INTRODUCTION
Petasites Mill. (butterbur) species are large-leaved rhizomatous perennial herbs from the daisy (Asteraceae) family, which are distributed all over Europe, Asia, and North America. Their name comes from the Greek word petasos, which refers to a large hat commonly worn in Ancient Greece. Out of 19 species accepted today (1), the widespread Petasites hybridus G.Gaertn., B.Mey. & Scherb. (common butterbur) is pharmaceutically and phytotherapeutically the most important (2). In recent years, the use of its extracts as green media for generating magnetic organometallic nanocomposites that are used as catalysts in one-pot multicomponent reactions (MCRs) is becoming increasingly popular, e.g., for the synthesis of compounds that could reduce organic pollutants or that could be used in the synthesis of new pharmaceuticals (3, 4). Flavonoids and phenolics present in the P. hybridus rhizome extract were suggested as the main compounds responsible for the reduction of metal ions to nano-zero-valent metallic particles (3). Modern pharmacotherapy also recognizes the use of P. hybridus rhizome extract (Petadolex®) for the prevention of migraine attacks (5), while P. hybridus leaf extract (Tesalin® – Ze 339) is clinically approved as a herbal medicine for the treatment of symptoms of seasonal allergic rhinitis such as rhinorrhea, sneezing, and nasal congestion (1). In fact, out of twenty-nine randomized controlled trials that evaluated the use of single medicinal plants for allergic rhinitis among adults and children, the greatest number of studies included P. hybridus (6).
Rare and idiosyncratic cases of herb-induced liver injury caused by the rhizome extract Petadolex®, often confounded by hepatotoxic co-medications, were reported (N = 48 cases over a period of > 30 years and an estimated 2.6 million patient month exposure), while clinically relevant liver function abnormalities were not observed in clinical trials with migraine patients (5). On the other hand, there have been no reports of serious adverse drug reactions with the butterbur leaf extract so far (6). This was recently acknowledged by the Swiss health authority as Tesalin® (Ze 339) was switched from prescription to nonprescription status. It was concluded that P. hybridus leaf extract Ze 339 may be regarded as safe if used in the clinically recommended dose regime (7). Also, results from a recent study that evaluated in vivo single and repeated oral dose toxicity and in vitro genotoxicity of P. japonicus (Siebold & Zucc.) Maxim. leaves suggested that they may be safe for human consumption (8). The clinically approved butterbur extracts mentioned above are standardized to petasins and are declared as PA-free, i.e., free of hepatotoxic and carcinogenic pyrrolizidine alkaloids (PAs). Due to their initially lower contents of PAs, leaves may be a more suitable source of petasins (9), the pharmacologically active ingredients thatare at least partially responsible for the anti-inflammatory effects of butterbur extracts (5). Various populations of P. hybridus were shown to vary considerably in petasin content both in their rhizomes (7.4 to 15.3 mg g–1dry mass (DM)) and leaves (3.3 to 11.4 mg g–1DM), while even greater differences were observed between rhizomes (4.8–89.9 µg g–1DM) and leaves (0.02–1.50 µg g–1DM) in the content of PAs (9). Besides between different organs, PA content may vary considerably within and between populations, while seasonal variations seem to be of minor importance (10). Similarly, great variabilities in essential oil constituents were observed for different plant parts and populations of P. hybridus and P. albus (L.) Gaertn. from Croatia (11). Recently, also the content of total phenolic compounds, and antioxidant and antimicrobial activity were reported for extracts obtained by ultrasound-assisted extraction (UAE) of different plant parts of P. hybridus from Turkey (12). However, little is known about the possible variabilities of phenolics and flavonoids between different populations of P. hybridus and related species.
Besides P. hybridus, which has been recognized as one of the most important Central European medicinal herbs used from classical antiquity to the modern and contemporary era (13), other species from the genus Petasites such as P. japonicus, P. tricholobus Franch., P. formosanus Kitam., and P. frigidus (L.) Fries have been used worldwide both as food and traditional medicines (1, 14, 15). In Bosnia and Herzegovina, ointments prepared from leaves of wild P. hybridus and P. albus are used for rheumatism (16). Ethnomedicinal use of the same two species in Serbia was also recently reported (17). The aim of this study was to evaluate the total phenolic and flavonoid contents of P. hybridus, P. albus, P. kablikianus Bercht., and P. paradoxus (Retz.) Baumg. together with their antioxidant potential. Samples of the former two species were collected from four different locations in Croatia, two of which were shared by both species (Medvednica, Fužine). To our knowledge, this is the first study that compared the phytochemical content and antioxidant activity of several Petasites species, and the first such study that compared the contents of polyphenols and flavonoids in different populations of P. hybridus and P. albus.
EXPERIMENTAL
Plant material
Leaves of four different Petasites species were collected during the flowering period from ten wild populations in Croatia: Petasites hybridus (Mount Medvednica, Fužine, Mount Ivanščica, Zagreb - Maksimir), P. albus (Mount Medvednica, Fužine, Mount Risnjak, Northern Velebit), P. kablikianus (Plitvice Lakes), and P. paradoxus (Baške Oštarije). Samples were authenticated by Prof. Kroata Hazler Pilepić and voucher specimens were deposited at the herbarium of the Department of Pharmaceutical Botany, University of Zagreb Faculty of Pharmacy and Biochemistry.
Extract preparation
After air-drying at room temperature, the leaves were initially cut into smaller pieces and then ground to powder using an electric mill. Ultrasound-assisted extraction was performed twice by adding 5 mL of methanol to 0.5 g of powdered plant material for the duration of 2 × 30 minutes. After filtering, the two extracts were combined and made up to the mark with methanol in a 10 mL flask.
Chemical reagents and standards
Folin-Ciocalteu’s reagent and sodium carbonate decahydrate were purchased from Kemika (Croatia). Aluminum chloride hexahydrate was obtained from Sigma-Aldrich (USA) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) from Fluka (Switzerland). Standard compounds gallic acid, quercetin, and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), were purchased from Merck Schuchardt (Germany), Sigma-Aldrich (USA) and Acros Organics (Belgium), respectively. All chemicals used were of analytical grade.
Evaluation of total phenolic and flavonoid content
Total phenolic content was evaluated using the Folin-Ciocalteu’s reagent, similarly as previously described (18). In brief, 0.25 mL of extract was mixed with 1.25 mL of Folin-Ciocalteu’s reagent (10 % (V/V)). After 5 min, 1 mL sodium carbonate decahydrate (7.5 g 100 mL –1 ) was added. After 60 min of incubation, the absorbance was read at 765 nm. The results are expressed as mg gallic acid equivalent g –1 dry mass (mg GAE g –1 DM).
Flavonoid content was evaluated using the previously described aluminum chloride method (18). In brief, 1 mL of extract was mixed with 1 mL AlCl3 × 6 H2O (2 g 100 mL –1 ). After 15 min, absorbance was measured at 415 nm. The results are expressed as mg quercetin equivalent g –1 dry mass (mg QE g –1 DM).
DPPH assay
Antioxidant potential was evaluated based on the DPPH radical scavenging activity as previously described (18). To 2 mL of methanolic DPPH solution, adjusted to an initial absorbance of 0.70 ± 0.02, 10 µL of extract was added. After 30 min incubation, the decrease in absorption of the radical was measured at 517 nm. The results are expressed as mg Trolox equivalent g –1 dry mass (mg TE g –1 DM).
Statistical analysis
All measurements were performed in triplicate. The results are expressed as means ± standard deviations (SD). Correlations between measured parameters were assessed using Pearson’s correlation coefficient (r) with the significance level, ɑ, set at 0.05. Statistical analysis was performed in GraphPad Prism 9.0. (GraphPad Software, San Diego, USA).
RESULTS AND DISCUSSION
In this study, four European Petasites species were harvested from ten wild populations in Croatia in order to evaluate their (poly)phenolic content, flavonoid content and antioxidant potential: P. hybridus (Medvednica, Fužine, Ivanščica, Zagreb - Maksimir), P. albus (Medvednica, Fužine, Risnjak, Northern Velebit), P. kablikianus (Plitvice Lakes), and P. paradoxus (Baške Oštarije).
Total phenolic content
The total phenolic content of collected samples was assessed using the Folin-Ciocalteu assay, one of the most commonly used methods for the determination of (poly)phenolic compounds in plant-based foods and beverages (19). The assay is based on a single electron transfer (SET) in which the antioxidant species acts as the electron donor and the Folin-Ciocalteu’s reagent acts as the oxidant, causing a change in color from yellow to blue, directly proportional to the reducing activity of the phenolic compounds. This is frequently displayed as gallic acid equivalents (GAE) (20).
In the present study, the total phenolic content of P. hybridus samples ranged from 4.43 ± 0.09 to 10.76 ± 0.60 mg GAE g–1 DM, while for P. albus samples it varied between 6.66 ± 0.43 and 19.92 ± 2.90 mg GAE g–1 DM (Fig. 1). Furthermore, the total phenolic contents of the remaining two species were within the ranges observed for P. hybridus and P. albus, 7.56 ± 0.17 mg GAE g–1 DM in P. kablikianus and 10.22 ± 0.46 mg GAE g–1 DM in P. paradoxus. In a recent study from Turkey, the content of total phenolic compounds of P. hybridus leaf extract, under optimal conditions, was found to be 3.78 μg GAE mg–1 extract (12). The total phenolic acid content of extracts obtained from P. japonicus leaves and stalks, assessed from peak areas of the UPLC-DAD chromatogram, was 16.76 ± 0.42 mg g–1 DM. The major phenolic acid was 3,5-di-O-caffeoylquinic acid followed by 5-O-caffeoylquinic acid and fukinolic acid, while kaempferol 3-O-(6”-O-acetyl) glucoside, quercetin 3-O-(6”-O-acetyl) glucoside, astragalin, and kaempferol 3-O-rutinoside (nicotiflorin) were the most represented flavonoids (21). The presence of caffeoylquinic and feruloylquinic acid derivatives was reported in P. hybridus leaves as well (22).
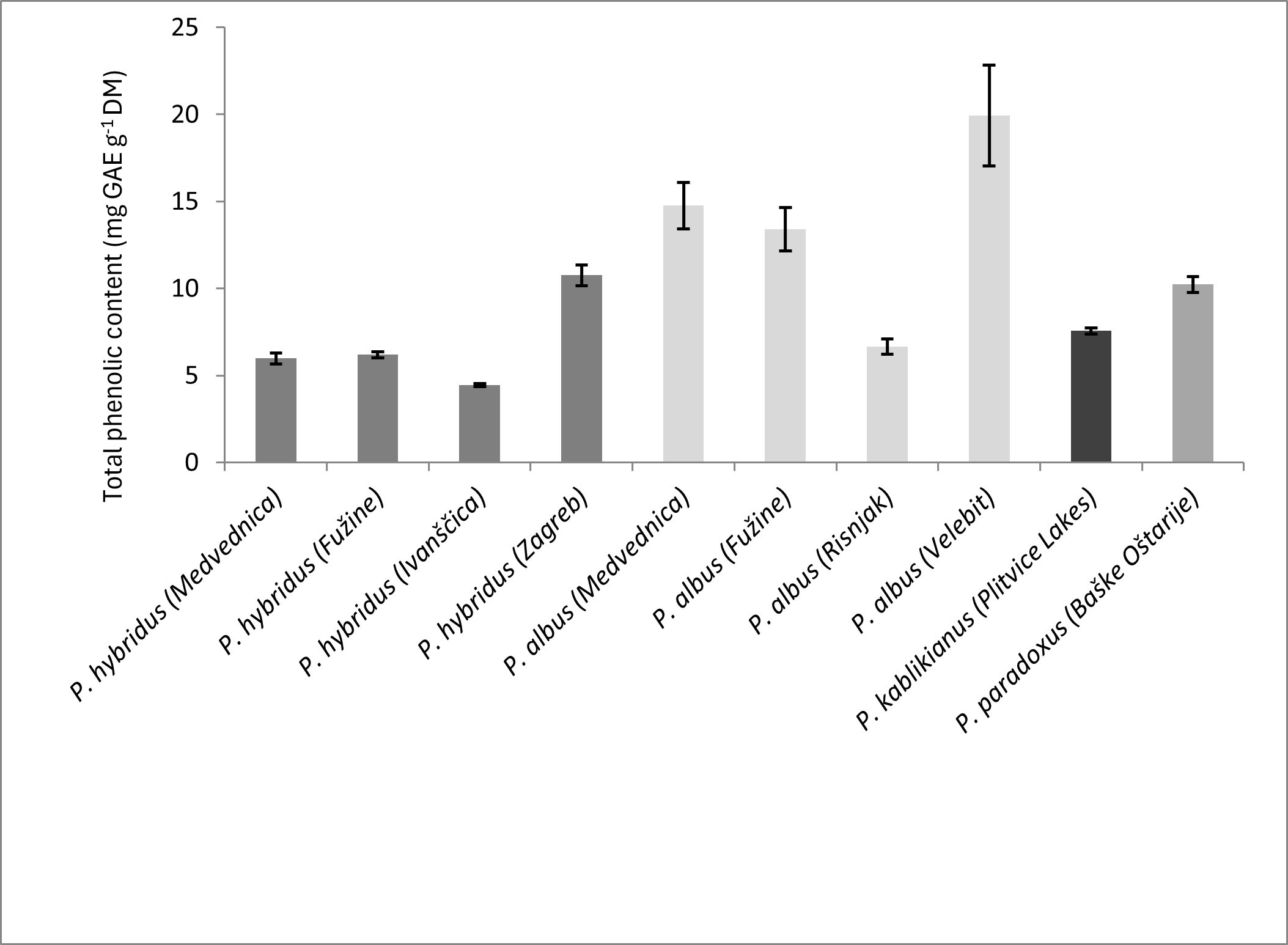
Fig. 1. Total phenolic content of ten investigated Petasites samples (averages ± SD, n = 3); GAE – gallic acid equivalent, DM – dry mass.
Flavonoid content
Flavonoid content in this study was assessed based on a spectrophotometric aluminum chloride chelation method, one of the most commonly used methods for the so-called total flavonoid determination, using the procedure without NaNO2 (23), which may only be used for the estimation of the contents of certain classes of flavonoids, i.e., flavones and flavonols (24). Flavonols such as quercetin and kaempferol and/or their glycosides were previously reported for Petasites species (21, 25). In the assay, flavonols form complexes with Al(III) with C-3 and C-5 hydroxy groups and with the dihydroxy groups in the B ring (23).
The highest flavonoid contents were recorded for P. kablikianus and P. paradoxus, 5.59 ± 0.05 mg QE g–1 DM and 5.50 ± 0.09 mg QE g–1 DM, respectively (Fig. 2). For P. hybridus, the same varied between 2.51 ± 0.10 and 4.03 ± 0.08 mg QE g–1 DM, while for P. albus, a wider range of flavonoid contents was recorded (from 2.21 ± 0.09 to 5.22 ± 0.02 mg QE g–1 DM). Not a lot is known about the flavonoids from investigated Petasites species. For P. hybridus, quercetin, quercitrin and rutin were reported (1). Flavone glycosides quercetin-3-O-β-glucopyranoside and kaempferol-3-O-β-glucopyranoside and their rhamnosylated derivatives (rutin, nicotiflorin) were isolated from P. tricholobus (25). Recently, a more detailed list of flavonoid compounds was given for 80 % ethanolic extract obtained by Soxhlet extraction from P. japonicus leaves and stalks, which was based on ultra-performance liquid chromatography coupled with diode array detector, quadrupole time-of-flight mass spectrometry (UPLC-QToF-MS) characterization. The total flavonoid content of the same extract was estimated to be 10.65 ± 1.25 mg g–1 DM (21).
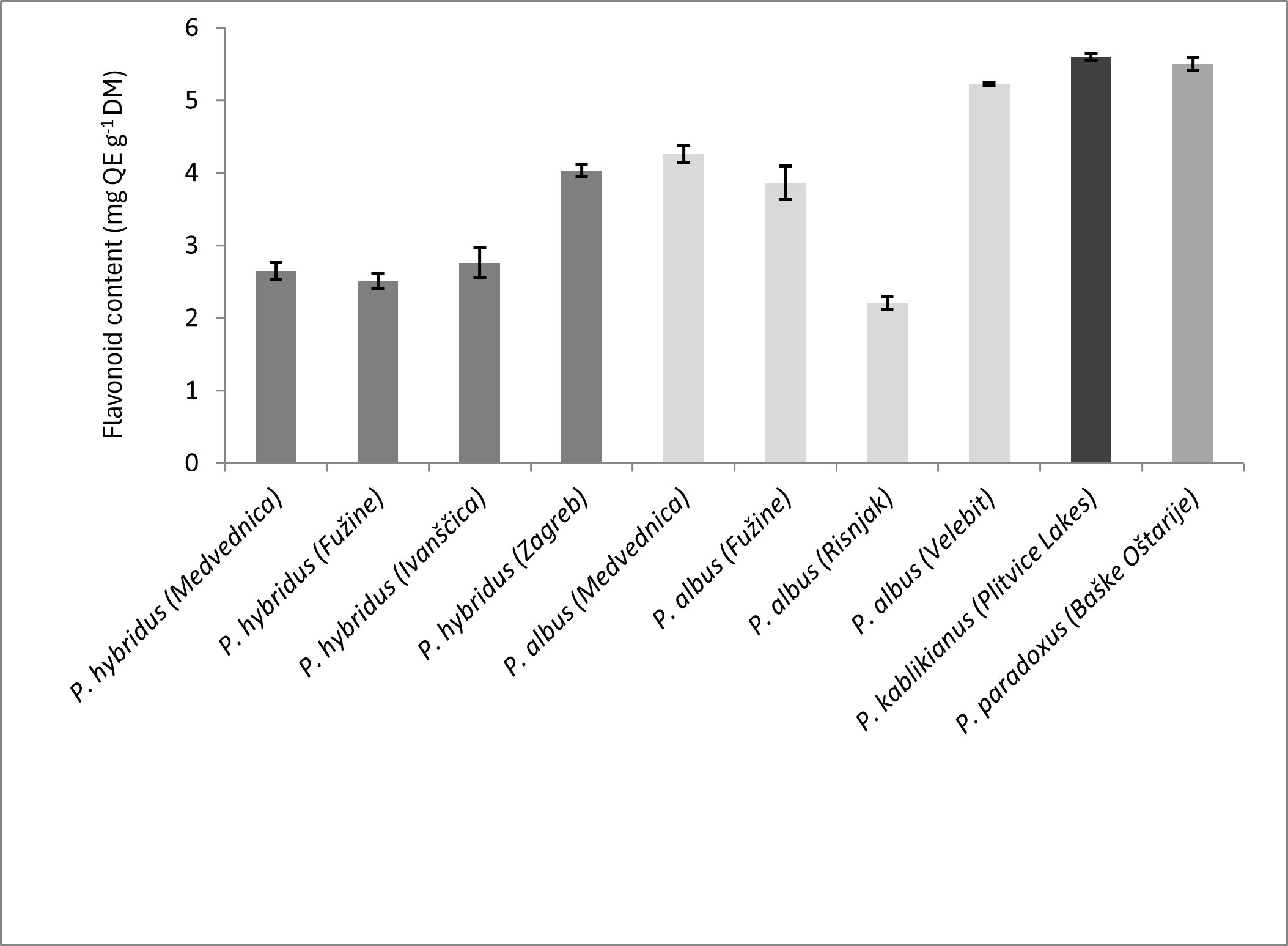
Fig. 2. Flavonoid content of ten investigated Petasites samples (averages ± SD, n = 3); QE – quercetin equivalent, DM – dry mass.
Antioxidant potential
Antioxidant potential was evaluated using the DPPH radical scavenging assay, the most known and commonly used method to determine the antioxidant ability of food and pharmaceutical ingredients. The DPPH assay is based on spectrophotometric measurements of the capacity of antioxidants to scavenge DPPH radicals. The method is popular due to its ease of use, sensitivity, reproducibility, and execution speed, and can be readily used without the need for free radical preparation prior to performing the test (26). The reaction mechanism is primarily based on electron transfer (ET), which can be further subdivided into single electron transfer followed by a proton transfer (SET-PT) and sequential proton loss electron transfer (SPLET) (27). Also, a marginal reaction pathway in the assay is hydrogen atom transfer (HAT), i.e., reduction of the single electron of the nitrogen atom in DPPH radical (intense deep violet color) to the corresponding hydrazine DPPH-H (pale yellow color) by taking a hydrogen atom from the antioxidants (H-donors) (28). A standard antioxidant Trolox may be used as a reference standard and the results of the assay may be expressed as Trolox equivalents (TE) (26).
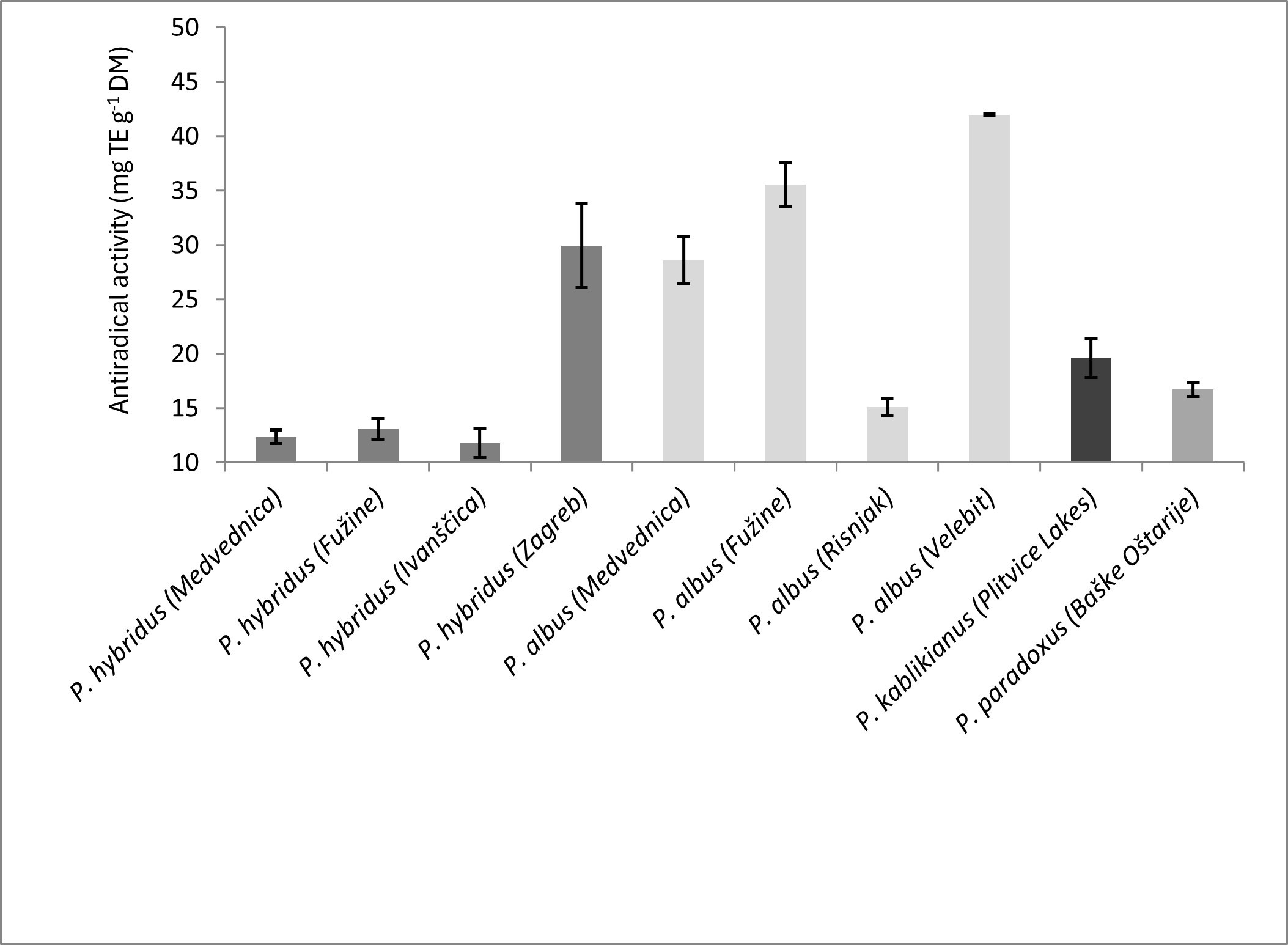
Fig. 3. DPPH radical scavenging activity of ten investigated Petasites samples (averages ± SD, n = 3); TE – Trolox equivalent, DM – dry mass.
The DPPH radical scavenging activity found in this study ranged from 11.78 ± 1.34 to 29.94 ± 3.83 mg TE g–1DM in P. hybridus samples and from 15.07 ± 0.80 to 41.98 ± 0.12 mg TE g–1DM in P. albus samples, while it was within the same ranges for the remaining two species, P. kablikianus (19.59 ± 1.77 mg TE g–1DM) and P. paradoxus(16.72 ± 0.66 mg TE g–1 DM) (Fig. 3). The antioxidant activity estimated by the DPPH assay of P. hybridus ethanolic leaf extract obtained under optimized conditions was found to be 2.27 µg TE mg–1extract (12). In the present study, the DPPH radical scavenging activity was found to be in high correlation with the total phenolic content (r = 0.93, p < 0.001, Table I). This may be explained by the fact that both assays are based on SET reactions (20, 27).
Table I. Pearson’s correlation coefficients (r) between the measured parameters (total phenolic content, flavonoid content, and DPPH radical scavenging activity) of ten Petasites samples
| Measured parameters | Total phenolic content | Flavonoid content |
|---|---|---|
| Flavonoid content | 0.59 (p = 0.071) | 1 |
| DPPH radical scavenging activity | 0.93 (p < 0.001) | 0.52 (p = 0.125) |
Until today, most of the investigations of antioxidant activity of Petasites species were done on P. japonicus and the DPPH assay was the most frequently used in vitro method (14). Caffeic acid derivatives such as 5-O-caffeoylquinic acid, fukinolic acid, 3,5-di-O-caffeoylquinic acid, and 4,5-di-O-caffeoylquinic acid present in methanolic extracts of leaves and roots, were some of the compounds responsible for the antioxidant activity with 3,5-di-O-caffeoylquinic acid having the greatest radical scavenging capacity in leaf (23.09 %) and root extracts (26.47 %). On the other hand, flavone glycoside quercetin-3-O-(6’’-acetyl)-β-glucopyranoside, which was present only in the leaf extract, showed weak activity, while no activity was observed for kaempferol-3-O-(6’’-acetyl)-β-glucopyranoside (29). This is consistent with our results, according to which the antiradical activity of investigated Petasites species was not significantly correlated with flavonoid content. Indeed, the aforementioned caffeoylquinic acid derivatives were, likewise, reported to be present in P. hybridus leaves together with 5-O-feruloylquinic acid, 1-O-caffeoyl-3-O-feruloylquinic acid, and 1-O-caffeoyl-4-O-feruloylquinic acid. It is interesting to notice that other well-known medicinal plants such as Achillea millefolium L. (yarrow) and Cynara scolymus L. (artichoke) also possessed most of the aforementioned hydroxycinnamic acid derivatives (22). Fukinolic acid is yet another phenolic compound, which was recognized as the major antioxidant constituent in ethanolic extracts of P. japonicus flower buds (30). However, to our knowledge, its presence in other Petasites species has not been reported so far (31). On the other hand, in investigated plants, other non-phenolic antioxidants may be present such as the benzofuran derivative euparin found in essential oils of P. albus (aerial parts), which was observed to possess moderate antioxidant activity according to the DPPH radical scavenging assay (32).
Evaluation of the antioxidant activity of extracts prepared from leaves, stems, and roots of P. japonicus based on various methods, including DPPH, 2,2′‐azinobis(3‐ethylbenzothiazoline‐6‐sulfonic acid) radical cation (ABTS•+), and superoxide anion radical scavenging assay, and the ferric reducing ability of plasma (FRAP), showed that the antioxidant activity of leaf extract is superior to those of other extracts (33). Similarly, essential oils obtained from leaves of P. hybridus subsp. ochroleucus exhibited greater antioxidant activity in the DPPH assay in comparison to essential oils obtained from rhizomes of the same plant (34).
Variabilities in specialized metabolites content within and between species
As observed in this and in some of our previous studies, the contents of specialized (secondary) metabolites may not only differ between different species of the same genus (35), but they may also vary considerably between different populations of the same species (18, 36). For this reason, to gain a better insight into the richness of biologically active compounds of a certain species, it is beneficial to include, when possible, more than one population of the species of interest in the study. This is certainly more challenging in terms of sample collection and is not always possible, e.g., when the species of interest are growing in single and/or hard-to-reach locations. Generally, investigated Petasites species are known to inhabit moist, damp, and shady areas, and are often covering large surfaces on riverbanks, near lakes and streams thanks to their creeping underground stems (rhizomes) (1, 37). Common butterbur (P. hybridus) and white butterbur (P. albus) are widespread in Europe and in Croatia (1, 38). On the other hand, in Croatia, P. kablikianus (glabrous butterbur) is found only in the Plitvice Lakes area (38). In fact, it is the dominant fast-decomposing species growing on the tufa barriers, whose formation is promoted by leaf litter breakdown (and vice versa) (39). Petasites paradoxus (Alpine butterbur), on the other hand, as its name suggests, may be found in mountainous regions (37, 38).
In this study, P. kablikianus and P. paradoxus were each collected from a single location, while four different populations of P. hybridus and P. albus were investigated due to the better distribution and accessibility of these species. Conveniently, two of the four harvesting locations were shared by the same species, which enabled a more reliable comparison between them, bearing in mind that they had been growing in equal environmental conditions. In our previous studies, the contents of secondary metabolites in leaf extracts were observed to be correlated with monthly precipitation amounts and mean monthly temperature (11, 18). Based on the comparison of the results obtained for the samples harvested from the shared locations (Medvednica and Fužine), it could be observed that P. albus contained more (poly)phenolic compounds and more flavonoids than P. hybridus. The total phenolic content of these populations of P. albus was also higher than those of P. kablikianus and P. paradoxus. On the other hand, the latter two species contained the most flavonoids. These results make the three species of Petasites potentially interesting in terms of their possible exploitation as sources of natural antioxidants and/or utilization as media in the green synthesis of nanoparticles.
Considerations regarding the use of Petasites species in green synthesis
Green synthesis is a subdivision of green chemistry, which aims to develop safer and more sustainable chemical products and procedures. The fundamental green chemistry principles that are applied in green synthesis include environmental pollution mitigation, renewable feedstock usage, usage of non-toxic (or safer) solvents/auxiliaries, derivatives usage minimization, and waste prevention or reduction (40). Green synthesis of nanoparticles from biomass and waste, as an eco-friendly, biocompatible, and cost-effective approach for use in medicine, agriculture, environmental remediation, and other fields, is believed to allow up to 30 % reduction in energy consumption, up to 40 % cost savings, and up to 50 % production increase and could therefore, contribute to a more sustainable future (41). Compared to other green synthesis methods of nanoparticles, plant-mediated synthesis is the most efficient (40).
Lately, the number of papers suggesting the use of P. hybridus rhizomes in green synthesis is on the rise (3, 4). The root extract of this plant is considered a renewable, mild, and safe reducing agent and effective stabilizer (4). Moreover, the utilization of P. hybridus leaf extract has also been demonstrated for the same purpose (42). However, caution should be taken considering that Petasites species are natural sources of highly hepatotoxic and carcinogenic pyrrolizidine alkaloids (PAs), especially in their underground parts (9, 43). Although the right mechanism by which butterbur preparations might cause liver injury is not known, it is suggested that liver-related adverse effects of commercially available butterbur products were likely connected to PAs contamination or mislabelling of the products (44). With that in mind, although the use of these extracts may be possibly advantageous, it may also be potentially harmful, even when low levels of PAs are present.
Leaves, which were used for Petasites extract preparation in this study, could potentially serve as a better and more renewable source of biologically active compounds (e.g., those acting as reducing agents in the synthesis of nanoparticles/nanomaterials) in comparison to roots/rhizomes, considering their expected higher content of reducing substances/antioxidants (33) and significantly lower PAs content (9, 43). Leaves of other species such as Eucalyptus sp., Thymus vulgaris L., and Ginkgo biloba L. have been successfully used to synthesize nanoparticles. However, the main research focus should be on materials that are not limited by seasonal and geographical availability (45).
Utilization of leaves from Petasites species could be potentially interesting due to the worldwide distribution of these species and their large size (1). The results of our study, although based on simple spectrophotometric reactions and a relatively small number of samples, indicate that P. albus, P. kablikianus, and P. paradoxus may exhibit similar, if not better, antioxidant/reducing properties to those of P. hybridus. Considering the need to minimize the risk from exposure to PAs, it would be interesting to compare the PA contents of P. hybridus and related species that may be locally available in future studies. Also, since information on the phytochemical composition/constituents, especially those including phenolic acids, flavonoids, and other polyphenols of P. albus, P. kablikianus, and P. paradoxus as well as most other Petasites species are lacking or are relatively modest in the case of P. hybridus, it would be interesting to evaluate those as well, having in mind their potential antioxidant (reducing) properties important for green synthesis.
Considerations regarding the use of standardized extracts of Petasites hybridus leaves
Up to now, five randomized controlled trials evaluated the use of P. hybridus standardized leaf extract (Tesalin – Ze 339) for allergic rhinitis among adults and children and, to the best of our knowledge, the extract has been used without reported serious side effects (6, 46). Also, in a randomized, placebo-controlled trial, a fixed herbal drug combination composed of four plant extracts including P. hybridus leaf extract (Ze 185) was recorded to be efficacious and safe in the short-term treatment of patients with somatoform disorders (47), and the same preparation was observed to reduce self-reported anxiety response to stress in healthy men (48). The mentioned extract Ze 339 is obtained by supercritical CO2 fluid extraction (SFE-CO2) and is standardized to 8 mg petasins (petasin, isopetasin, and neopetasin) as active substances (49). Phenolic acids and flavonoids have not been considered as important biologically active compounds present in this extract.
Together with ultrasound-assisted extraction, pressurized liquid extraction, and microwave-assisted extraction, SFE-CO2, is a green chemistry method used for the extraction and isolation of bioactive compounds from plants-based materials. It is considered one of the best techniques for obtaining flavonoids, essential oils, and other natural chemical components from natural plant materials (50). Flower extracts obtained by SFE-CO2, which have shown anti-inflammatory activity, were often characterized by flavonoids (e.g., quercetin, kaempferol, quercetin 3-O-rhamnoside, quercetin 3-O-glucoside, quercetin 3-O-rutinoside) and phenolic acids as their major constituents (51). Similarly, a recent study on the flavonoid yield and profile of Ziziphus jujuba leaves indicated that, compared with conventional Soxhlet extraction and UAE, SFE-CO2 with ethanol as a cosolvent may provide an extract with significantly increased flavonoid yield, antioxidant activity, and antiproliferative activity (52). The flavonoid compounds identified in the study were kaempferol and quercetin glycosides including some that may be found in Petasites species such as rutin and quercetin-3-O-glucoside.
It has been shown that the extract Ze 339 is five times as active as purified petasin indicating that other constituents present in the plant material (extract matrix) may influence the biopharmaceutical properties of active ingredients. However, variations of fatty acids (17.1–27.2 %), crude oil and fat (17.7–44.2 %), sterols (3.0–4.9 %), and essential oils (1.3–10.5 %) observed in a quantitative analysis of twelve extract batches did not result in significant differences in inhibition of leukotriene synthesis (49). Polyphenolic compounds are important plant-specialized metabolites, whose use may provide health-promoting effects due to their diverse biological activities including, but not limited to antioxidant, anti-inflammatory, and antiallergic activity, making them interesting for industries such as food and pharmaceutical (53). It is possible that some of these compounds also contribute to the observed beneficial activities of butterbur and its preparations. Recently, P. japonicus leaf extract, which was standardized to 3,4-dicaffeoylquinic acid (0.02 μg g–1), 3,5-dicaffeoylquinic acid (0.15 μg g–1), and 4,5-dicaffeoylquinic acid (0.43 μg g–1), showed neuroprotective activity against amyloid beta 25-35 protein fragment (Aβ25–35) plaque neurotoxicity in vitro and in vivo (54). The results of our study indicate that antioxidant activity could be connected to the total phenolic content of these extracts. Amounts of these compounds in Petasites samples harvested from different locations may vary significantly, just as those of other biologically active substances, which in turn could influence the overall activity of the extracts. Therefore, it may be interesting to analyze the contents of these compounds in the original plant material used for marketing as well as in different batches of marketed products and evaluate their possible contribution to the biological activities of pharmaceutical interest.
CONCLUSIONS
It is known that plant species and, consequentially, their herbal preparations may vary considerably in the amounts of chemical constituents such as specialized (secondary) metabolites, which are mostly associated with their diverse biological activities and consequent effects on human health. Besides comparing, for the first time, the total phenolic content and flavonoid content of four different species of the genus Petasites, the results of our study also give insights into the possible variations of these compounds in the two species used in European folk medicine, P. hybridus and P. albus. The former species is especially pharmaceutically important as it is contained in herbal medicines and dietary supplements in the form of standardized leaf or rhizome extracts. Our evaluation was done on extracts prepared from leaves as a more ecologically sustainable source of Petasites bioactive compounds that, from a health perspective, may also be more appropriate considering their initially lower pyrrolizidine alkaloid content. The antioxidant activity of prepared extracts observed in this study was in high correlation with their total phenolic content. Considering that the biological effects of polyphenols and flavonoids, which are potentially beneficial to health, could probably be added to the effects of the bioactive sesquiterpenes (petasins) to which the marketed Petasites products are standardized, and considering their possible variabilities observed in this study, it could also be interesting to investigate these compounds in more detail in future studies.
Acknowledgments. – The authors would like to honor the memory of Prof. Kroata Hazler Pilepić under whose supervision this research was conducted.
Conflicts of interest. – The authors declare no conflict of interest.
Funding.– This work was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (project No. 006-0061117-1239).
Authors contributions. – Conceptualization and sample collection, M.F. and K.H.P.; investigation and analysis, M.F. and K.V.; writing, original draft preparation, M.F.; writing, review and editing, M.F., K.V., S.J. and Ž.M.; funding acquisition, Ž.M.; supervision, K.H.P. and Ž.M. All authors have read and agreed to the published version of the manuscript.
