Introduction
Hypertension (high blood pressure) adds to the workload of the heart and arteries. If it continues long, the heart and arteries may not function properly. This can damage the blood vessels of the brain, heart, and kidneys, resulting in a stroke, heart attack or kidney failure. These problems may be less likely to occur if blood pressure is controlled. Hydrochlorothiazide (HCTZ) combination is used to treat hypertension. Hydrochlorothiazide is a thiazide-type diuretic that has been used clinically for more than half a century. The drug has been widely used to treat hypertension globally and is relatively safe. Hydrochlorothiazide acts on the distal convoluted tubules and inhibits the sodium chloride co-transporter system. This action leads to a diuretic action that lowers blood pressure, but there is also a potassium loss in the urine. Hydrochlorothiazide is also helpful in removing the excess water from the body; however, calcium retains in the body. Moreover, as a therapeutic option for congestive heart failure, diabetes insipidus, renal tubular acidosis, and symptomatic edema. In addition, it prevents kidney stones [1-5]. All these aspects encourage investigating the electrochemical features of HCTZ on novel materials, and it has been studied with an increasing trend in recent research activities around the world.
The various techniques available for the selective and sensitive detection of HCTZ include HPLC, capillary zone electrophoretic, spectrophotometric/HPLC and electrochemical methods [6-11]. Electrochemistry-based methods can be employed considering their lower cost, speediness, portability, reasonable selectivity, simple preparation process, suitable accuracy, and precision for the analysis of biological compounds, gas pollutants, drugs, food and water pollutants compounds [12-22]. The chemical modification of inert substrate electrodes offers significant advantages in the design and development of electrochemical sensors. The properties of chemically modified electrodes that have driven their development include increased selectivity and sensitivity, chemical and electrochemical stability, larger usable potential windows, and resistance to fouling [23-35]. The incorporation of nanomaterials has had a great impact on the development of electrochemical sensors [36-40]. Significant progress has been made toward synthesizing nanomaterials with controllable morphologies, dimensions, surface charges, and physicochemical properties [41-62].
Conducting polymers (CPs) derivatives of polypyrrole nanotubes (PPy-NTs) are 1-D nanostructured materials and are considered as one of the ideal candidates due to their large usage in various industrial applications and tremendous physico-chemical properties, such as high electrical conductivity, good mechanical stability, nanoscale particle size, lightweight, and wide surface area. PPy-NTs has an excellent material for device fabrication in electrochemical sensors or biochemical fields due to their small diameter with an average length, which may allow more scope for proper chemical interaction with a doping material and enhance the surface area and sensitivity compared to the bulk material. PPy-NTs show a high conductivity due to extended π conjugation and a tunable doping process that promotes the electrochemical oxidation activity [63,64].
Based on the above-mentioned information and in light of the information presented, the current study was based on the development and application of PPy-NTs/CPEs for the voltammetric determination of HCTZ. Inspired by the aforementioned discussions, we modified the bare electrodes with PPy-NTs, which are applied to improve the selectivity and sensitivity of the electrochemical sensor. Hence, the PPy-NTs were synthesized and developed as a highly sensitive and selective platform for detecting HCTZ. The constructed sensor has good performance characteristics, simplicity of preparation, high selectivity, stability, wide linear range and a small limit of detection. It was successfully applied for the voltammetry determination of HCTZ in biological samples.
Experimental
Equipment and materials
In order to do electrochemical tests at ambient temperature, we utilized the Auto-lab potentiostat /galvanostat (PGSTAT 302N, Eco Chemie, the Netherlands) with GPES (General Purpose Electrochemical System-version 4.9) software to control the system. Electrochemical measurements were performed at room temperature in a conventional electrochemical cell with a PPy-NTs/CPE as the working electrode, 3.0 M Ag/ AgCl/KCl as a reference electrode (Azar Electrode, Urmia, Iran) and platinum wire as a counter electrode (Azar Electrode, Urmia, Iran). Moreover, pH was measured using the Metrohm 713 pH-meter with a glass electrode (Switzerland). Hydrochlorothiazide and all other solutions used during the procedure were prepared by reagent-grade chemicals from Merck and Sigma-Aldrich and deionized water was supply from Millipore, Germany. Orthophosphoric acid was utilized to prepare the phosphate buffer solutions (PBSs), and sodium hydroxide was used to adjust the desired pH values (pH range between 2.0 and 9.0).
Preparation of PPy nanotubes
Polypyrrole nanotubes (Ppy-NTs) were prepared by the oxidation of pyrrole monomer with iron(III) chloride in the presence of a structure-guiding agent, methyl orange. In a typical synthesis, 0.784 g (2.3 mmol) methyl orange and 3.888 g (23 mmol) FeCl3 were dissolved into 480 mL of deionized water. Then 0.84 mL (12.1 mmol) of pyrrole was added to the solution and stirred for 24 h at room temperature. The formed PPy precipitate was washed with deionized water/ethanol several times until the filtrate was colorless and neutral and finally dried under a vacuum atmosphere at 65 °C for 20 h.Figure 1 shows the FE-SEM image of PPy nanotubes.
Preparation and surface modification of electrode
To prepare PPy-NTs/CPE, 0.95 g graphite powder and 0.05 g PPy-NTs were mixed. Next, a suitable amount of paraffin oil was poured into the resulting mixture, followed by mixing well for 30 min to obtain a uniformly wetted paste. An appropriate amount of the paste was tightly packed into a glass tube and a copper wire was positioned over the carbon paste to make electrical contact.
Results and discussion
Electrochemical behavior of HCTZ on polypyrrole nanotubes
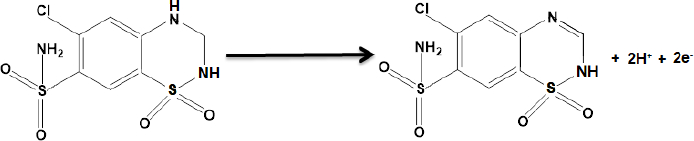
According to our knowledge, the electrooxidation of HCTZ is closely related to the pH value of the solution. So, the effect of pH was investigated using the differential pulse voltammetry (DPV) method. The results show that the oxidation peak current increased slowly from pH 2.0 to 7.0, and then the current conversely decreased when the pH value increased from 7.0 to 9.0. According to obtained results, pH 7.0 was chosen as the optimal experimental condition for other experiments. The electrochemical reaction of HCTZ involves two electrons and two protons, according toScheme 1.
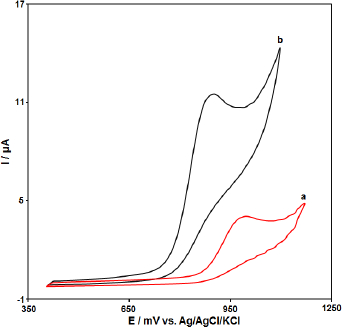
The electrochemical behavior of the CPE, PPy-NTs/CPE was studied by the cyclic voltammetry (CV) technique in the 0.1 M phosphate buffer (pH=7.0) as the supporting electrolyte at a scan rate of 50 mV s−1 (Figure 2). As shown inFigure 2, in comparison to the bare CPE (a), PPy-NTs/CPE (b) presents a well-defined Irreversible oxide peak with a higher current signal (HCTZ concentration equal to 200.0 μM).
Role of variable scan rates
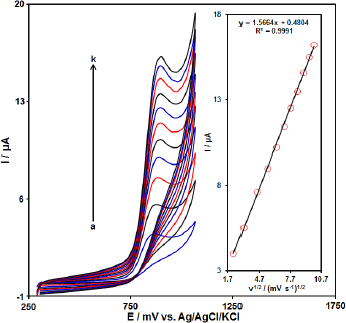
The effect of the potential scan rates (5-100 mV s-1) on the electrochemical oxidation of HCTZ was studied by linear sweep voltammograms (LSV).Figure 3 shows the LSV of 200.0 μM of HCTZ in the 0.1 M phosphate buffer solution at the PPy-NTs/CPE. These results show that the anodic current increases with increasing scan rate. The oxidation current of HCTZ increased linearly with the square root of the scan rate (Figure 3, Inset), demonstrating a diffusion-controlled electrochemical process.
Chronoamperometric analysis
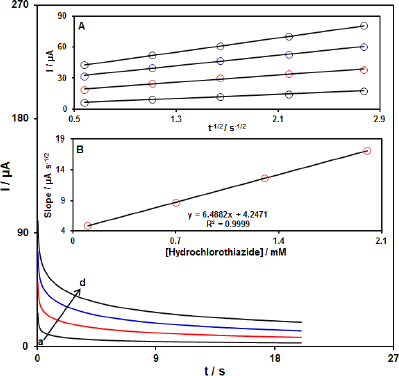
The chronoamperometric measurements of HTCZ at the PPy-NTs/CPE surface were done to estimate the apparent diffusion coefficient of HTCZ.Figure 4 shows the current-time profiles obtained by setting the working electrode potential at 950 mV for different concentrations of HTCZ. At long enough experimental times (t=0.3-3s), where the electron transfer reaction rate of HTCZ is more than its diffusion rate toward the working electrode surface, the current is diffusion controlled.Figure 4, inset A, shows the experimental plots of I versus t-1/2 with the best fit for different concentrations of HTCZ employed. The slopes of the resulting straight lines were then plotted versus the HTCZ concentration (Figure 4, inset B). Based on the Cottrell equation [65], the slope of this plot (Figure 4 inset B) can be used to estimate the apparent diffusion coefficient of HTCZ. From the slope of this plot (11.644 A s1/2 mM-1), the value of diffusion coefficient was found to be 1.7w10-6 cm s1.
DPV analysis of HCTZ
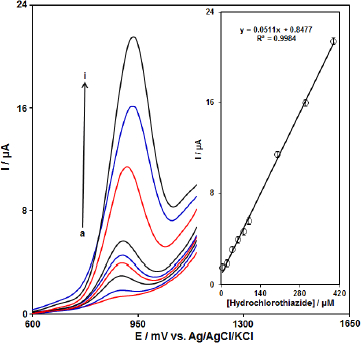
DPV was used for the determination of HCTZ at PPy-NTs/CPE due to its high sensitivity. The DPV responses for different concentrations of HCTZ are illustrated inFigure 5. The linear range was found to be 5.0 μM to 400.0 μM. The linear equation was Ip (μA)=0.8477-0.0511 CHCTZ (μM) with a correlation coefficient of 0.9984. The detection limit was 1.5 μM (S/N=3).
Conclusion
A novel electrochemical protocol using PPy-NTs/CPE was fabricated for the sensitive determination of HCTZ. The modified electrode electrocatalytically oxidizes the HCTZ at a less positive potential with an increased oxidation current. The electrocatalytic oxidation current of HCTZ was linearly increased with the increased concentration of HCTZ. The sensor under the optimized circumstances possessed a fast current response to HCTZ, with a linear dynamic range between 5.0-400.0 μM, a thin limit of detection of 1.5 μM, and an appreciable sensitivity of 0.0511 μA/μM. According to the analyses, the modified electrode demonstrated acceptable electrocatalytic activities and sensitivity. Also, excellent features, like a wide linear range, low detection limit, high reproducibility and repeatability and longtime stability, proved the successful application of this sensor for the determinations of HCTZ.