Introduction
Mastitis significantly affects dairy herds, leading to economic losses due to high treatment costs, reduced milk production and quality, increased labor and premature culling (Magaš et al., 2013; Benić et al., 2018; Poljak et al., 2022). It also raises concerns about antimicrobial use, leading to resistant strains and antimicrobial residues in dairy products, posing public health risk (Turk et al., 2017). Therefore, monitoring mastitis in dairy cows is crucial for maintaining herd health, milk quality and public health (Maletić et al., 2017; Knežević et al., 2021).
Somatic cell count (SCC) reflects the udder health and is a quantitative method for diagnosing mastitis by enumerating different cell types in milk (leucocytes, including neutrophils, macrophages and lymphocytes) (Darbaz et al., 2023). SCC exceeding 200,000 cells/mL is typically associated with bacterial infection (Sharma et al., 2011). Both, clinical and subclinical mastitis can disrupt the SCC pattern (De Haas et al., 2004). In general, the major mastitis pathogens ( Streptococcus uberis, Escherichia coli, Staphylococcus aureus, Streptococcus dysgalactiae and Streptococcus agalactiae) elicit greater somatic cell response (Bradley and Green, 2005), than the minor pathogens ( Corynebacterium species and coagulase-negative staphylococci) (Sharma et al., 2011). Interestingly, infections involving the major pathogens are more likely to result in SCC levels over 200,000 cells/mL, whereas minor pathogens typically maintain SCC levels in the range from 50,000 to 150,000 cells/mL (Bradley and Green, 2005). Therefore, it's essential to keep these factors in mind when using SCCs as marker for making decisions regarding health status in dairy herds (Haxhiaj et al., 2022). Significantly, SCC has been linked to intramammary infection resolution, indicating its potential role in antibiotic treatment decisions (Williamson et al., 2022; De Jong et al., 2023).
Hence, this study was conducted to observe the distribution of SCCs and potential correlation to the different mastitis associated pathogens in the selected dairy farms in Serbia. Additionally, the prevalence of pathogens in the milk samples obtained from cows affected by mastitis was assessed.
Material and methods
Isolation and identification of mastitis associated pathogens
The Animal Ethics Committee of the Ministry of Agriculture, Forestry, and Water Management-Veterinary Directorate granted the approval for the experimental protocol (Approval No. 9000-689/2, dated 06 July 2020). This study was conducted from June to December 2021 on two dairy farms located in the Vojvodina Province, Serbia. A total of 194 individual cow milk samples were collected from cows with clinical and subclinical mastitis. Farm veterinarians examined the udders and milk to check for clinical mastitis, while the California Mastitis Test was used to confirm subclinical mastitis by analyzing the milk's SCC. Clinical signs of udder inflammation included swelling, pain and redness, while changes of interest in the first jets of milk were clots, color change and density. The milk samples for microbiological testing were obtained under aseptic conditions. After teat cleaning, drying and disinfection, the first jets of milk were discarded, and a 10 mL sample was collected in sterile tubes. These samples were stored in an ice container at 4 °C during transport to the Laboratory for Milk Hygiene at the Department of Veterinary Medicine, Faculty of Agriculture, University of Novi Sad. The samples were inoculated on the 2 % blood agar, and standard bacteriological diagnostic techniques, as previously described by Kovačević et al. (2021a), were employed for the isolation, identification and determination of mastitis pathogens. Yeast strains were cultured on Sabouraud dextrose agar plates, which were then incubated at 30 °C for 48 hours. Isolates were subsequently identified, using the “API 20 C AUX” (bio Meraux, France).
Determination of the somatic cell count
The SCC in the milk samples was assessed following the microscopic reference method as per the Institute for Standardization of Serbia (SRPS EN ISO 13366-1:2010) (ISO, 2010). To determine the SCC, 0.01 mL of mixed milk from each sample was spread across a 1 cm2 area on a glass slide. These slides were then air-dried, stained with the Newman-Lampert stain, and examined under a microscope. The SCC result below 200,000 cells/mL was considered low, indicating a healthy mammary gland (Piccinini et al., 2005). In contrast, SCC exceeding 200,000 cells/mL was classified as high, signifying the presence of an intramammary infection.
Statistical analysis
The obtained data were summarized by application of Microsoft Office Excel and statistically processed by Tibco Statistica (v. 13.5). Data were analysed by descriptive statistical methods, while differences between evaluated groups in terms of number of somatic cells were assessed by application of ANOVA.
Results and discussion
Prevalence of mastitis-associated pathogens
Based on laboratory results, 104 (53.60 %) milk samples were negative for both yeast and bacteria (group 1), while the rest of samples (46.40 %) were positive on pathogens presence. Of those, 56 (28.87 %) were positive for bacteria (group 2), 24 (12.38 %) for yeast (group 3), and 10 (5.15 %) were positive for yeast and bacteria (group 4).
Table 1. Prevalence of mastitis-associated pathogens in the milk samples
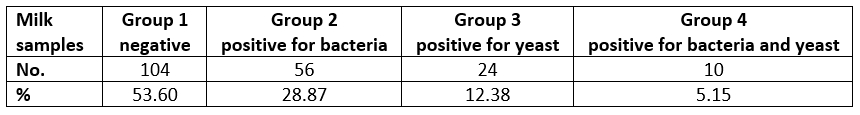
Of the isolated bacteriological causes of mastitis, Streptococcus spp. was isolated in 20 cases (30.30 %), E. coli and S. marcescens were present in 12 samples (18.18 %) each, followed by 8 cases (12.12 %) of S. aureus, while Proteus mirabilis was isolated in 6 cases (9.10 %). Klebsiella spp., Staphylococcus spp., S. uberis and β-haemolytic Streptococcus spp. were isolated in 2 samples, each (3.03 %). With regard to yeast, we isolated Candida spp. in 28 samples (82.35 %) of total samples positive for yeast, and C. albicans in 6 cases (17.64 %).
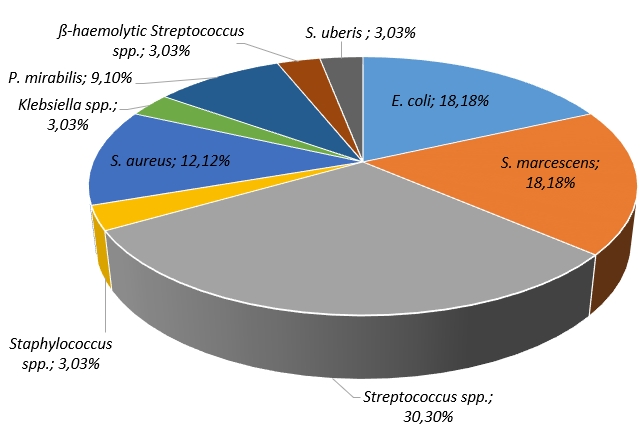
Figure 1. Frequency of the mastitis-associated bacteria isolates in the tested samples
Bovine mastitis involves range of microorganisms with changing prevalence linked to factors like sample timing and region-specific variations in infection patterns (Malinowski et al., 2006). Since this disease has gained great attention, mastitis causative agents are well described in the studies conducted in Serbia (Milanov et al., 2014; Kovačević et al., 2021a, 2021b; Kovačević et al., 2022) and worldwide (Janosi and Baltay, 2004; Malinowski et al., 2006; Tenhagen et al., 2006; Bi et al., 2016; Abed et al., 2021). While some researchers explored the relationship between the SCC and mastitis-associated pathogens (Moretti et al., 1998; De Haas et al., 2004; Huang and Kusaba, 2022), to our knowledge no similar studies have been performed yet in Serbia.
Our findings indicate that Streptococcus spp. were prevalent in 30.30 % of evaluated samples, which is consistent with study in Italy (33.84 %) (Ceniti et al., 2017). These bacteria are globally recognized as major mastitis pathogens (Kaczorek et al., 2017) with the prevalence of 50 % in Australia, followed by Europe (38 %) (Kabelitz et al., 2021). Smistad et al. (2023) suggests that strategies to control infectious pathogens, like the five-point and 10-point plans, reduced contagious mastitis but had limited impact on environmental pathogens like Streptococcus spp. and coliform bacteria. Our findings show a 12.12 % prevalence of S. aureus, despite being considered as one of the most common causes of mastitis (Liu et al., 2020). In addition, lower prevalence of S. aureus was also reported in Croatia, being isolated in 4.48 % of udder quarter samples (Cvetnić et al., 2021). According to McDougall et al. (2022) prevalence of S. aureus can vary with the age being more prevalent and of greater duration in older animals due to its contagious nature.
Coliform-associated mastitis ( E. coli and S. marcescens) in our study had a relatively high prevalence (18.18 %) being comparable to study in Norway (14.50 %) (Smistad et al., 2023), but lower than 21.9 % prevalence in North West Cameroon, where it was significantly associated with factors such as lactation stage, cow breed, history of mastitis and contaminated environment (Abegewi et al., 2022). Furthermore, E. coli is recognized as the most common gram-negative coliform bacteria in intensive milk production systems on dairy farms (Morales-Ubaldo et al., 2023). Apart from E. coli, S. uberis and K. oxytoca were also found, supporting the increasing presence of environmental pathogens (Cervinkova et al., 2013).
The prevalence of S. uberis in our study (3.03 %) aligns with other reports ranging from 2 % to 9 % (Bi et al., 2016; Smistad et al., 2023). This pathogen's ability to produce biofilms, capsules, invade mammary gland cells and resist phagocytosis makes controlling S. uberis mastitis challenging, even in case of a low farm-level presence (Wente et al., 2019). Furthermore, Klebsiella spp., particularly K. oxytoca, plays a significant role in bovine mastitis (Song et al., 2023), primarily originating from environmental sources. Improved hygiene practices can reduce transmission (Song et al., 2023). In our study, K. oxytoca was found in 3.03 % of samples, but it's less significant in milk samples when compared to S. aureus and E. coli, as noted by other authors (Song et al., 2023).
Yeast, although infrequently implicated, have been linked to mastitis in dairy cattle. While data on yeast prevalence in Serbian dairy farms is limited, our study's results (12.38 %) are relatively comparable with those from (Milanov et al., 2014), who reported yeast isolation in 6.02 % of milk samples. Candida is a significant pathogen in mycotic mastitis among dairy cows, particularly non-albicans species, as supported by the literature (Zhou et al., 2013). Our study found all yeast to be Candida spp., including C. albicans. In contrast, Milanov et al. (2014) pointed out that among all the yeast species recovered from cow's milk , C. albicans rarely takes dominant role. However, it's important to note that extensive production systems, environmental temperatures and disease duration are significant risk factors contributing to the prevalence of mycotic mastitis (Zhou et al., 2013).
Somatic cell count in relation to type of pathogens
The application of ANOVA has demonstrated a negative statistically significant differences in SCC of milk samples (Figure 2), while positive on bacteria, positive on yeast and positive on bacteria and yeast (F(3, 98)=10.895, p=0.00). Moreover, the recorded difference was a result of high SCC in milk samples being positive both for bacteria and yeast.
SCC levels and bacteriological examinations serve as different methods for evaluating the mammary gland's condition (Schwarz et al., 2010; Tommasoni et al., 2023). Besides, using the test-day SCC records helps identify deviations from the usual SCC pattern, indicating potential mastitis-causing pathogens (De Haas et al., 2004). Numerical increase in lactations associated to isolated pathogens, compared with unaffected cows have been reported worldwide (De Haas et al., 2004; Skrzypek et al., 2004; Malinowski et al., 2006; Lopes Júnior et al., 2012; Sumon et al., 2020). Yet, comparing milk SCC in relation to specific bacterial species in the literature is challenging due to variations in methodologies, including the analysis of different milk types by different authors. Typically, the major pathogens are associated to the most significant increase in SCC, while infection by minor pathogens leads to a notably lower SCC rise and rarely to the clinical manifestation of the mastitis (Supré et al., 2011). Our study results indicated that there was a statistically significant difference between the SCC observed among all tested samples (negative, positive for bacteria, positive for yeast and positive both for bacteria and yeast) (Figure 2). Notably, the highest SCC was recorded in milk samples that tested positive for both bacteria and yeast.
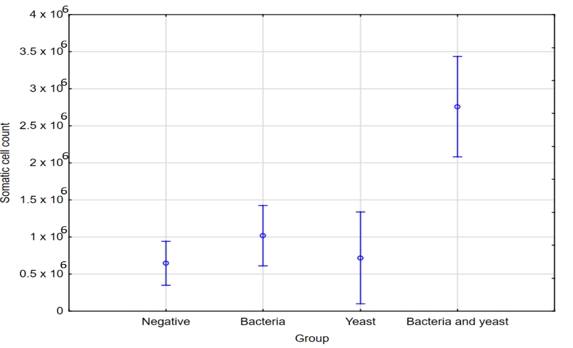
Figure 2. ANOVA - SCC in relation to type of pathogens
Milk samples with SCC levels below 200,000 cells/mL were mostly cultured negative (75 %). In contrast to these results, some of the mastitis cases without detectable bacterial growth exhibited an increase in SCC. The majority of samples showing no bacterial growth indicated SCC levels in the range from 200,000 to 500,000 cells/mL. Besides, samples where SCC levels exceeded 1,000,000 cells/mL were primarily associated with infections caused by E. coli, S. marcescens and S. aureus. Furthermore, notably elevated SCC levels were linked to infections attributed to both yeast and bacteria, whereas yeast-only infections resulted in a comparatively smaller increase in SCC, typically measuring less than 1 million cells per milliliter.
Passing over the limit of 200,000 cells/mL indicates the transition from health to disease (Skrzypek et al., 2004). Our study revealed that milk samples with SCC below 200,000 cells/mL were mainly culture negative, consistent with other research (Malinowski et al., 2006). We have also observed higher SCC in cases of mastitis with no bacterial growth, aligning with previous findings suggesting that high SCCs may not always indicate the presence of mastitis pathogens (Souza et al., 2016; Sumon et al., 2020). According to McDougall et al. (2001), the presence of high SCC in milk samples, even in the absence of microorganisms, does not necessarily indicate the udder's health. Nonetheless, even though we couldn't detect any pathogens in those quarters, they could still be infected. Elevated SCC in cases without detected bacteria could result from undetectable bacterial levels during sampling or effective immune elimination (Campos et al., 2022). Negative bacteriological findings may also be influenced by sporadic pathogen shedding, antimicrobials, growth-inhibiting substances, or intracellular survival (Schwarz et al., 2010; Kandeel et al., 2018). It must be pointed out, that SCC can also be affected by non-infectious factors, such as animal’s age, stage of lactation, the time of year, milking frequency and nutrition (Bradley and Green, 2005). Our study confirmed mastitis pathogens presence in cows with lower SCC, emphasizing the need for bacteriological culture even when SCC suggests lower likelihood of subclinical mastitis (Katsande et al., 2013; Huang and Kusaba, 2022). It is important to note that SCC and bacteriology may not always produce matching results, as infected udders may not consistently release pathogens, leading to negative test outcomes (De Haas, 2005).
In the group of cows with SCC between 200,000 and 500,000 per milliliter, a substantial number did not test positive for any of pathogens. This pattern is consistent with other authors (Janosi and Baltay, 2004; Souza et al., 2016). Samples with SCC exceeding 1,000,000/mL were primarily associated with E. coli, S. marcescens, and S. aureus infections. Janosi and Baltay (2004) reported that nearly 50 % of cows infected by coliform bacteria had milk SCC exceeding 400,000/mL. In Serbian study, milk samples had SCC of over 2,000,000 cells per milliliter due to S. aureus infection (Radinović et al., 2008). This SCC elevation was also observed in other countries with S. aureus infections (Souza et al., 2016; Karzis et al., 2017) De Haas et al. (2004) found that different pathogens affect lactation SCC differently. For instance, S. aureus mastitis leads to a long-lasting SCC increase, while E. coli mastitis results in a short-term elevation. Infections early in lactation, particularly if caused by persistent pathogens such as S. aureus, have a significant impact on lactation SCC (De Haas et al., 2004).
Our study found that infections attributed to both bacteria and yeast were associated with significantly elevated SCC, which is consistent with other studies (Malinowski et al., 2006; Lopes Júnior et al., 2012; Sumon et al., 2017). This increased response may result of interaction between these pathogens as the immune system simultaneously combats both, compared to single-pathogen infections. SCC increases depend on bacteria pathogenicity and affected udder tissue (Pyörälä, 2003). Variation in susceptibility to different bacteria is linked to individual immune responses and distinct infection mechanisms (Campos et al., 2022). Based on findings by Safak et al. (2022), it was determined that maintaining strong cellular immunity can be beneficial in preventing E. coli-induced mastitis, while strong humoral immunity is advantageous in reducing the occurrence of S. aureus and S. agalactiae-induced mastitis. Additionally, SCC response to significant pathogens varies among individual cows, making it impractical to identify pathogen types based solely on SCC (Sharma et al., 2011). As Hariharan et al. (2004) stated, there is weak correlation between SCC and bacteriological results.
Table 2. Microorganisms isolated from milk samples in relation to SCC (x 103/mL)
Somatic cell count in relation to specific isolated mastitis associated pathogens.
The application of ANOVA did not show statistically significant differences in SCC in the milk samples when the specific isolated pathogens, as well as samples being negative were taken into account (Figure 3, F(11, 90)=.87, p=0.57).
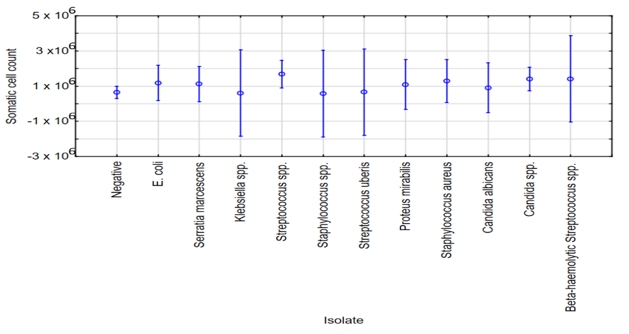
Figure 3. ANOVA - SCC in relation to specific isolated pathogens
Conclusion
In conclusion, mastitis is significant challenge in the dairy industry. Monitoring udder health using SCC is crucial and analyzing SCC records in the mastitis control programs is effective. However, distinguishing specific pathogens based on SCC is challenging. Current study offers insights into the complex management of the mastitis in the dairy herds.
