INTRODUCTION
Honey is a natural, sweet, and viscous mixture of substances created by honeybees, processing nectar or honeydew (breaking down complex carbohydrates into simpler ones) in their glands with saliva hydrolytic enzymes (1). The main components of honey are water, fructose, glucose, and then other sugars (maltose, sucrose, and higher sugars), amino acids, proteins, minerals, vitamins, organic acids, and polyphenols (2). Flavonoids and phenolic acids are the most common compounds from the polyphenol group and they are the most responsible for honey’s natural antioxidant activity and its protective effects on human health (3). In addition to antioxidant properties, honey ingredients have anti-inflammatory, antimicrobial, antimutagenic, antiparasitic, and antitumor properties, and an increasing number of studies indicate the importance of the therapeutic role of honey in the treatment of many diseases (4).
Nectar honey can be divided into unifloral or polyfloral honey, depending on whether the grazing of bees is directed mostly towards one or more plant species. More than 100 different types of unifloral honey are known in Europe (5). Persano Oddo et al. (5) analyzing the data from the International Honey Commission, reported 10 types of unifloral honey most commonly present in production and commercial availability in the European market.
The composition, quality, and biological effects of honey depend on many production parameters, such as the plant origin of honey, geographical origin, species of bees that produce honey, climatic conditions as well as the technical process of honey processing, time age of the product and exposure to high temperatures. More precise control of these parameters affects the generation of high prices for honey production, which in turn leads to the problem of increased market share of counterfeits (6).
In order to eliminate counterfeits and ensure standards for honey products, important properties of honey, the method of technological processing, and methods for determining the validity of the declaration and product quality are legally defined by Croatian Regulations (7) and in the European Union by Harmonised methods of the European Commission (8, 9). Melisopalinological analysis determines the composition of pollen in honey, and various chemical analyses define the composition of sugars, water, free acids, water-insoluble substances, hydroxymethylfurfural content, and electrical conductivity of honey, and enzymatic activity of diastase. In these analyses, the analysis of the composition of phenolic compounds, which are among the main carriers of antioxidant properties of honey, is not legally required for testing the quality of honey. In the last ten years, there has been an increase in the number of papers examining the composition of non-volatile components, such as polyphenols, which significantly contribute to antioxidant activity. Some of these papers (1, 10–20) have researched Croatian and other honeys from Europe investigating both total and individual polyphenol content as well as antioxidant activity. According to Brščić et al. (21), consumers in Croatia prefer unifloral acacia honey the most (56 %), followed by multifloral floral honey (44 %) and meadow honey (35 %). They also prefer unifloral sage (25 %), chestnut (21 %), and linden (16 %) honey. A mild flavor (52 %) and brighter color (44 %) of honey are also preferred by Croatian consumers. Based on these preferences, we decided to use two unifloral kinds of honey in our work: acacia and linden honey, which have mild flavors and brighter colors, and chestnut honey, which has a stronger aroma and darker color. Additionally, these three types of honey were available in both Croatia and Germany.
The aim of our study is to a) determine the polyphenol content and composition by HPLC analysis and by spectrophotometric determination of total soluble phenols (TP) by Foline-Ciocalteau reagent, total flavonoids (TF) by AlCl3 method, total hydroxycinnamic acids (THA) and total flavonols (TFL) by HCl method and the total flavanol (TFLA) content by p-dimethylaminocinnamaldehyde (DMACA) method and antioxidant activity (ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), DPPH: 2,2-diphenyl-1-picrylhydrazyl and FRAP: ferric ion reducing antioxidant power) in acacia (Robinia pseudoacacia L.) honey, chestnut (Castanea sativa Mill.) honey and lime-tree (Tilia spp.) honey originated from Croatia and Germany, b) compare the obtained results on the basis of plant botanical and geographical origin. To determine the polyphenol content and composition, we have developed a new HPLC method for detecting flavonoids in honey samples. The novelty of the research lies in the fact that for the first time, the same honey type produced in different geographical and climatic regions was compared from Croatia and Germany. So far, the honey samples were compared from the point of botanical origin (11, 18, 19, 22–24), or from the point of production seasons (10). In our study, we took into account both, geographical and botanical origin.
EXPERIMENTAL
Honey samples
Seven samples of honey from the area of Central Europe were collected, four from Croatia and three from Germany. All samples were purchased from beekeepers, only certain samples were available as a commercial product in stores (Table I). All samples have the same method of technological processing, which was checked when buying honey and is legally defined in Europe with the Codex Alimentarius and the European Honey Directive. The origin of each honey is shown on a geographical map created with the help of the program QGIS 2.18 (Fig. 1). Samples were stored for two weeks at room temperature in a dark place for purification.
Table I. Botanical and geographical origin of Croatian and German honey samples and their commercial availability
Fig. 1. Geographical origin of sampled honey products in: a) Croatia and b) Germany.
Honey purification
Purification was performed as described in Kenjerić et al. (10) with slight modification. Before sampling, each honey is well mixed. Then 25 g of each sample was dissolved in 125 mL 0.01 mol L–1 solution of hydrochloric acid. The solution is then vacuum filtrated via a polyetersulphonic (PES) filter with pores of 0.2 μm. Chromatography with the aim of extracting phenolic compounds from samples was performed in a glass column (25 × 2 cm) with the help of the stationary phase AMBERLITE® XAD® 2, SUPELCO®. About 50 g of stationary phase per sample was washed off 15 minutes with 96 % ethanol (V/V), twice dH2O, and once with 150 mL 0.01 mol L–1 of hydrochloric acid solution. After passing the samples, the stationary phase with adsorbed compounds was washed with 250 mL 0.01 mol L–1 with a solution of hydrochloric acid and 250 mL dH2O. The elution of compounds from the stationary phase was carried out with 175 mL of 96 % ethanol (V/V). The collected fractions are paired on a rotavapor at a temperature range of 32–35 °C with a rotation of 60 rpm. The final masses obtained by extraction were mixed with 96 % ethanol (V/V) so that the final mass concentration of each sample was 60 mg mL–1. For further analysis, extracts were prepared at a mass concentration of 10 mg mL-1 and were purified three times by centrifugation with 5 min cycles on 15.000 g and 4 °C. The prepared extracts are stored at a temperature of –20 °C until use.
Chemicals and apparatus
Commercial polyphenol standards were purchased from Sigma-Aldrich GmbH (Germany) and Extrasynthese (France). All chemicals and reagents were of analytical grade and supplied by Sigma Aldrich GmbH (Germany) or Kemika (Croatia). RP-HPLC analyses were performed using the Agilent 1100 Series system equipped with a quaternary pump, multi-wave UV/Vis detector, autosampler, fraction collector, analytical Zorbax Rx-C18 guard column (4.6 × 12.5 mm, 5 µm particle size) and Poroshell 120 SB-C18 column (4.6 × 75 mm, 2.7 µm particle size) (Agilent Technologies, Waldbronn, Germany). All absorbance measurements of polyphenols were performed with a NanoDrop 2000c (Thermo Scientific®) and of antioxidant activity with a Fluostar Optima microplate reader (BMG Labtech GmbH, Germany).
Spectrophotometric determination of polyphenols
Total soluble phenols (TP) of honey samples were determined with Foline-Ciocalteau reagent adapted for small volume as described in Vujčić Bok et al. (25). A volume of 2 μL of tested honey extracts was diluted with 158 μL of distilled water and then 10 μL of Foline-Ciocalteau reagent was added. Afterwards, 30 μL Na2CO3 (1.88 mol L–1) was added and the mixture was incubated for 30 min at 45 °C. The absorbance of the mixture was measured at 740 nm. The TP content was calculated from the calibration curve and expressed as gallic acid equivalents (GAE).
The content of total flavonoids (TF) of honey extracts was determined with AlCl3 adapted for small volume as described in Vujčić Bok et al. (25). To dilute the tested solution (2 μL in 80 μL of dH2O), a volume of 6 μL NaNO2 (5 %) was added. After 5 min incubation, a volume of 6 μL AlCl3 (10 %) was added and the mixture was incubated at room temperature for an additional 6 min. Afterward, 40 μL NaOH (1 mol L–1) and distilled water were added to a final volume of 200 μL. The absorbance of the reaction mixture was read at 520 nm. The TF content was calculated from the calibration curve and expressed as quercetin equivalents (QE).
Total hydroxycinnamic acids (THA) and total flavonols (TFL) of honey extracts were measured as described in Vujčić Bok et al. (26) adapted for small volumes using caffeic acid and quercetin as standards. The volume of 0.25 mL of the extract was mixed with 0.25 mL HCl (1 g L–1; prepared in ethanol) and 4.55 mL HCl (2 g L–1). The absorbance of the solution was read at 320 and 360 nm, respectively. THA and TFL contents were calculated from the corresponding calibration curves and expressed as caffeic acid (CAE) and quercetin equivalents (QEE), respectively.
The total flavanol (TFLA) content was determined using p-dimethylaminocinnamaldehyde (DMACA) adapted for small volume as described in Rusak et al. (27). A volume of 100 μL of tested honey extracts was mixed with 150 μL of DMACA solution (0.1 % in 1 mol L–1 HCl in MeOH). After 10 min of incubation at room temperature, absorbance at 640 nm was measured. TFL content was calculated from the calibration curve and expressed as catechin equivalents (CE).
RP-HPLC analysis of flavonoids
Before HPLC analysis, honey samples were hydrolyzed as follows: 150 µL of each extract was mixed with 16.97 μL of HCl (36.5 %, V/V) and incubated for 2 h at 80 °C and 300 rpm, stored at –20 °C and centrifuged 15 min on 15.000 g until HPLC analysis.
Qualitative and quantitative RP-HPLC analyses of honey extracts were performed using the Agilent 1100 Series system. The solvents used were: (A) 0.2 % (V/V) aqueous glacial acetic acid, and (B) 80 % (V/V) methanol + 0.2 % (V/V) glacial acetic acid. Gradient profile was (A/B): 85/15 at 0 min, 51.7/48.3 at 20 min, 46.5/53.5 at 24 min, 36.5/63.5 at 30 min, 0/100 at 37.3 min, 0/100 at 40 min. 100/0 at 43 min. The injection volume was 15 µL, the constant flow rate was 1.0 mL min–1, and the column temperature was set at 30 °C. The multi-wave UV/Vis detector was set at 254, 280, 310, 335 and 360 nm. Phenolic compounds were characterized according to their retention times and UV spectra compared with commercial standards. For the quantitative analyses, calibration curves were obtained by injection of 8 known concentrations (in the range 1–250 µg mL–1) of the mixed 96 % EtOH standard solution in triplicate. The injection volume was 15 µL. The honey extracts were compared with available phenolic standards (pinobanksin, pinocembrin, chrysin, p-coumaric acid, syringic acid, chlorogenic acid, and quercetin). The results were expressed as μg mL–1 of honey weight.
Antioxidant activity
The ABTS assay was carried out as described in Radić Brkanac et al. (28). A volume of 2 μL of the tested honey extracts was added to 200 μL of ABTS solution and incubated for 6 min at room temperature. The absorbance of the reaction mixture was read at 740 nm. The radical scavenging activity was calculated as the percentage of ABTS inhibition as follows: % inhibition = [(A0 – At)/A0] × 100, where A0 was the absorbance of the control (blank, without tested solution) and At was the absorbance in the presence of the tested solution.
DPPH assay was performed as described in Radić Brkanac et al. (28); 10 μL of tested honey extracts was added to 190 μL of freshly prepared ethanolic DPPH solution (0.1 mmol L–1) and incubated in the dark for 30 min at room temperature. The decrease in absorbance was measured at 520 nm and the radical scavenging capacity was calculated using the above-mentioned equation.
The ferric reducing antioxidant power (FRAP) assay was performed as described in Radić Brkanac et al. (28). The tested honey extracts (10 μL) were mixed with the 190 μL of freshly prepared FRAP reagent (and the absorbance was measured at 595 nm after 4 min of reaction time. The percent of Fe3+-TPTZ reduction was calculated using the formula: % reduction = [(At – A0)/At] × 100, where A0 was the absorbance of the control (blank, without tested solution) and At was the absorbance in the presence of the tested solution. Trolox was used as a positive control for all antioxidant activity methods.
All results were evaluated using the Statistica 13.3 software package (Stat Soft Inc., USA). RP-HPLC and results from spectrophotometric determination were subjected to one-way ANOVA for comparison of means and significant differences were calculated according to Duncan's multiple range test. The data are presented as the mean ± standard deviations (SD). Pearson’s correlation coefficient and Principal component analysis (PCA) between individual and total polyphenols and antioxidant activity were performed. Data were considered statistically significant at p ≤ 0.05.
RESULTS AND DISCUSSION
Spectrophotometric determination of polyphenols
Total phenols (TP), total flavonoids (TF), total flavanols (TFLA), total flavonols (TFL), and total hydroxycinnamic acids (THA) in Croatian and German honey were presented in Fig. 2.
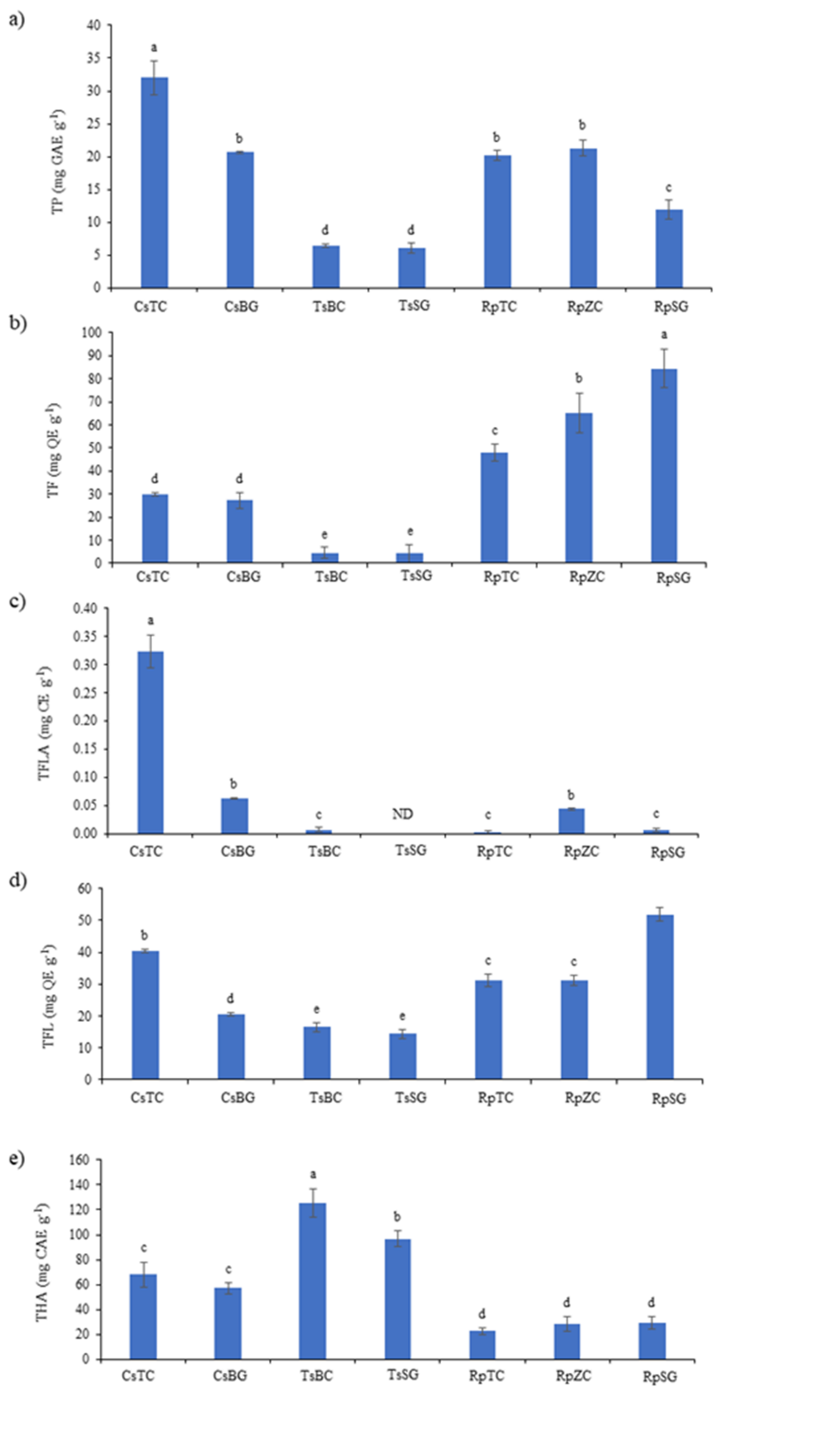
Fig. 2. Phenolic content: a) total phenolics (TP); b) total flavonoids (TF); c) total flavanols (TFLA); d) total flavonols (TFL); e) total hydroxycinnamic acids (THA) in Croatian and German honey. Values represent mean ± SD of 3 replicates. Different letters indicate significant differences at p < 0.05. Castanea sativa honey, Topusko, Croatia – CsTC; Castanea sativa honey, Brandenburg, Germany – CsBG; Tilia spp. honey, Bilogora, Croatia – TsBC; Tilia spp. honey, Saska, Germany – TsSG; Robinia pseudoacacia honey, Topusko, Croatia – RpTC; Robinia pseudoacacia honey, Konjščina, Zagorje, Croatia – RpZC; Robinia pseudoacacia honey, Saska, Germany – RpSG.
The highest TP (Fig. 2a) content was measured in Castanea sativa honey originating from Topusko, Croatia, and the lowest in Tilia spp. honey originating from Bilogora, Croatia, and Saska, Germany. Croatian Castanea honey had statistically higher values of TP than German Castanea honey. The same trend was observed between Craotian Robinia honey (Topusko and Konjščina, Zagorje) and German Robinia honey from Saska. No significant difference in TP between Tilia spp. honey from Bilogora, Croatia, and Saska, Germany was detected.
In German Robinia honey from Saska, the highest values of TF (Fig. 2b) were detected, whereas the lowest TF values were detected in Tilia spp. honey originating from Bilogora (Croatia) and Saska (Germany). Statistically significant decreased between all Robinia honey samples were observed as follows RpSG, RpZC, and then RpTC. All Robinia honey samples had higher values compared to all Castanea and Tilia honey samples. Castanea honey samples had higher TF values compared to Tilia honey samples. No significant difference between all Tilia spp. honeys was observed with the TF method. Also, no significant difference in TF between all Castanea sativa kinds of honey was observed.
Castanea sativa honey from Topusko (Croatia) had the highest TFLA (Fig. 2c) values. In Tilia spp. honey from Saska (Germany), TFLA was not detected. Significant higher values of TFLA were observed between CsTC and CsBG and between RpZC and other Robinia honey samples (RpTC and RpSG).
Robinia honey from Saska (Germany) had the highest values of TFL detected (Fig. 2d), whereas the lowest was measured in all Tilia spp. honey samples (TsBC and TsSG). German Robinia honey (RpSG) had significantly higher values than Croatian Robinia honey (RpTC and RpZC). Croatian Castanea honey (CsTC) had significantly higher values than German Castanea honey (CsBG). No significant difference in TFL between all Tilia spp. honey (TsBC and TsSG) was detected.
Croatian Tilia honey (TsBC) had the highest THA (Fig. 2e). The lowest THA values were observed in all Robinia honey samples (RpTC, RpZC, and RpSG). Significant decline in THA was measured as follows TsBC, and then in TsSG, then in CsTC and CsBG, and then in all Robinia honey samples (RpSG, RpZC, and RpTC). In Croatian Tilia honey, significantly higher values of THA were observed compared to German Tilia honey. No significant difference for THA between all Castanea honey samples was observed. Also, no significant difference for THA between all Robinia honey samples was observed.
Antioxidant activity
In Fig. 3, antioxidant activity (ABTS; % inhibition and mmol L–1 TE g–1, DPPH; % inhibition and mmol L–1 TE g–1 and FRAP; % reduction Fe3+, mmol L–1 Fe2+ g–1 and mmol L–1 TE g–1) of Croatian and German honey were presented.
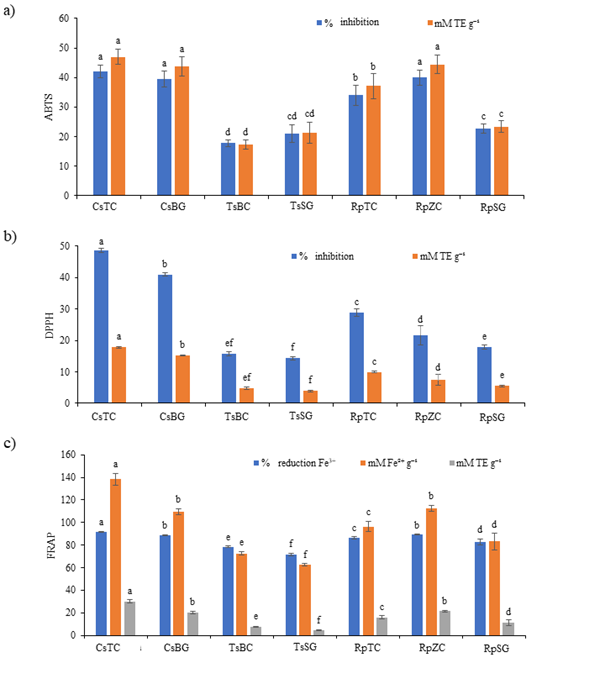
Fig. 3. Antioxidant activity: a) ABTS; b) DPPH; c) FRAP in Croatian and German honey. Values represent mean ± SD of 3 replicates. Different letters indicate significant differences at p < 0.05. Statistics are performed separately for results presented in % and separately for results presented in mM. Castanea sativa honey, Topusko, Croatia – CsTC; Castanea sativa honey, Brandenburg, Germany – CsBG; Tilia spp. honey, Bilogora, Croatia – TsBC; Tilia spp. honey, Saska, Germany – TsSG; Robinia pseudoacacia honey, Topusko, Croatia – RpTC; Robinia pseudoacacia honey, Konjščina, Zagorje, Croatia – RpZC; Robinia pseudoacacia honey, Saska, Germany – RpSG.
The highest antioxidant activity was measured in Castanea honey (CsTC, CsBG) and Robinia honey (RpZC) with ABTS method (expressed as a percentage of inhibition and in mmol L–1 TE g–1) and the lowest in Tilia honey (TsBC). Sample RpZC had statistically higher ABTS values than the RpTC sample. A significant decrease in ABTS was observed in Robinia honey originating from Croatia (RpZC, RpTC) compared to German honey (RpSG). No significant difference in antioxidant activity measured with ABTS (% inhibition and mmol L–1 TE g–1) between all Castanea honey samples was observed. Also, no significant difference in antioxidant activity measured with ABTS (% inhibition and mmol L–1 TE g–1) between all Tilia honey samples was observed.
In Croatian Castanea honey (CsTC) was measured the highest antioxidant activity with DPPH method (% inhibition and mmol L–1 TE g–1) and lowest in German Tilia honey (TsSG). A significant decrease in DPPH (% inhibition and mmol L–1 TE g–1) was observed in Robinia honey originating from Croatia (RpZC, RpTC) compared to German honey (RpSG). This trend was also observed between Croatian and German Castanea honey with the DPPH method (% inhibition and mmol L–1 TE g–1). Honey sample RpTC had statistically higher DPPH values than the RpZC sample.
Castanea honey (CsTC) originated from Croatia had the highest antioxidant activity with the FRAP method (% reduction, mmol L–1 Fe2+ g–1 and mmol L–1 TE g–1) and lowest had German Tilia honey (TsSG). All Croatian honey samples had statistically higher FRAP values (% reduction, mmol L–1 Fe2+ g–1 and mmol L–1TE g–1) than the honey samples from Germany.
RP-HPLC flavonoids
With the new RP-HPLC method, we identified 3 flavonoids (Fig. 4, Table II) from 7 available phenolic standards (flavonoids: pinobanksin, pinocembrin, chrysin, and quercetin; phenolic acids: p-coumaric acid, syringic acid, and chlorogenic acid). The novelty of the HPLC method refers to a new solvent gradient adapted to the honey. The solvent gradient is described in Experimental. The new method is shorter (43 min compared to 60 min in the study of Kenjerić et al.(10) and Šarić et al. (19); 52 min in the study of Tomás-Barberán et al. (22) and still separates both flavonoids and phenolic acids.
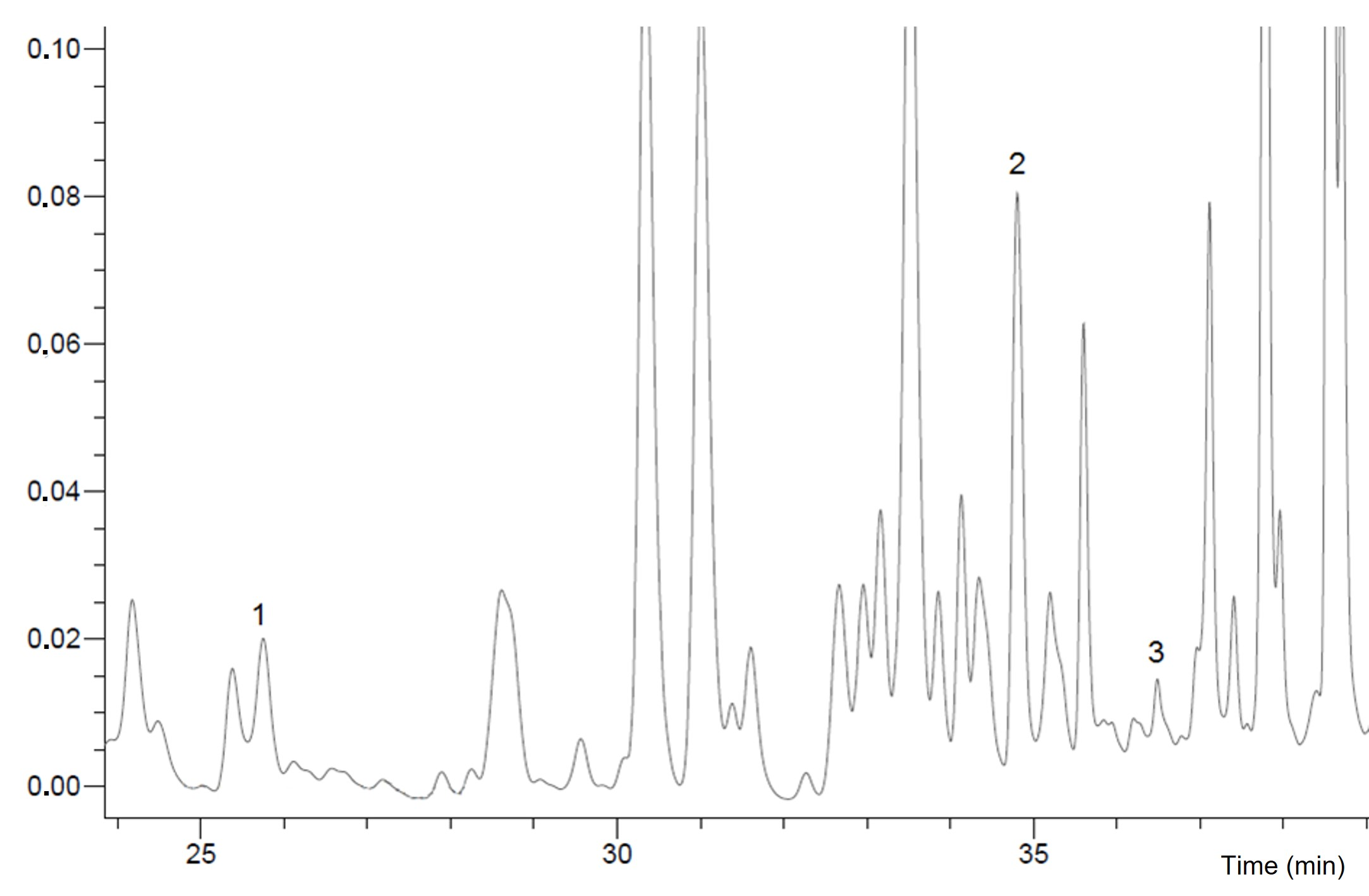
Fig. 4. HPLC profiles of flavonoids recorded at 280 nm in Tilia spp. honey. 1 – pinobanksin, 2 – pinocembrin, 3 – chrysin.
Table II. Content of individual and total identified flavonoids by HPLC
Values represent the mean of 3 technical replicates (SD < 5 %). Different letters indicate significant differences at p < 0.05. TiF – total identified flavonoids; n.d. – not determined
Flavonoids chrysin and pinocembrin were identified in all tested Croatian and German honey samples. Flavonoid pinobanksin is detected only in Croatian and German Castanea and Tilia honey samples. The highest chrysin and pinocembrin content was measured in the Croatian Robinia honey sample (RpZC) and the lowest in the Tilia honey sample originated from Saska (Germany) for chrysin and in Robinia honey originated from Saska (Germany) for pinocembrin. Tilia spp. honey originated from Bilogora (Croatia) and Castanea sativa honey originated from Brandenburg (Germany) and had the highest pinobanksin content. Total identified flavonoids (TiF) were calculated as the sum of identified chrysin, pinocembrin, and pinobanxin. Sample CsBG had the highest values of TiF and in RpSG were detected lowest values of TiF.
Polyphenols and antioxidant activity
For TP and almost all antioxidant methods (ABTS, FRAP and FRAP) expressed as a percentage of inhibition and in mmol L–1, Croatian Castanea honey (CsTC) had the highest values, followed by German Castanea honey (CsBG) and Croatian Robinia honey (RpZC), followed by Croatian Robinia honey (RpTC), followed by German Robinia honey (RpSG), followed by Croatian Tilia honey (TsBC) and German Tilia honey (TsSG). The same trend was observed for Castanea and Robinia honey and for Castanea and Tilia honey, and the opposite for Robinia and Tilia honey in literature (11, 24, 29–31). Based on sensory properties – given the dark color, intense smell, and taste, it was expected that the Castanea honey would have high values of phenolic compounds and antioxidant activity. Gorjanović et al. (23) reported a very strong positive correlation between honey color, TP, and antioxidant activity (FRAP, ORAC, TEAC, and DPPH). The quantitatively high values of almost all results of the Castanea honey can most probably be explained by the fact that chestnut honey is categorized into types of honey with extremely high pollen content (85 %) because C. sativa Mill. honey plant is characterized by hyperproduction of pollen and nectar (32) and pollen contributes to the content of proteins, phenolic compounds, vitamins, and minerals in honey and its antioxidant capacity (33–35). Also, the composition of collected nectar may influence the content of polyphenols and the antioxidant capacity of honey (20). The samples with the lowest antioxidant capacity, the content of total phenols and the examined phenolic subgroups are German and Croatian Tilia honey samples. The most probable reason for such results is the fact that Tilia honey according to Louveaux et al. (32) is grouped into a type of unifloral honey with low pollen content (20–30 %). The obtained results are in accordance with the assumption that Tilia honey will have a lower concentration of phenolic compounds and antioxidant capacity compared to Castanea honey due to its mild organoleptic properties and yellowish transparent color. The permitted levels of pollen grains in Tilia honey can be 10 % if it possesses all the important organoleptic properties. Robinia honey is also grouped into a type of unifloral honey with low pollen content (20–30 %) (32). This is in accordance with the lower measured values for most of the tested methods for Robinia honey compared to Castanea honey. According to the available literature, there are no results of the spectrophotometric determination of total flavonoids (TF), total flavanols (TFLA), total flavonols (TFL) and total hydroxycinnamic acids (THA) in Croatian and German honey. These methods are rapid and low cost and, in the future, it would be desirable to apply them to determine the composition of polyphenolic groups of compounds because they allow testing purified and unpurified honey samples.
In Croatian Robinia honey, Kenjerić et al. (10) detected six flavonoids (quercetin, luteolin, kaempferol, apigenin, chrysin, and galangin), and the presence of phenolic acids (caffeic acid and p-coumaric acid) was also confirmed. Flavonoids myricetin, quercetin, luteolin, kaempferol, apigenin, isorhamnetin, chrysin, and galangin were identified in Croatian Castanea honey samples by Kenjerić et al. (36). According to the available literature, there are no results of the Croatan Tilia honey flavonoid profile. Tomás‐Barberán et al. (22) detected caffeic acid, p-coumaric acid, ferulic acid, quercetin, luteolin, kaempferol, pinobanksin, pinocembrin, and chrysin in some German Robinia honey samples, caffeic acid, p-coumaric acid, pinobanksin, pinocembrin and chrysin were detected in some German Castanea honey samples and p-coumaric acid, 8-methoxykaempferol and chrysin in German Tilia honey sample. In our study, we identified chrysin and pinocembrin in all tested Croatian and German honey samples, whereas pinobanksin was identified only in Croatian and German Castanea and Tilia honey samples. Variability of flavonoid profile and concentrations is to be expected due to the seasons, climatic conditions, and other factors.
The values of individual flavonoids obtained by HPLC analysis do not necessarily follow the relationships obtained by measuring total phenols and antioxidant activity. The identified chrysin, pinobanksin, and pinocembrin are just some of the compounds that contribute to the total phenol composition and antioxidant activity, so reported values of the mentioned flavonoids give a more specific view of the mutual differences between honey samples. Thus, for example, the sample with the highest concentration of pinobanksin is the Croatian Tilia honey and THA, although antioxidant activity and TP, TF, TFLA, and TFL were the lowest compared to other samples. Also, some flavonoids such as Pcb do not have pronounced antioxidant properties (37).
Based on the detected and identified flavonoids, we can assume the positive biological effects of certain honey samples on human health. Croatian Robinia honey originated from Zagorje had a high chrysin content compared to other honey samples which is why it could have a positive effect on anti-inflammatory processes because chrysin inhibits cyclooxygenase-2, the enzyme responsible for inflammation and accompanying pain (38). The same sample had the highest pinocembrin content compared to other honey samples, which suggests its potentially beneficial effect on cell protection due to poor blood circulation. According to Khalil et al. (37), pinocembrin inhibits the onset of apoptosis in such cells. Croatian Tilia honey and German Castanea honey had the highest pinobaksin content compared to other samples, which is why it could have a positive effect on stopping tumor formation. According to Silva-Carvalho et al. (39), pinobanksin acts by slowing the growth of tumor cells.
In our study, Castanea honey (CsTC, CsBG) and Robinia honey (RpZC and RpTC) had moderate (42.07 %, 39.38 %, 33.99 %, and 39.95 %), and all other samples weak (17.73–22.72 %) antioxidant activity in relation to Trolox (82.43 %) by ABTS method. Moderate (40.92–48.56 %) antioxidant activity with the DPPH method was observed in Castanea honey (CsTC, CsBG) and Robinia honey (RpTC; 28.75 %), and all other samples had weak (14.37–21.69 %) antioxidant activity in relation to Trolox (82.06 %). With the FRAP method, all tested Croatian and German honey samples showed strong (73.33–94.29 %) antioxidant activity in relation to Trolox (97.45 %). Our classification of the antioxidant activity of honey samples is based on the Vujčić et al. (40) classification. In this paper, antioxidant activity is classified as weak (< 35 %), moderate (35–70 %), and strong (70–100 %) in relation to the positive control (100 %) for herbal-originated extracts.
Analyzed Croatian honey samples had higher levels of polyphenols and stronger antioxidant activity in comparison to German honey samples. A possible explanation lies in the fact that greater diversity of Croatian flora and climatic characteristics are more favorable for beekeeping in Croatia than in Germany. This supports the influence of geographical origin on the quality of honey. If we observe climatic characteristics as the only factor influencing the quality of honey in a geographical area, we can spot that with an increase in northern altitude, probably due to the decrease in average annual temperatures, the quality of honey also decreases. This is explained by the lower activity of bees in collecting pollen and nectar at lower temperatures because bees, instead of collecting and producing honey, spend most of their time heating the hive to the optimum temperature of 33–35 °C (41). According to the Köppen-Geiger climate classification, Germany is characterized by a moderately warm humid climate with warm summers (Cfb), and Croatia by a moderately warm climate with hot summers without drought (Cfa) and with dry summers (Csa) (41). For the period from 1901 to 2000, the Croatian average temperature was 10.90 °C, and the German 8.50 °C (43). It is obvious from the above that Croatia has higher average temperatures compared to Germany, which is logical if we take into account its latitude and the influence of the Mediterranean Sea; the climate certainly remains one of the factors contributing to the difference between Croatian and German honey. An additional argument that is closely related to climatic characteristics is the trend of decreasing diversity of flora from the equator to the north, which affects the quality of honey (44). By reducing the biodiversity of flora in the range of bees, the availability of diverse pollen is reduced, which causes a weak colony due to loss of nutrition and immunodeficiency caused by the non-diverse diet of bees (44). According to a direct comparison of flora according to data from 2001, Croatia has 5347 different types of vascular flora, while Germany has 2742 (46). Therefore, the flora of Croatia has 0.07561 species per km2 of its area, while Germany has 0.00771 species per km2, which means that on one km2 within the Croatian territory, bees will theoretically have 89.80 % more varied pollen.
Statistics
Pearson’s correlation coefficient between polyphenolic content and antioxidant activity of Croatian and German honey is presented in Table III.
Table III. Pearson’s correlation coefficient between total and individual polyphenolic content and antioxidant activity of Croatian and German honey
TP correlated very strongly (r > 0.80) with all antioxidant activity methods (ABTS %: 0.94, ABTS mmol L–1 TE 0.94, DPPH % 0.89, DPPH mmol L–1 TE: 0.89, FRAP % 0.92, FRAP mmol L–1 Fe2+: 0.98 and FRAP mmol L–1 TE: 0.98) and strongly (r > 0.60 < 0.79) with TFLA (0.78). A positive very strong correlation was observed between TF and TFL (0.84) and a negative very strong correlation was observed between TF and THA (–0.87) and TF and Pbs (–0.87). TFLA had a very strong (0.80) correlation with DPPH %, FRAP mmol L–1 Fe2+, and FRAP mmol L–1TE and strong (0.79) with DPPH expressed as mmol L–1 TE. THA correlated very strongly with Pbs (0.85) and Chr correlated strongly with Pcb (0.76). All antioxidant methods had very strong (0.82–1) or strong correlation (DPPH % and FRAP %: 0.78, DPPH mmol L–1 TE and FRAP %: 0.79) among themselves.
The first (Factor 1) and the second (Factor 2) principal components (PC) described 54.68 % and 23.94 % of the variance (Fig. 5).
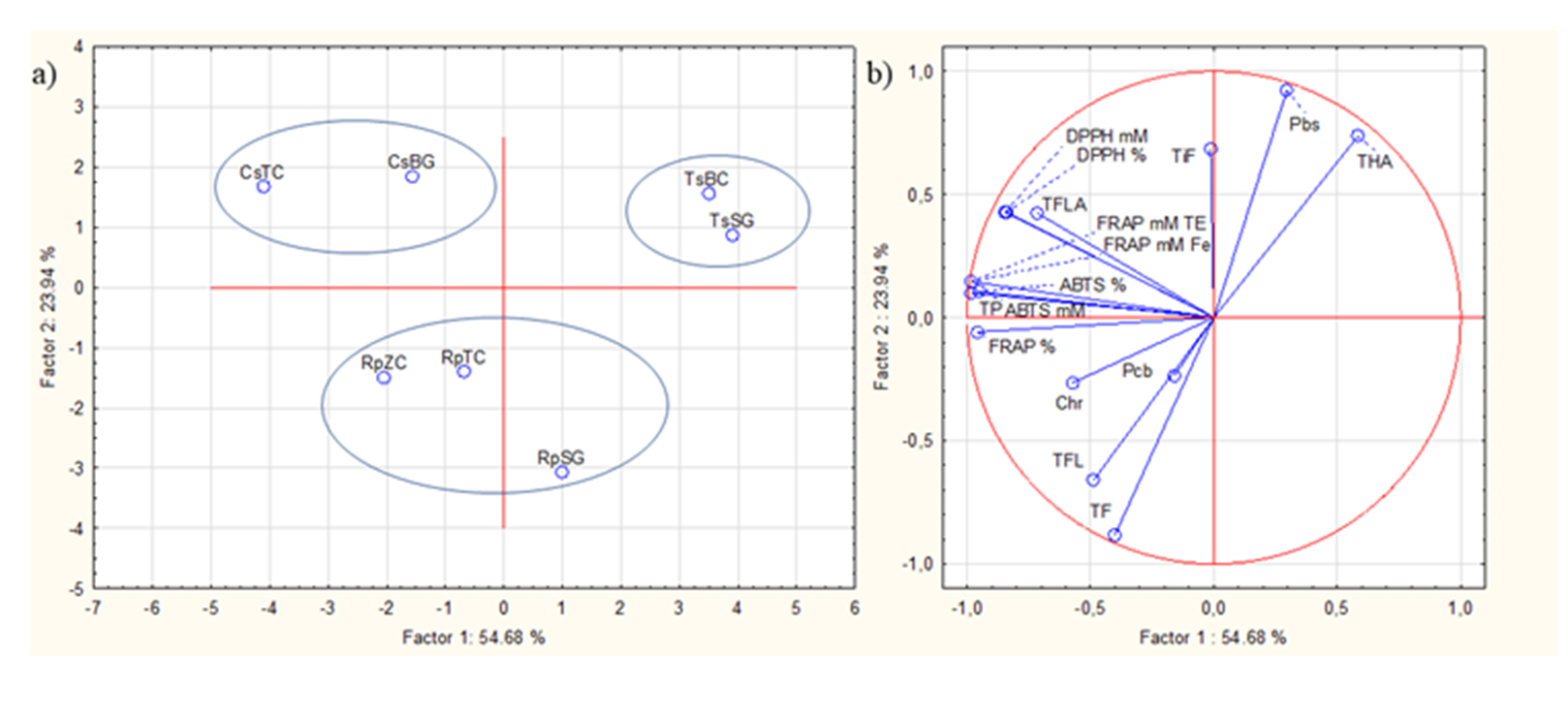
Fig. 5. Principal component analysis of the measured polyphenols and antioxidant activity in the Croatian and German honey. a) Score plot separating the Croatian and German Castanea, Tilia, and Robinia honey samples; b) the loading plot of polyphenols and antioxidant activity as variables. Castanea sativa honey, Topusko, Croatia = CsTC; Castanea sativa honey, Brandenburg, Germany – CsBG; Tilia spp. honey, Bilogora, Croatia – TsBC; Tilia spp. honey, Saska, Germany – TsSG; Robinia pseudoacacia honey, Topusko, Croatia – RpTC; Robinia pseudoacacia honey, Konjščina, Zagorje, Croatia – RpZC; Robinia pseudoacacia honey, Saska, Germany – RpSG and TiF – total identified flavonids, Pbs – pinobanksin, Pcb – pinocembrin, Chr – chrysin, total flavanols – TFLA, total flavonoids – TF, total flavonols – TFL, total hydroxycinnamic acids – THA, total phenols – TP.
Together, the first two PCs represent 78.62 % of the total variability. With the PCA plot (Fig. 5a) honey samples were divided into three groups of honey based on their botanical origin. So, the highest distance was detected between Castanea, Tilia, and Robinia honey, and the smallest distance was detected between Croatian and German Tilia honey (TsBC and TsSG), then in Croatian and German Castanea honey (CsTC and CsBG) and then between Croatian (RpZc and RpTC) and German (RpSG) Robinia honey. Both, Croatian and German Castanea honey (CsTC and CsBG) had strong loadings with the most tested total (TP, TFLA, and TIF) compounds and antioxidant activity (ABTS: % and mmol L–1 TE, FRAP: %, mmol L–1 TE and mmol L–1 Fe2+, DPPH: % and mmol L–1 TE) (Fig. 5b). Croatian (RpZc and RpTC) Robinia honey had strong loadings with total (TF and TFL) and individual (Chr and Pcb) polyphenolic compounds (Fig. 5b). Strong loadings with Pbs and THA were detected in Croatian and German Tilia honey (TsBC and TsSG) (Fig. 5b).
Correlation analysis confirmed the expected positive correlation between the results of antioxidant methods with the total phenol content (TP) and phenolic subgroup (TFLA). According to Moniruzzaman et al. (47) and Flanjak et al. (24), phenolic compounds are responsible for the antioxidant properties of honey. TF method showed low values of positive and negative correlation coefficients, which are without statistical significance. This can be explained by the non-specificity of this method (48). The THA method shows a negative correlation, i.e. an inversely proportional relationship with the results of antioxidant methods. Since hydroxycinnamic acids are powerful antioxidants that can mediate the scavenging of harmful reactive oxygen species (49), our correlation results could be the result of certain non-specific reactions.
Each honey declared as a unifloral type may contain a different percentage of pollen grains due to the impossibility of direct control of bee grazing, so it is important to define the validity of the declared botanical origin by melisopalinological analysis and by analyzing and defining different markers, i.e. specific reference values of certain compounds in honey (50). Overview of the similarities and differences between different Croatian and German Castanea, Tilia, and Robinia honey samples as well as the interrelationships between the measured properties (polyphenol composition and antioxidant activity) were provided by the PCA plots for the purpose of indirectly determining the botanical origin of honey. Three groups of honey (Castanea, Tilia, and Robinia) based on their botanical origin were divided with PCA. According to the results of PCA, we can conclude that Castanea is the best quality unifloral honey compared to Robinia and Tilia honey. Because, Castanea honey (CsTC and CsBG) had strong loadings with most tested total (TP, TFLA, and TIF) compounds and antioxidant activity (ABTS: % and mmol L–1 TE, FRAP: %, mmol L–1 TE and mmol L–1 Fe2+, DPPH: % and mmol L–1 TE). From PCA plots, it can be seen that the different position of Tilia honey based on the ordinate is most affected by the content of THA and Pbs content, which for these honey samples are the largest compared to other samples, while the position of Robinia honey is most affected by TF and TFL and the content of Pcb and Chr. In almost all conducted analyses, Croatian unifloral types of honey are of better quality than German ones. In PCA plots (Fig. 5a,b), Croatian honey samples are always grouped closely to the most of measured methods. Croatian CsTC samples had smaller distances in PCA plots with TP, TFLA, and antioxidant activity methods (ABTS: % and mmol L–1 TE, FRAP: %, mmol L–1 TE and mmol L–1 Fe2+, DPPH: % and mmol L–1 TE) compared to German CsBG samples. German CsBG samples had a smaller distance in TiF compared to Croatian CsTC samples. Robinia honey samples originating from Croatia had smaller distances in PCA plots with TF, TFL, Chr, and Pcb, whereas German Robinia honey was from the opposite axis of the aforementioned methods. Tilia honey originating from Croatia had a smaller distance in PCA plots with Pbs and THA compared to Tilia honey originating from Germany.
CONCLUSIONS
Through most methods, the sample with the highest antioxidant capacity and the quantitative content of total phenolic compounds is Croatian chestnut honey. According to plant origin, chestnut honey is of higher quality in terms of the quantitative composition of phenolic compounds and antioxidant capacity than acacia and linden honey, and acacia honey is of better quality than linden honey. The above order of unifloral types of honey is most likely caused by the amount of pollen present in the honey because pollen contributes to the final content of phenolic compounds, and thus to the antioxidant capacity of the honey. Additionally, the composition of the collected nectar may influence the polyphenol content and the antioxidant capacity of honey. The analyzed Croatian honey samples are of better quality in terms of the composition of phenolic compounds and antioxidant capacity compared to the German honey samples, most likely due to more favorable climatic characteristics for bee breeding and greater diversity of Croatian flora.
Acronyms, abbreviations, symbols. – ABTS – 2,2’-azinobis(3- ethylbenzothiazoline-6-sulfonic acid), CsBG – Castanea sativa honey, Brandenburg, Germany, CsTC – Castanea sativa honey, Topusko, Croatia, FRAP – Ferric Reducing/Antioxidant Power Assay, RpZC – Robinia pseudoacacia honey, Konjščina, Zagorje, Croatia, RpSG – Robinia pseudoacacia honey, Saska, Germany, RpTC – Robinia pseudoacacia honey, Topusko, Croatia, TiF – total identified flavonids, TsBC – Tilia spp. honey, Bilogora, Croatia, TsSG – Tilia spp. honey, Saska, Germany, TFLA – total flavanols, TF – total flavonoids, TFL – total flavonols, THA – total hydroxycinnamic acids, TP – total phenols.
Acknowledgements. – This work was supported by the University of Zagreb, Croatia (Grant No. 20283112) and by the Foundation of the Croatian Academy of Sciences and Arts, Croatia (Grant: Phenolic and antioxidant profile of honey from certain Croatian regions).
Conflict of interest. – The authors declare that they have no known conflict of interest.
Authors contributions. – Conceptualization, G.R., I.Š. and V.V.B.; investigation, I.Š., V.V.B, A.B. and R.N.; original draft preparation, V.V.B. and A.B.; review and editing, G.R., I.Š. V.V.B, J.L.M. and Ž.M. All the authors have read and agreed to the published version of the manuscript.
