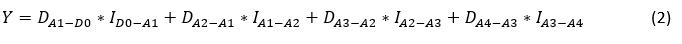
Where:
Y = estimated milk yield (kg);
DA1-D0 - the difference between the estimated daily milk yield at the first successive milk recording and the daily milk yield determined at the reference milk recording;
ID0-A1 - the interval between the reference recording and the first successive milk recording;
DA2-A1 - the difference between the estimated daily milk yield at the second and first successive milk recordings;
IA1-A2 - the interval between the first and second successive milk recordings;
DA3-A2 - the difference between the estimated daily milk yield at the third and second successive milk recordings;
IA2-A3 - the interval between the second and third successive milk recordings;
DA4-A3 - the difference between the estimated daily milk yield at the fourth and third successive milk recordings;
IA3-A4 - the interval between the third and fourth successive milk recordings.
The total difference in milk production in quantity (kg) and value (euro, (Jurinić Kojić et al., 2023)) of milk in the analysed period was presented separately by each milk recording season.
Results and discussion
The analysis of the prevalence of healthy, cows at mastitis risk, and cows with mastitis confirmed the effect of milk recording season on animals’ health status (Figure 1). The mastitis prevalence varied from 19.9 % to 23.0 %, the prevalence of cows at mastitis risk varied from 13.3 % to 14.1 %, while the prevalence of healthy cows amounted from 63.1 % to 66.7 %. The highest prevalence of cows with mastitis in the amount of 23.0 % of the total population was determined in autumn, while the lowest mastitis prevalence was during the summer season (19.9 %). The highest prevalence of healthy cows was also determined in the summer season (66.7 %).
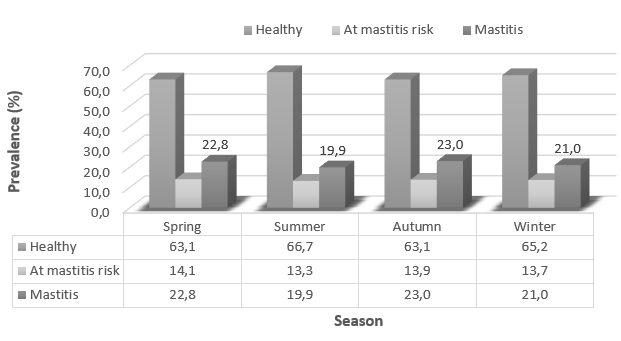
Figure 1. The prevalence of healthy, cows at mastitis risk, and cows with mastitis regarding the season of milk recording
According to Tomazi et al. (2018), several factors like season, herd size, production system, cow's production level, and somatic cells in milk, may influence the occurrence of mastitis-causing pathogens and incidence rate of clinical mastitis cases in dairy herds. The authors further suggested that high temperatures and humidity, which create a favourable environment for heat stress in dairy cows, increase the risk of intramammary infections, especially those caused by environmental pathogens. Nobrega and Langoni (2011) found a higher level of lactose in cows during the dry season, indicating a higher prevalence of mastitis during that period. Similarly, Sharma et al. (2018) report that mastitis cases are most common during the early autumn or winter, and there is an increased risk of mastitis during winter calving. The authors explain that free and open housing on farms increases the risk of infectious agents in the cows' bedding, which contributes to mastitis-related problems.
Gantner et al. (2011) reported that the changes in environmental conditions and heat stress during the summer period could change the quantity and quality of milk, somatic cell counts, and mastitis prevalence. Weber et al. (2020), in a study on Holstein breed in Brazil, found that the season significantly affected the composition and quality of milk. They observed that milk was of higher quality during the winter and spring seasons, while in the hotter months of summer and autumn, the quality and availability of forage, and the frequency of mastitis (increased somatic cell counts) negatively affected milk quality. According to Haygert-Velho et al. (2018), the variation in monthly milk production and quality can be attributed to heat stress that affects lactating cows in summer and autumn. Antanaitis et al. (2021) state that summer is the most crucial time for the appearance of the causative agent of subclinical mastitis in milk. However, mastitis can also be related to management systems, nutrition, and housing in different seasons. For instance, during the outdoor season, milk is more likely to contain higher proportions of environmental bacteria.
Statistically speaking, the effect of the mastitis index (D-0, A-1, A-2, A-3, A-4) significantly (<0.0001) affected the analysed traits; daily milk, fat, and protein yield with the notable variability along the recording season (Table 1). LsMeans of daily milk yield varied from 20.814 kg/day at D-0 to 22.221 kg/day at A-4 in the spring season; from 20.430 kg/day at D-0 to 21.738 kg/day at A-1 in summer; from 19.581 kg/day at D-0 to 20.957 kg/day at A-2 in autumn; and from 20.062 kg/day at D-0 to 21.984 kg/day at A-2. In all seasons, the lowest daily milk yield was determined at D-0 (test-day record when the mastitis prevalence was determined), followed by the increase at successive milk recordings that varied regarding the season of recording. The variability of daily fat yield showed a different pattern with the highest values determined at D-0 (0.891 kg/day in spring; 0.853 kg/day in summer; and 0.860 kg/day in autumn) followed by a drop at successive milk recordings, with the exception of the winter season when the highest value (0.911 kg/day) was determined at A-2 successive recording. LsMeans for daily protein yield (0.732 kg/day, 0.705 kg/day, 0.709 kg/day, and 0.724 kg/day in spring, summer, autumn, and winter) showed similar variability like daily milk yield with the lowest values determined at D-0 milk recording followed by the increase in value at successive milk recordings.
Table 1. LsMeans of daily yields (milk, fat, and protein) at analysed milk recordings (D-0, A-1, A-2, A-3, A-4) regarding the milk recording season
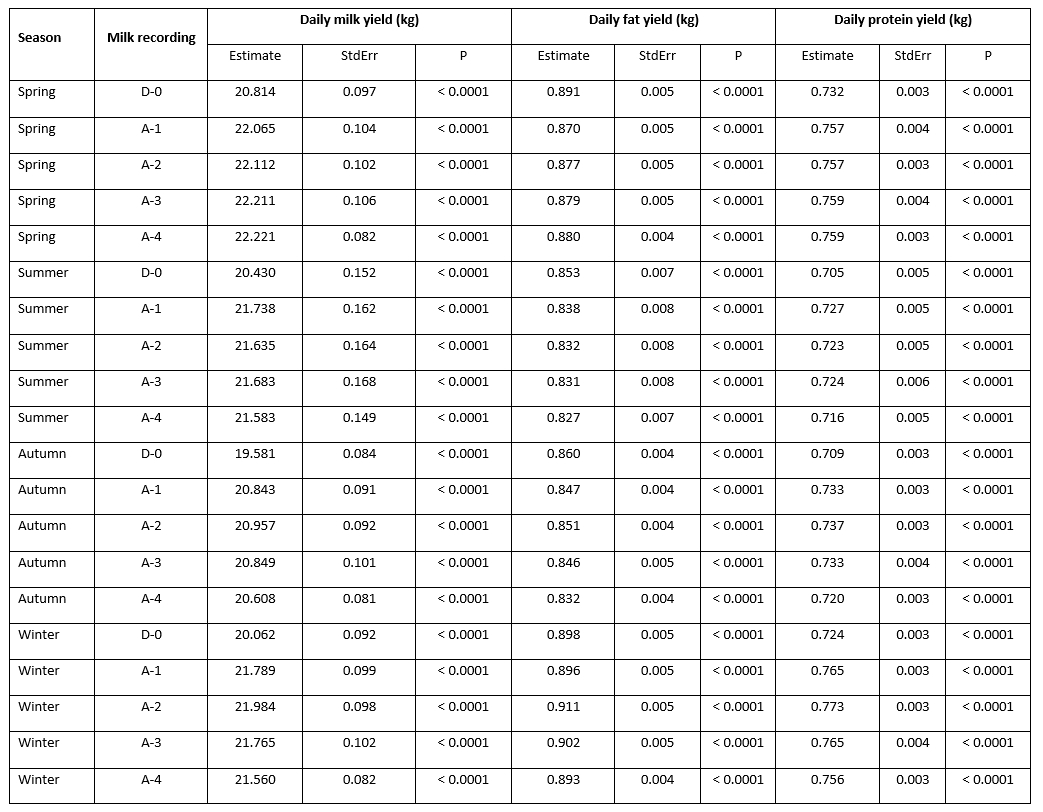
*D-0 - milk recording when the mastitis prevalence was determined; A-1, A-2, A-3, A-4 - successive milk recordings
Estimated differences in daily milk yield between the D-0 and A-1 milk recordings varied regarding the recording season with the highest increase determined in winter (1.73 kg/day = 51.80 kg/month) and the lowest during the spring season (1.25 kg/day = 37.53 kg/month). The differences between the other analysed milk recordings (A-1&A-2, A-2&A-3, and A-3&A-4) amounted from 0.05 kg/day to 0.24 kg/day (increase or decrease) and varied regarding the number of milk recordings and the season of milk recording. The constant increase in daily milk yield during the analysed period (from D-0 to A-4) was determined only in the spring season. Daily fat yield dropped between the D-0 and A-1 milk recordings in all seasons in amount from -2.12 kg*10-2/day (in spring) to -0.19 kg*10-2/day (in winter) while daily protein yield increased from 2.22 kg*10-2/day (in summer) to 4.12 kg*10-2/day (in winter). Furthermore, daily fat yield was continuously decreasing during the entire analysed period (from D-0 to A-4) in the summer season. The differences in daily fat and protein yield between the other analysed milk recordings (A-1&A-2, A-2&A-3, and A-3&A-4) amounted from 0.11 kg*10-2/day to 2.12 kg*10-2/day for fat and from 0.03 kg*10-2/day to 4.12 kg*10-2/day for protein (increase or decrease) and varied regarding the number of milk recordings and the recording season.
The established differences in the increase in the daily amount of milk during successive controls after the diagnosis of mastitis, depending on the recording seasons, can be explained by different feeding and microclimatic conditions in the production facilities. The highest increase in daily milk yield determined in the winter period indicates that Holstein cows recover more efficiently in an environment characterized by lower temperatures and humidity. Antanaitis et al. (2021) state that the differences in the daily amount of milk are related to the differences in management systems, feeding and keeping during different seasons. According to the results of this research, Wani et al. (2022) determined that the greatest milk loss during mastitis was recorded in spring, followed by summer and autumn. Yang et al. (2013) found that milk yield, composition, and related measures were influenced by both parity and season. Chen et al. (2023) noted that the impact of season on mastitis occurrence varied among different regions, likely due to the diverse climate conditions.
Table 2. Estimated differences in daily milk, fat, and protein yield between the analysed milk recordings (D-0, A-1, A-2, A-3, A-4) regarding the milk recording season
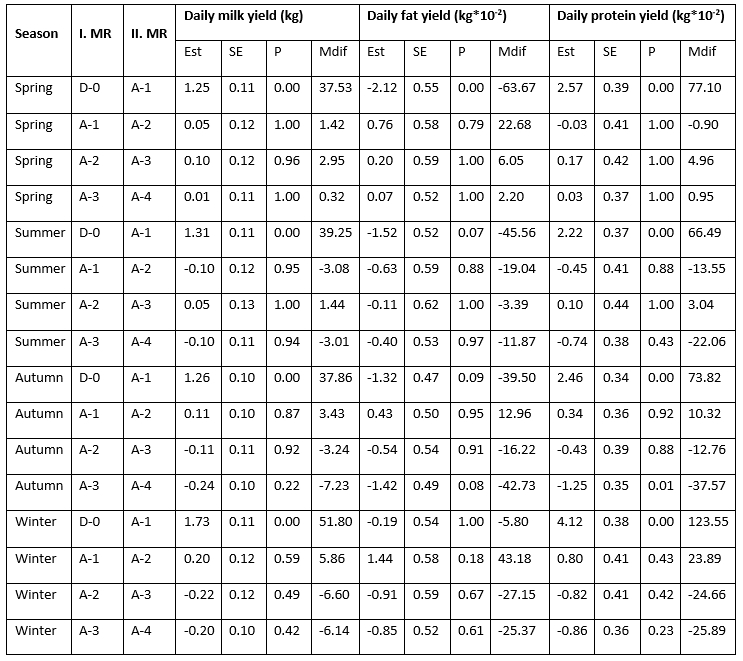
*D-0 - milk recording when the mastitis prevalence was determined; A-1, A-2, A-3, A-4 - successive milk recordings; Est - estimated difference, Mdif - estimated monthly difference (Est*interval between the successive milk recordings)
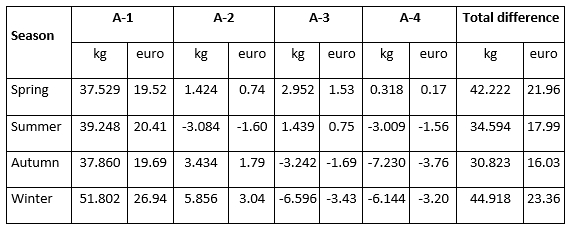
The total difference in milk production in quantity (kg of milk) and value (euro) of milk in the analysed period from D-0 to A-4 milk recording regarding the milk recording season is presented in Table 3. During the spring period, the constant increase of daily milk yield after the detection of mastitis, in the amount of 37.529 kg (19.52 euro)/month, 1.424 kg (0.74 euro)/month, 2.952 kg (1.53 euro)/month, 0.318 kg (0.17 euro)/month was observed. This increase resulted in a total increase of 42.222 kg of milk or 21.96 euro. Although in spring cows experienced a constant increase in milk production in the following four months after the mastitis prevalence, that increase was lower when compared to the increase in production in the winter season. The highest increase (51.802 kg (26.94 euro)/month) in the first month after the mastitis detection was determined in the winter period followed by a decrease in the 3rd and 4th successive milk recordings, resulting in the highest total increase of 44.918 kg or 23.36 euro. Furthermore, the lowest total increase amounting 30.823 kg (16.03 euro) was determined during the autumn season. These results indicate that the recovery potential of an animal is highly variable and significantly affected by the season with the highest possibility of recovery and restoration of production following the animal's genetic potential in the winter season.
Table 3. Total difference in milk production in quantity (kg) and value (euro) of milk in the analysed period of four successive milk recordings (from D-0 to A-4) regarding the milk recording season
According to the results of this study, mastitis prevalence varies and the impact of mastitis differs depending on the season in which milk recording is performed. The previous research conducted by Nóbrega and Langoni (2011), Gantner et al. (2011, 2017), Tomazi et al. (2018), Sharma et al. (2018), Weber et al. (2020), Stocco et al. (2022), and Chen et al. (2023) has also shown that season plays a significant role in the incidence of cows with intramammary infections. Thus, it is crucial to monitor the health of individual animals using test day records (and SCC data) while enabling timely action to prevent further development of mastitis.
Conclusion
The aim of the study was to determine how different seasons affected the prevalence of mastitis and the recovery potential of cows. The results showed that mastitis was most prevalent in autumn, with a lower total increase in milk production. Conversely, the highest prevalence of healthy cows was observed in summer and winter, with the highest total increase in milk production in the amount of 44.918 kg or 23.36 euros per animal in the winter period. These findings indicate that mastitis prevalence and recovery potential in cows are highly variable and significantly impacted by the season. In the winter season, with lower temperatures and humidity, animals have the highest chance of recovery and restoration of production, in line with their genetic potential.
