Introduction
The European Horse-chestnut ( Aesculus hippocastanum L.) is the only native European species of the Aesculus genus, which counts 13 tree and shrub species living in mesophilous, temperate deciduous forests (Hardin 1960). It is an endemic species of the mountainous range of Western Balkans, forming small, isolated populations. Its natural distribution is restricted to the mountains of Greece, Albania and North Macedonia as well as to one remote locality in Bulgaria (Ravazzi and Caudullo 2016). While the genus Aesculus was widespread in Europe during the Neogene (Postigo Mijarra et al. 2008), fossil records of Ae. hippocastanum in mainland Europe date back at least 1 million years (Harris et al. 2009). Therefore, the species’ occurrence in Europe, which is documented since the Early Pleistocene, accompanied by the subsequent shrinkage of the distribution of the species, like that of the Aesculus genus, makes Ae. hippocastanum a biogeographic relict (Postigo Mijarra et al. 2008). Decline of the species’ populations may be related to climatic changes (Harris et al. 2009), low tolerance of seeds to desiccation as well as specific ecology and seed dispersal strategy (Walas et al. 2018, 2019, Thomas et al. 2019).
Despite its very restricted natural distribution, Ae. hippocastanum has been either introduced or cultivated across several European countries (Euro + Med PlantBase 2006-2022). Historically (Lack 2002), this extended reintroduction of the species in Europe has been attributed to the import of seeds of uncertain provenance in 1557 from Türkiye to Prague. After that, the cultivation of Ae. hippocastanum as an ornamental tree started throughout Europe and resulted in numerous horticultural varieties.
Aesculus hippocastanum is nowadays widespread in urban areas of temperate Europe, while it also constitutes an important medicinal plant (Ravazzi and Caudullo 2016). However, the species is assessed as “Vulnerable” within its natural habitat at the European level (Rivers 2019). Its natural populations are declining due to strong infections by Cameraria ohridella Deschka et Dimic (Lepidoptera), which feeds on Ae. hippocastanum leaves, causing midsummer defoliation and exhaustion of the affected individuals, leading to reduced reproductive success of natural populations (Barredo et al. 2015). The species is affected also by Guignardia aesculi, a fungus that causes leaf blotch disease. The combined negative effects of these two biotic factors lead to the large-scale periodical defoliation of Ae. hippocastanum stands (Walas et al. 2018). Despite the botanical, conservational, ornamental, and pharmaceutical significance of Ae. hippocastanum, the vegetation patterns in the species’ natural habitats have never been thoroughly studied throughout its overall distribution range. There is some more recent information in the works of Thomas et al. (2019), who summarized some already existing data from Greece (Tsiroukis 2008), Bulgaria (Gussev and Valchev 2015), etc. Vegetation studies, so far only at a local scale, of plant communities hosting Ae. hippocastanum have been made in Greece (Barbero and Quézel 1976, Raus 1980, Bergmeier 1990, Mastrogianni 2020), North Macedonia (Em 1957, Matvejeva and Nikolovski 1976, Em et al. 1985, Rizovski and Džekov 1990, etc.), Albania (Peçi et al. 2012) and Bulgaria (Adamović 1908, Gussev and Valchev 2015). Its habitat characteristics throughout this area, and more specifically the assemblages that it inhabits, remain relatively undescribed. This study aims at the identification of the different syntaxonomic units of the relict Ae. hippocastanum communities, throughout its natural distribution range. Additionally, the main ecological gradients of floristic differentiation of Ae. hippocastanum communities are inferred. Finally, based on the species composition of these communities, as well as on literature sources, we discuss the evolutionary history of the species and the putative refugial origin of its communities.
Material and methods
In total, 55 phytosociological relevés were used in the present study, 25 published (Barbero and Quézel 1976, Matvejeva and Nikolovski 1976, Raus 1980, Bergmeier 1990, Rizovski and Džekov 1990) and 30 unpublished from Bulgaria and Greece. All published and unpublished relevés were carried out according to the Braun-Blanquet approach (Westhoff and van der Maarel 1978) in forest types where Ae. hippocastanum dominates or co-dominates in cover. Vegetation data used in the present study cover most of the total natural distribution of Ae. hippocastanum (Fig. 1). More specifically, vegetation data from most of the localities with dominance or co-dominance of Ae. hippocastanum in Greece, Bulgaria and North Macedonia were included in our dataset, but not from Albania, from where no published relevés were found. Header data of all relevés used in this study are presented in On-line Suppl. Mat. (On-line Suppl. Tab. 1). Plant nomenclature follows the Euro + Med PlantBase (2006-2022) and the nomenclature of mosses – Hodgetts et al. (2020). The area of sampling plots varied between 100 to 1000 m2, with most of the plots having an area of 400 m2. Moss taxa were identified for relevés from Bulgaria and North Macedonia, but they were not used in the cluster analysis as they were not recorded in all plots. However, despite their exclusion from the cluster analyses, moss taxa were included in the vegetation table presented in Tab. 1 as well as in On-line Suppl. Tab. 2 to present their occurrence in the relevés where moss taxa were recorded. The different layers of species occurring in the vegetation strata (especially for trees and shrubs), were merged for the analysis. Cover/abundance of plant taxa was recorded as percentages or on the basis of the 7-degree or 9-degree Braun-Blanquet scale (Westhoff and van der Maarel 1978). For the last two cases, cover-abundances were subsequently transformed to percentages according to the default correspondence in Turboveg software (Hennekens and Schaminée 2001) in order to allow the application of numerical analyses.
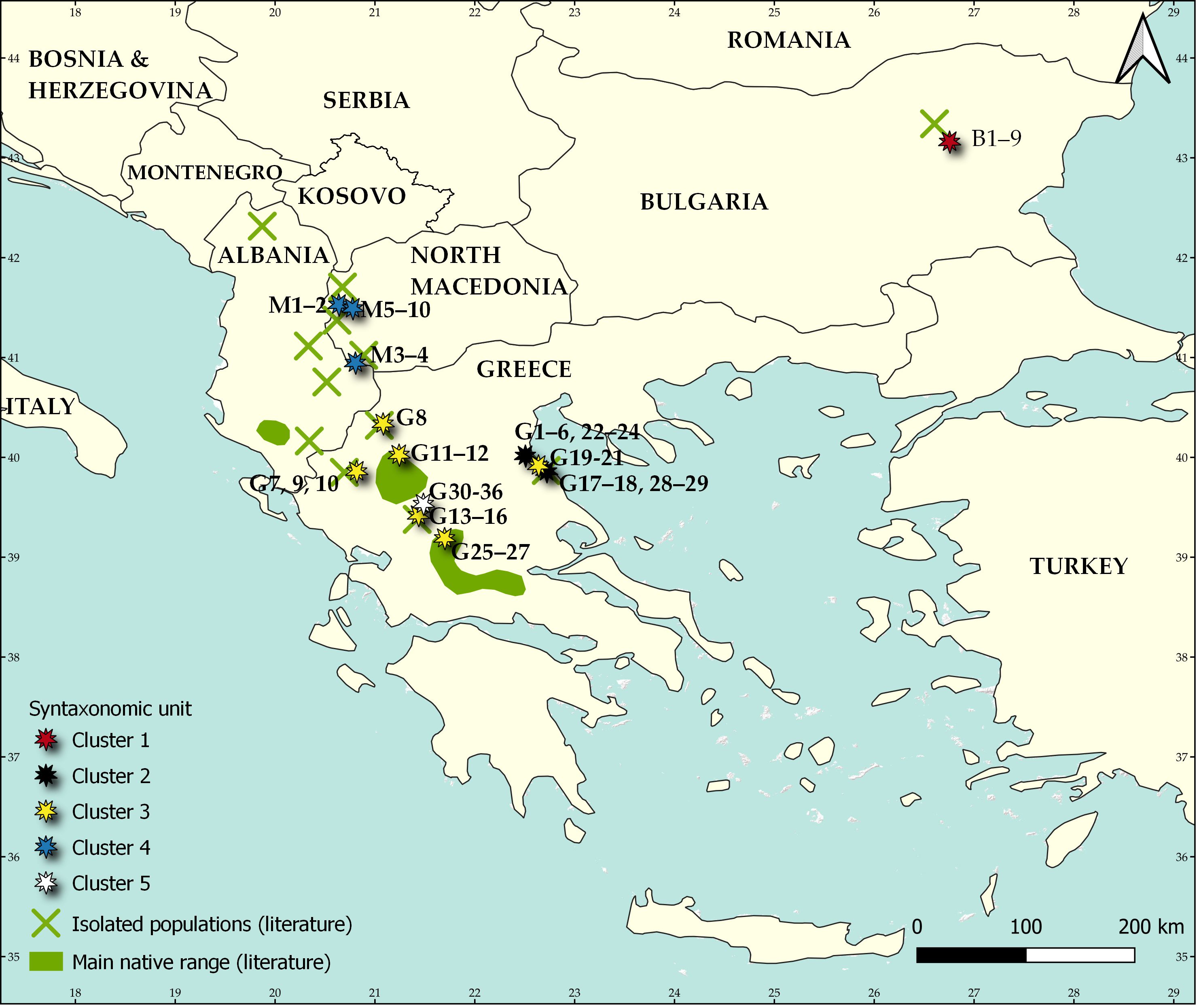
Fig. 1. Distribution of Aesculus hippocastanum including its main native range (green areas) and isolated populations (green crosses) and the identified syntaxonomical units: Cluster 1 = Staphyleo pinnatae-Aesculetum hippocastani ass. nova, Cluster 2 = Rusco hypoglossi-Aesculetum hippocastani, Cluster 3 = Aesculus hippocastanum-Tilia platyphyllos community, Cluster 4 = Juglando-Aesculetum hippocastani, Cluster 5 = Aesculus hippocastanum-Abies borisii-regis community. The map is based on the map published by Caudullo et al. (2018).
Cluster analysis was performed using the vegan package (Oksanen et al. 2020) in R (R Core Team 2022). Bray-Curtis dissimilarity was used as distance measure, and flexible beta with β equal to -0.25 as linkage method. Cover data of taxa were square-root transformed prior to cluster analysis. Number of clusters was defined on the basis of fusion level values plot, as well as ecological interpretation of clusters. Fusion level values represent the dissimilarity values, where a fusion between two branches of a dendrogram occurs (Borcard et al. 2011). The function “hcoplot” was used to reorder the clusters in the dendrogram according to their similarity.
Diagnostic taxa of the distinguished clusters were determined with the help of the “indicspecies” package in R, by means of the point-biserial correlation coefficient calculated on abundance as well as presence-absence data and for equalized groups of plots. We have determined the diagnostic species for all possible group combinations (i.e. starting from combinations of two up to n-1, where n is the number of defined clusters), as this allows us to identify important diagnostic taxa that are shared between the groups (Tsiripidis et al. 2009, De Cáceres et al. 2010). The number of permutations was set to 999 and those taxa with P ≤ 0.01 were considered as diagnostic. Finally, we chose to keep the results derived by using the presence-absence data, based on interpretation as well as on the fact that point-biserial correlation coefficient calculated in this way corresponds to the phi coefficient which is widely used in vegetation science (Chytrý et al. 2002). Few taxa were designated as characteristic or differential ones of certain vegetation units without following fully the results of numerical determination, based on the bibliography (i.e., the diagnostic taxa combinations given for Ae. hippocastanum vegetation units in the original publications), their ecology and geography as well as their abundance in the relevés.
Direct and indirect ordination analysis was conducted to facilitate the interpretation of clustering and the exploring of floristic gradients. An initial running of a detrended correspondence analysis (DCA) resulted in a length of gradient higher than 3, allowing the application of unimodal methods. Canonical correspondence analysis was used to explore which of the examined explanatory variables explained relatively high and significant proportion of species data variance. This was tested by applying 999 unrestricted permutations in a forward selection of explanatory variables. As explanatory variables the following were used: altitude, ground inclination (slope), plots’ geographical coordinates (latitude and longitude) as well as 19 bioclimatic variables (Karger et al. 2017). The explanatory variables that were found to explain a statistically significant portion of the variance at P ≤ 0.01 based on the forward selection of the CCA were used as passive (supplementary) variables in a DCA analysis. In both ordination analyses species cover values were square root transformed and rare species were down weighted. Ordination analyses were performed with the use of CANOCO 5 software (Šmilauer and Lepš 2014).
Classification results were interpreted and discussed in the light of the most influential syntaxonomic interpretations of vegetation types of the Balkan Peninsula. The new syntaxa were named according to the rules of the 4th edition of the International of Phytosociological Nomenclature (Theurillat et al. 2021).
Tab. 1. Synoptic table for the forest vegetation hosting Aesculus hippocastanum. Diagnostic taxa have their constancy values shaded in grey. In the second column, the taxa abbreviations used in Fig. 3 are given. Furthermore, Con.: relative constancy of taxa in the whole dataset; P.val: P values according to the permutation test for the determination of diagnostic taxa. Taxa with constancy values equal or lower than 20% in any cluster were omitted.
Results
Classification of Aesculus hippocastanum-dominated communities
The fusion levels graph, which is presented in the On-line Suppl. Mat. (On-line Suppl. Fig. 1) indicated that the distinction of four up to six clusters is reasonable. From an interpretation of these possible classification schemes we concluded with the distinction of five clusters (Fig. 2). The first cluster includes all relevés from Bulgaria, the second and third - most of the relevés from Greece, the fourth - the relevés from North Macedonia and the fifth cluster - the remaining relevés from Greece. In Tab. 1, a synoptic table of differential taxa of the distinguished clusters is presented, while in the On-line Suppl. Tab. 2 and Tab. 3 with all the complete relevés, the geographical distribution of these clusters within the study area is given. On one hand, clusters are differentiated positively as well as negatively by an adequate number of absolute differential taxa (Tab. 1) and thus the ecological and plant geographical differentiation of the vegetation units distinguished is highlighted. There are also enough taxa differentiating combinations of clusters and thus revealing the common ecological conditions but maybe also the common history that these vegetation units share.
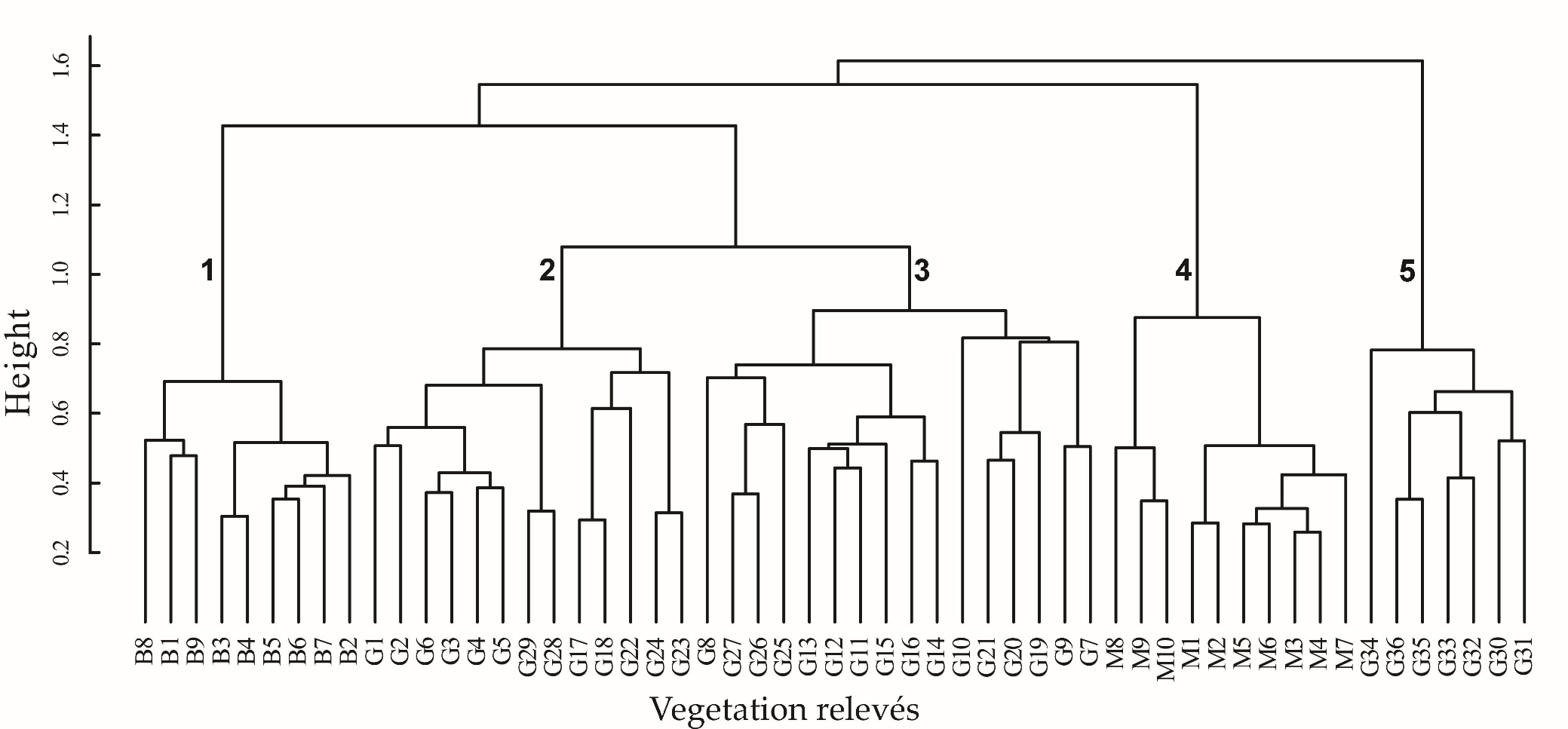
Fig. 2. Cluster dendrogram of the studied vegetation relevés from Bulgaria (B1-B9), Greece (G1-G36) and North Macedonia (M1-M10) and their classification in vegetation units. The numbers inserted in the dendrogram correspond to the following vegetation units: 1. Staphyleo pinnatae- Aesculetum hippocastani ass. nova - Bulgaria; 2. Ass. Rusco hypoglossi- Aesculetum hippocastani Raus et Bergmeier 1990 - Greece; 3. Aesculus hippocastanum- Tilia platyphyllos community - Greece; 4. Ass. Juglando- Aesculetum hippocastani Matvejeva & Nikolovski 1976 – N. Macedonia; 5. Aesculus hippocastanum - Abies borisii- regis community of Barbero & Quézel 1976 – Greece.
Ordination of Aesculus hippocastanum-dominated communities
Floristic differentiation between the five clusters and the two main groups (1 - 4 and 5) was confirmed also by the ordination diagram of the plots (Fig. 3a). Total variation in DCA was equal to 3.24715. The first DCA axis (eigenvalue equal to 0.3669; explained variation 11.30%) discriminates the 5th cluster from the rest of the clusters, supporting its significant floristic and ecological differentiation. Clusters 2 and 3, which include relevés from Greece, are positioned more or less in the central part of the horizontal axis (first DCA axis), albeit closer to the right end of the axis, where the relevés from Bulgaria and N. Macedonia occur. From the above-mentioned distribution of relevés along the first DCA axis as well as from the ordination diagram of the explanatory variables passively projected onto the ordination space of the first two DCA axes (Fig. 3b), it seems that the first DCA axis represents a latitudinal gradient, which is also related to some bioclimatic variables, such as Bio12 (annual precipitation) and Bio15 (precipitation seasonality), as well as altitude. The latter 3 variables (Bio12, Bio15 and latitude), however, are also correlated with the second DCA axis, as they are directed diagonally in relation to the first two DCA axes. The bioclimatic variables Bio14 (precipitation of driest month), Bio17 (precipitation of wettest quarter) and Bio4 (temperature seasonality), which explain a small but significant proportion of unique variation of species data are also positively correlated with the first DCA axis, albeit weakly. The conditional effects of the explanatory variables are presented in the On-line Suppl. Tab. 3 and ten variables were found to significantly explain a unique proportion of species data variation.
The second DCA axis (eigenvalue equal to 0.1974; cumulative explained variation together with the first axis 17.38%) discriminates mainly the relevés sampled in Bulgaria from those sampled in N. Macedonia (Fig. 3a) and it is correlated to longitude. Furthermore, it also contributes to the discrimination between the two clusters from Greece (the 2nd and 3rd) but not so clearly. The second axis may represent a longitudinal gradient of transition from the drier climatic conditions of the western part of the Balkan Peninsula towards the moister ones of the eastern part. This is also revealed from the correlation of the second axis to temperature annual range (Bio7), isothermality (Bio3) and precipitation seasonality (Bio15). However, the second axis represents an even more complex gradient as it is partly correlated to altitude and annual precipitation (Bio12).
Figure 3c clearly depicts the strong floristic differentiation between most of the distinguished vegetation units, as their differential taxa are distributed at certain and distinct parts of the ordination diagram. It also complements the abovementioned results, which revealed that both ordination axes represent ecological and phytogeographical differentiation between the localities of Ae. hippocastanum vegetation in the different countries or geographic regions. This is depicted from the position at the left part of the first DCA axis of typical East Mediterranean incl. oro-Mediterranean species (e.g. Doronicum orientale, Acer monspessulanum, Cardamine graeca), while European (s. str.) and Euro-Siberian species (e.g. Geranium sylvaticum, Hylotelephium maximum, Fraxinus excelsior, Daphne mezereum) are at the right part of the axis. Interestingly, the more mesophilous species, which are typical of the habitat of Ae. hippocastanum (i.e. low mountain ravine forests with high air and soil humidity), such as Ruscus hypoglossum, Taxus baccata, Athyrium filix-femina, Galium odoratum, Drymochloa drymeja, Ilex aquifolium, Luzula sylvatica, are distributed at the center of the ordination space and are diagnostic of the 2nd cluster, demonstrating possibly the range of the ecologically favorable conditions for the refugial Ae. hippocastanum communities.
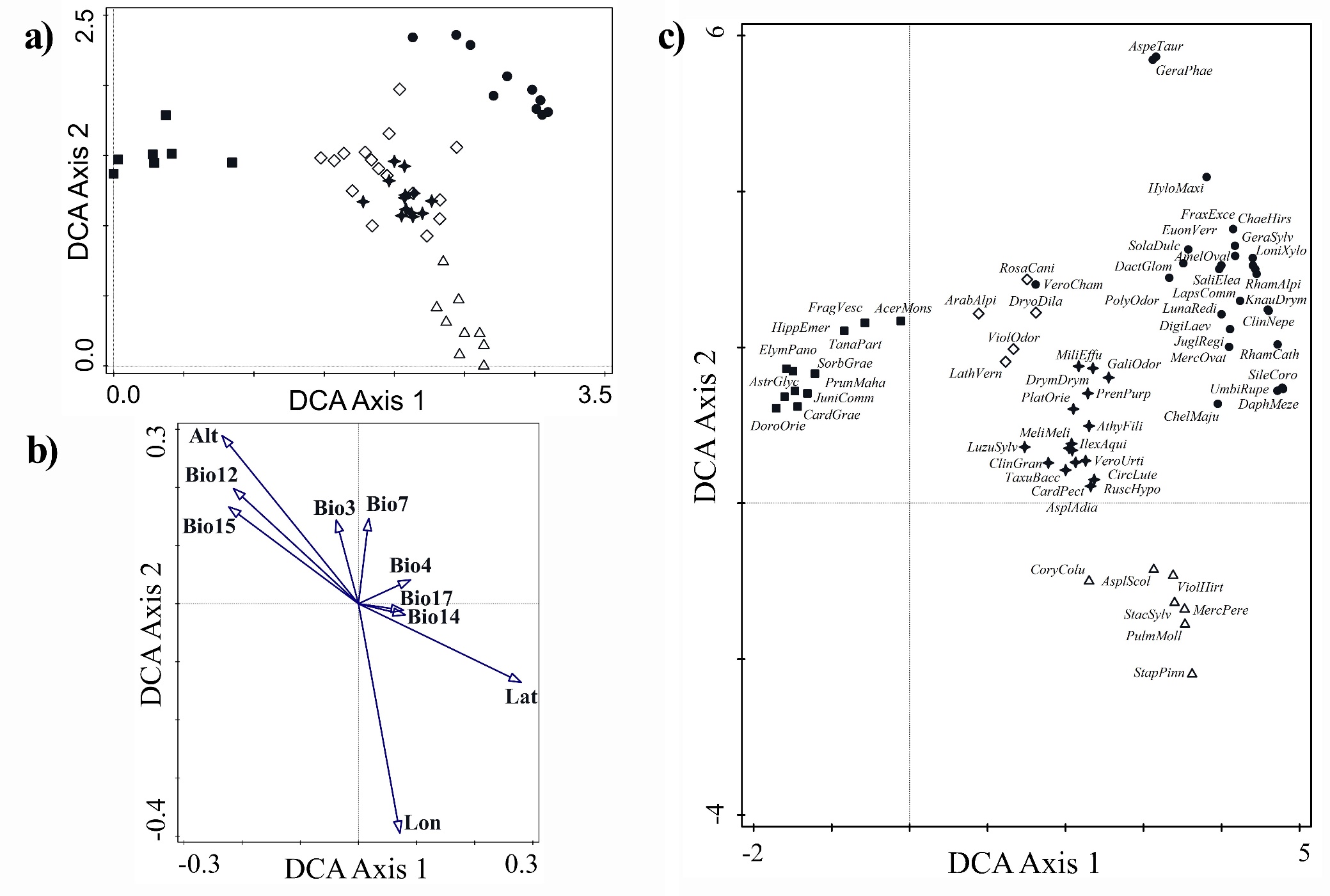
Fig. 3. Ordination diagrams of relevés (a), explanatory variables [altitude (Alt); latitude (Lat); longitude (Lon); isothermality (Bio3); temperature seasonality (Bio4); temperature annual range (Bio7); annual precipitation (Bio12); precipitation of driest month (Bio14); precipitation seasonality (Bio15), and precipitation of wettest quarter (Bio 17)] passively projected onto the ordination space (b) and absolute differential taxa (c) on the basis of the first two DCA axes. In the ordination diagrams of relevés (a) and differential taxa (c) the different symbols denote the clusters and vegetation units in which the relevés were classified or differentiated by the taxa, as follows: ∆ (empty triangles): Cluster 1 = Staphyleo pinnatae-Aesculetum hippocastani, Bulgaria, ✢(filled stars): Cluster 2 = Rusco hypoglossi-Aesculetum hippocastani, Greece, ◊ (empty rhombuses): Cluster 3 = Aesculus hippocastanum-Tilia platyphyllos community, Greece, ● (filled circles): Cluster 4 = Juglando-Aesculetum hippocastani, ■ (filled squares): Cluster 5 = Aesculus hippocastanum-Abies borisii-regis community, Greece.
Association Staphyleo pinnatae-Aesculetum hippocastani ass. nova, Tzonev, Mastrogianni, Tsiripidis, Dimitrov, Gussev, Mandzhukovski et Pachedjieva
(Cluster 1), holotypus rel. № B4 hoc loco (Appendix 1)
Diagnostic species: Asplenium scolopendrium (Ch, Df), Staphylea pinnata (Ch, Df), Stachys sylvatica (Df), Viola hirta (Df), Mercurialis perennis (Ch, Df), Pulmonaria mollis (Df), Corylus colurna (Df) and Homalothecium lutescens (Ch).
The unique locality of Aesculus hippocastanum in Bulgaria is situated in the Preslavska Mountains of the East Balkan Range. It is so remote and isolated compared to the main range of this species that it was considered to be of non-native origin. However, firstly Adamović (1908) and subsequently many other authors (Gussev and Valchev 2015) proved that this is a relict locality of the species, emphasizing its Tertiary origin.
The communities in Bulgaria are located along Dervishka and Lazarska rivers, on the northern slopes of the low Preslavska Mountains. It is a part of the Forebalkan area – a great calcareous foothill of Balkan (Stara Planina) range with many plateaus and low mountains, mostly with altitude lower than 1000 m. Mt. Preslavska is also known as a remnant locality of Cercis siliquastrum in Bulgaria. The communities of Ae. hippocastanum occupy the steep slopes (25–60°) of these limestone valleys and form stands of variable density in the most humid parts (Gussev and Valchev 2015).
This is a newly described association, which is floristically and ecologically related mainly to the Ae. hippocastanum stands in Greece and especially those classified in the 2nd cluster but also with the species stands in N. Macedonia. Among the common differential taxa that this association shares with clusters 2 to 4, the taxa Polystichum setiferum, Acer platanoides, Tilia platyphyllos and Carpinus betulus indicate its mesic character and its high representativeness concerning the ravine forests of the southern Balkans. Probably because of the more mesic conditions and the higher latitude of its locality, it lacks many thermophilous species occurring in all other vegetation units distinguished here, such as Ostrya carpinifolia and Helleborus odorus subsp. cyclophyllus. The name-giving taxon is Staphylea pinnata, which forms a lower tree sub-layer in some parts of the stands. The species is mostly distributed in southeastern Europe, but not in the drier parts of the Mediterranean region, for example in Greece, where it occurs outside the range of the Ae. hippocastanum (Heiss et al. 2014).
Association Rusco hypoglossi-Aesculetum hippocastani Raus et Bergmeier 1990
(Cluster 2)
Diagnostic species: Athyrium filix-femina (Ch, Df), Cyclamen hederifolium (Ch), Polystichum setiferum (Ch), Galium odoratum (Df), Drymochloa drymeja (Df), Taxus baccata (Ch, Df), Milium effusum (Df), Veronica urticifolia (Df), Ruscus hypoglossum (Ch, Df), Melittis melissophyllum subsp. albida (Df), Ilex aquifolium (Df), Luzula sylvatica (Df), Prenanthes purpurea (Df), Circaea lutetiana (Df), Platanus orientalis (Ch, Df), Asplenium adiantum-nigrum (Df), Clinopodium grandiflorum (Df) and Cardamine pectinata (Df).
This association comprises the most representative habitat type of Ae. hippocastanum in its native distribution range. It comprises an azonal forest described from Mt. Ossa, 800 m a.s.l. near Karitsa village (Raus 1980). Raus (1980) classified the two relevés as a community named Aesculus hippocastanum-Tilia platyhyllos. Furthermore, the author noticed the absence of some taxa, such as Juglans regia, Fraxinus exclesior, Acer pseudoplatanus, A. campestre and Tilia cordata, usually present in forests hosting Ae. hippocastanum in Albania, N. Macedonia and Bulgaria. This may indicate that this community is a floristically impoverished form of the corresponding vegetation from the above-mentioned northern countries. Bergmeier (1990) found forest stands with similar floristic composition and ecological characteristics in Mt. Kato Olympos and described a new association, Rusco hypoglossi-Aesculetum hippocastani, in which he also classified the two relevés of Raus (1980) from Mt. Ossa.
From the high frequency and cover of mesic species in this association (e.g. Athyrium filix-femina, Drymochloa drymeja, Ruscus hypoglossum, Circaea lutetiana, Taxus baccata, etc.), many of which are also absolute differentials of this cluster, it can be inferred that Ae. hippocastanum finds in this vegetation type a habitat with increased air and soil moisture conditions. These highly moist conditions may be due to the topographic characteristics (they occur in deep ravines, usually on steep north-facing slopes and on colluvial soils) of the localities of this association, but also to the fact that the slopes of Mts. Ossa and Kato Olympos are facing the Aegean Sea and so receiving higher precipitation (Styllas and Kaskaoutis 2018).
Some of the above-mentioned species (e.g. Ruscus hypoglossum, Taxus baccata, etc.) are considered typical of the ravine forests of southern Balkans (Bergmeier 1990). Bergmeier (1990) classified this association in his newly proposed sub-alliance: Aesculo-Tilienion tomentosae, of the alliance Tilio-Acerion. However, some of the diagnostic species of the proposed sub-alliance (e.g. Asplenium scolopendrium, Carpinus betulus, Euonymus latifolius, Tilia tomentosa) do not present a high or a differentiating constancy in this association. The floristic and ecological distinction of this community type is further supported by the classification of Mastrogianni et al. (2019) and Mastrogianni (2020), where 3493 relevés from mountainous broadleaved and coniferous forests of Greece were used. In the dataset used by the former authors all relevés from this cluster (except one that was not used in the dataset) were included and classified in the ravine forests community types and specifically in the one characterized as “mesic mixed broadleaved ravine forests, occurring on soils with very good water and nutrient availability at mid-altitudes”.
This association presents relatively high floristic similarity with the next vegetation unit (3rd cluster), as well as with the vegetation units distributed in Bulgaria and N. Macedonia (1st and 4th clusters). Most of these common species are mesic or diagnostic taxa of the Fagetalia sylvaticae order.
Community Aesculus hippocastanum-Tilia platyphyllos
(Cluster 3)
Diagnostic species: Viola odorata (Df), Dryopteris dilatata (Df), Rosa canina (Df), Arabis alpina (Df).
This vegetation unit seems to represent a transitional type from ravine forests to mesic broadleaved forests. Floristically and ecologically it is close to the former association. During tests of classification analyses we conducted by applying different distance measures, linkage methods or species cover transformations, the 2nd and 3rd clusters were the only ones between which there was an exchange of relevés i.e., the two clusters almost always came up but with slightly different composition in relevés. Their floristic similarity is evident also in the ordination plot of relevés (Fig. 3a). The tree layer of this vegetation unit is comprised of Ae. hippocastanum accompanied by mesophilous broadleaved deciduous species such as Tilia platyphyllos (most often), Carpinus betulus, Acer pseudoplatanus or the more thermophilous Tilia tomentosa. Despite the high number of taxa occurring in the vegetation plots of this cluster, it had the lowest number of absolute diagnostic taxa, with none of them typical of ravine forests. It is characterized by the co-occurrence of mesic and thermophilous species of beech and oak forests (e.g. Dryopteris dilatata, Hedera helix, Melica uniflora, Polystichum setiferum, Acer platanoides, etc.). However, it hosts and is dominated by ravine forest species such as those described above comprising its tree layer. The floristic composition and ecology of this unit to a great extent resembles the association Tilio-Castanetum Dafis 1973 found from Mt. Kato Olympos by Bergmeier (1990). This author stressed the floristic similarity of this association to the Rusco hypoglossi-Aesculetum hippocastani from the same mountain, and commented that Tilio-Castanetum is ecologically as well as spatially positioned between the mesic forests of Ostryo-Carpinion orientalis Horvat 1959 ( Dryopterido pallidae–Ostryetum carpinifoliae Bergmeier 1990) and the ravine forests of Tilio-Acerion Klika 1955 ( Rusco hypoglossi-Aesculetum hippocastani). Tilio-Castanetum forests described by Raus (1980) for Mt. Ossa and by Dafis (1973) from other localities, are more xeric and thermophilous than the Aesculus hippocastanum-Tilia platyphyllos community described here and also than the Tilio-Castanetum Bergmeier (1990). In general, such forests described in Greece and characterized by the dominance of Tilia tomentosa (see Raus 1980; Bergmeier 1990, etc.) are more or less equally shared between the “thermophilous mixed broadleaved ravine forests of mid-altitudes” and the “mesic mid-altitude thermophilous, broadleaved mixed forests” or “ Quercus dalechampii forests” according to the classification scheme of Mastrogianni et al. (2019) and Mastrogianni (2020).
Association Juglando-Aesculetum hippocastani Matvejeva et Nikolovski 1976
(Cluster 4)
Diagnostic species: Chaerophyllum hirsutum (Ch, Df), Fraxinus excelsior (Ch, Df), Euonymus verrucosus (Df), Mercurialis ovata (Ch, Df), Saxifraga rotundifolia (Ch), Rhamnus alpina subsp. fallax (Ch, Df), Juglans regia (Ch, Df), Lunaria rediviva (Ch, Df), Geranium macrorrhizum (Ch), Acer hyrcanum (Ch), Frangula rupestris (Ch), Asarum europaeum (Ch), Dryopteris carthusiana (Ch), Galium pseudoaristatum (Ch), Lapsana communis (Df), Clinopodium nepeta (Df), Lonicera xylosteum (Df), Rhamnus cathartica (Df), Solanum dulcamara (Df), Dactylis glomerata (Df), Digitalis laevigata (Df), Hylotelephium maximum (Df), Knautia drymeia (Df), Polygonatum odoratum (Df), Umbilicus rupestris (Ch, Df), Geranium sylvaticum (Df), Silene coronaria (Df), Salix eleagnos (Df), Daphne mezereum (Df), Veronica chamaedrys (Df), Chelidonium majus (Df), Asperula taurina (Ch, Df), Geranium phaeum (Df), Amelanchier ovalis (Df).
There are four localities of Ae. hippocastanum communities in the western part of N. Macedonia, especially in wet and calcareous valleys and ravines between 750 m and 1100 m a.s.l. As accompanying species Em (1957) determined Fraxinus excelsior, Acer obtusatum, Corylus colurna, Ostrya carpinifolia, Rhamnus alpinus subsp. fallax, etc. The associations described by Em (1957) were Aesculi-Ostryetum and Aesculi-Fagetum, which were subsequently accepted and described in greatest detail on Mt. Bistra by Rizovski and Džekov (1990). However, these associations are “nomina nuda” (see Art. 2b in Theurillat et al. 2021), because Em (1957) did not publish any phytocoenological relevé from them. Therefore, the only valid and priority published association is Juglando-Aesculetum hippocastani Matvejeva and Nikolovski 1976, which was also reported from Mt. Bistra. Cluster analysis did confirm the great floristic and ecological similarity between the phytocoenoses from all localities in N. Macedonia. Therefore, they all belong to this association. Several of the diagnostic species of this syntaxon, such as Rhamnus alpina subsp. fallax, Lunaria rediviva, Fraxinus excelsior, Chaerophyllum hirsutum, Umbilicus rupestris, etc., are mesic species mostly distributed across southern and central Europe.
The association from N. Macedonia occupies very similar habitats to those of the southern part of the species range in Greece. It is also distributed on steep slopes of ravines and gorges, on often exposed limestone bedrocks, with relatively poor soils, but with high soil moisture. Its floristic composition, however, is enriched by thermophilous and light demanding species of oak forests, possibly because of degradation caused by anthropogenic disturbances, such as logging. This thermophilous species group includes taxa such as Fraxinus ornus, Lonicera xylosteum, Silene coronaria, Helleborus odorus, Galium pseudaristatum, Quercus pubescens, Dictamnus albus, Amelanchier ovalis, Aegonychon purpurocaueruleum, Colutea arborescens, Acer mospessulanum, etc. However, the main group of mesic and relict species, especially trees and shrubs, such as Ulmus glabra, Tilia platyphyllos, Ostrya carpinifolia, Carpinus betulus, as well as many typical fern species, occur with a high constancy in this association.
Community Aesculus hippocastanum - Abies borisii-regis
(Cluster 5)
Diagnostic species: Astragalus glycyphyllos (Df), Prunus mahaleb (Ch, Df), Sorbus graeca (Ch, Df), Tanacetum parthenium (Ch, Df), Cardamine graeca (Ch, Df), Acer monspessulanum (Ch, Df), Hippocrepis emerus subsp. emeroides (Df), Elymus panormitanus (Df), Fragaria vesca (Df), Doronicum orientale (Df), Juniperus communis (Df).
This 5th cluster was described as a distinct association by Barbero and Quézel (1976) with the name “Association à Abies borisii regis et Aesculus hypocastanum”. It has a limited distribution in the central Pindus Mts., on calcareous outcrops (rocky formations of exposed limestone) with Cretaceous (Senonian) age. It is clearly differentiated from the other two clusters (2nd and 3rd) occurring in Greece, despite its spatial proximity to them. Barbero and Quézel (1976) emphasize the distinct floristic character and rare distribution occurrence of this community, and they attribute it to the special substrate of the area, characterized mostly by steep calcareous walls. The locality of relevés sampled by Barbero and Quézel (1976) was visited by Anna Mastrogianni in 2016. It was established that together with vegetation in which individuals of Ae. hippocastanum participate, they were still found to occur in open habitats, rich in thermophilous species. This fact supports the interpretation of Bergmeier (1990) concerning the peculiar structure and composition of this community by excluding other possible interpretations, such as disturbances or field sampling peculiarities. Because this vegetation type depends on local ecological conditions, we chose to distinguish it as a community.
Because of its vegetation this community is transitional to the sub-Mediterranean mountain oak forests and represents the driest habitats in which Ae. hippocastanum was found, constituting an ecological extreme for the species. This is further supported by its floristic composition, which includes, even among its diagnostic species, typical xerophytic and thermophilous species, such as Prunus mahaleb, Acer monspessulanum, Hippocrepis emerus subsp. emeroides, Sorbus graeca, etc. Although this community spatially occurs within a general area of high habitat suitability for Ae. hippocastanum, the extreme conditions, in terms of soil moisture, allow inferences regarding the niche breadth of the species, indicating the ability of the species to survive under less optimum environmental conditions. It should be noted however, that this community has the highest average altitude (1407 m) among the vegetation units of Ae. hippocastanum.
Aesculus hippocastanum vegetation in Albania
Communities from the most northwestern part of the Ae. hippocastanum natural distribution, located in Albania, are not represented in the present study due to the lack of published relevés. However, relatively detailed phytocoenological, phytoecological and population data are available in the work of Peçi et al. (2012). The species habitat in this area mostly includes scree and deforested terrains of altitudes between 700-1200 m, where Ae. hippocastanum participates in mesic forest vegetation, growing on steep and rocky slopes of ravines. The vegetation has been determined as belonging to Fagion sylvaticae, but it is more probable that the accompanying tree species ( Sorbus torminalis, Acer hyrcanum, Acer pseudoplatanus, Tilia cordata, Tilia platyphyllos, Viburnum lantana, Ilex aquifolium, Carpinus betulus, Ostrya carpinifolia), as well as the description of the habitats, indicate that it is related to Ostryo carpinifoliae-Tilion platyphylli. The plant communities of Ae. hippocastanum there are very similar to those in North Macedonia and probably belong to the same (ass. Juglando-Aesculetum) or some other, but close synvicariant syntaxon.
Discussion
Syntaxonomic relationships of Aesculus hippocastanum-dominated communities
On the basis of the floristic and ecological affinities of the first four clusters distinguished in the classification we consider that all of them belong to the same alliance.
Bergmeier (1990) placed the Rusco hypoglossi-Aesculetum hippocastani in the Tilio-Acerion alliance. However, it was latterly considered by Mucina et al. (2016) as sensu lato. and this alliance is divided into four others with more restricted distribution. Geographical position, diagnostic species (such as Hedera helix, Asarum europaeum, Tilia platyphyllos, Aremonia agrimonoides, Juglans regia, Campanula rapunculoides, C. trachelium, Lilium martagon, Sanicula europaea, Arum maculatum, Sorbus torminalis, Helleborus odorus, Scutellaria altissima, Digitalis grandiflora, Calamintha grandiflora, Pseudoturritis turrita), ecological peculiarities, etc., placed the most widespread communities of Ae. hippocastanum on the Balkans in the alliance Ostryo carpinifoliae-Tilion platyphylli. It also includes typical refugial forests in the ravines at lower altitudes, such as foothills and lower mountains, mostly from the Western Balkans. However, the 5th cluster is strongly differentiated floristically and ecologically from the rest of the vegetation units, indicating its relationship with different higher syntaxa. On the basis of the dominance of Abies borisii-regis in this community as well as its thermophilous character we propose classifying this community in Abietion cephalonicae, which includes supra-Mediterranean Hellenic fir forests. Below the syntaxonomic scheme of the distinguished vegetation units in this study is given.
Class Carpino-Fagetea sylvaticae Jakucs ex Passarge 1968
Order Aceretalia pseudoplatani Moor 1976
Alliance Ostryo carpinifoliae-Tilion platyphylli (Košir et al. 2008) Čarni in Willner et al. 2016
Association Rusco hypoglossi-Aesculetum hippocastani Raus et Bergmeier 1990
Association Juglando-Aesculetum hippocastani Matvejeva et Nikolovski 1976
Association Staphyleo pinnatae-Aesculetum hippocastani Tzonev et al. 2024 ass. nova
Aesculus hippocastanum - Tilia platyphyllos community (Tzonev et al. 2024)
Class Quercetea pubescentis Doing-Kraft ex Scamoni et Passarge 1959
Order Quercetalia pubescenti-petraeae Klika 1933
Alliance Abietion cephalonicae Horvat et al. 1974
Aesculus hippocastanum-Abies borisii-regis community (Barbero et Quézel 1976)
Historical dynamic and conservation value of Aesculus hippocastanum dominated communities
Aesculus hippocastanum constitutes a biogeographic relict species of the Balkan Peninsula, since it is the only surviving descendant of the once widespread genus of Aesculus in the Europe. The initial entry of the Aesculus genus in Europe from eastern Asia is considered to have taken place by the early Oligocene (Harris et al. 2009). Although studies on the paleo-distribution of Aesculus representatives have revealed a wide distribution of the genus in Europe as well as the Balkan Peninsula since the Miocene, it is estimated that the genus was more widely distributed across the whole European continent mainly during the Pliocene (Postigo Mijarra et al. 2008). This distribution shrank abruptly due to the subsequent unfavorable climatic conditions that occurred during the end of the Pliocene, like the distribution of several other woody taxa (Svenning 2003).
The habitat suitability of temperate and even northern Europe for Ae. hippocastanum is indicated by the species widespread current distribution throughout Europe as a cultivated species and its systematic use as an ornamental tree in many European cities. This raises questions concerning the causes of the species’ inability to recolonize naturally central and northern parts of Europe. Based on the available knowledge it can be inferred that species biology rather than habitat suitability is mostly responsible for this distribution pattern (Thomas et al. 2019, Walas et al. 2019). Specifically, the species reproduction ecology seems to constitute a significant limiting factor for its spreading. On one hand, its barochoric (mean weight per seed ≈ 42 g) seeds, which spread mainly by gravity, limit the ability of the species to disperse over great distances (Tsiroukis 2008, Thomas et al. 2019). It is considered that Ae. hippocastanum seeds can travel only short distances by gravity (up to 14.5 m), similarly with its relative Japanese Horse-chestnut ( Aesculus turbinata) (Hoshizaki et al. 1999). Nevertheless, seeds can be carried greater distances by rodents, although this is not particularly common (Thomas et al. 2019). On the other hand, its seeds are recalcitrant and intolerant of desiccation, limiting its ability to successfully establish new localities that lack the necessary conditions related with humidity (Tsiroukis 2008, Walas et al. 2019). Therefore, the species’ inability to travel great distances, as well as its seeds’ sensitivity to dry conditions during the early stages of any attempt by the species to establish itself in a new location, constitute the main known causal factors for its current native range. After the initial restriction of the species in refugial low and mid-altitude habitats, such as ravines and gorges, that allowed the species persistence by ensuring relative environmental stability through periods of climatic changes (Woolbright et al. 2014), it was unable to overcome topographical barriers and recolonize the rest of Europe.
The present study, based on vegetation data of communities of Ae. hippocastanum across its whole distribution range, led to the classification of its communities into five syntaxonomical units assigned to the Carpino-Fagetea sylvaticae and Quercetea pubescentis classes, thus dividing the vegetation of Aesculus hippocastanum, respectively, in mesic and xeric-thermophilous vegetation groups. Inferences regarding the historical communities of Aesculus in the central and southern Europe during Pleistocene also indicate the co-occurrence of the species with several thermophilous and mesic taxa, such as Ulmus, Pterocarya, Carya, Platanus, Juglans, Fagus and Carpinus (Renault-Miskovsky and Girard 1978). Overall, it has been proposed that temperate deciduous communities occurring in moist habitats constitute the main historical habitat of Aesculus, which was mainly established in environments such as valley bottoms, riverheads, mid-mountain riverine forests or forests on temporarily flooded soils (Postigo Mijarra et al. 2008). These habitats allowed the preservation of the species during the Pleistocene as well as during the current adverse environmental conditions by ensuring high air humidity and water availability for its populations, which has been revealed to constitute one of its main ecological requirements (Walas et al. 2018).
Further indications of the special floristic character of Ae. hippocastanum communities could be derived through other relict species that occur and even dominate in them, which have similar ecological preferences, reproductive and dispersal strategies. Such are the Persian walnut ( Juglans regia) and the European bladdernut ( Staphylea pinnata), which have been also objects of economic interest since Ancient times, humans having expanded their distribution significantly for various commercial needs (Heiss et al. 2014, Pollegioni et al. 2017).
The current distribution of the Staphylea pinnata is sporadic, but significantly wider than that of Ae. hippocastanum. It is also considered a Tertiary relict (from the Pliocene), and a representative element of the nemoral flora in the sub-Mediterranean region (Heiss et al. 2014). According to Meusel and Jäger (1989), forest plants with ranges in the eastern part of sub-Mediterranean area (mostly in the Balkans and western Anatolian Peninsula) were named “ Staphylea-type”. This group includes Quercus cerris, Q, frainetto, Ostrya carpinifolia, and Fraxinus ornus, but species such as Tilia tomentosa, Syringa vulgaris and Ae. hippocastanum are confined to the center of the this “ Staphylea-type” area. The range of Staphylea pinnata , like that of Aesculus hippocastanum, has been affected by human activity due to the exploitation of the species for a variety of economic uses, since Antiquity, leading to a significantly wider distribution and its subspontaneous establishment in many places in Central and Western Europe (Heiss et al. 2014).
Juglans regia is known from macrofossils from Western Bulgaria (Sofia Basin) from the late Pliocene, but the genus was distributed in the Balkans ( Juglans ungeri) even earlier - since the Lower Oligocene (Palamarev 1993). According to Pollegioni et al. (2017), at least in the eastern Mediterranean, in two phytogeographically well-defined plant refugia - the Balkans and Turkey, Juglans regia existed during all the cold and dry periods of the Pleistocene. Sudden increases of its fossil pollen between 2500 and 1000 years BP, presumably are due to the increase of its cultivation from Greek and Roman times onwards. This range’s “fluctuation” is also strongly comparable with the history of Ae. hippocastanum surviving in the Last Glacial Maximum in even more restricted refugia than Juglans regia and its secondary expansion by humans.
The evolutional history of Staphylea pinnata and Juglans regia is also good evidence that their important phytocoenological role in the recent communities of Ae. hippocastanum is a result of long coexistence together in these Tertiary refugia. However, in general, we can conclude that these species represent the most conservative relict component of the Tertiary flora and forest vegetation that survived in the Last Glacial Maximum (LGM), only in specific habitats and refugial plant communities. These communities have hosted more widespread species as well, such as representatives of the genera Tilia, Ulmus, Quercus, Carpinus, Corylus, Ostrya, Acer, etc. However, the more successful dispersal strategies (mostly anemochory) of the latter did help them to expand their ranges, especially in the temperate regions of the Mediterranean and southern temperate Europe. Therefore, we could demonstrate especially Ae. hippocastanum-dominated communities as a “model example” for the refugia of mesophilous forest vegetation during the Pleistocene and at least, during the LGM.
Currently, natural relict populations of the species are to be found in the Western Balkans, with the Bulgarian subpopulation constituting a location of special character, since the area is not included in the potential distribution of Ae. hippocastanum, even since the period of Mid Holocene (Walas et al. 2019). The diversity patterns among the syntaxa of Ae. hippocastanum, as they were identified by the present study, revealed both spatial (longitude and latitude) as well as ecological variables (meso-climate differentiation represented by altitude, but also microhabitat differentiation depicted by the relevés’ floristic composition), as driving factors of Ae. hippocastanum habitat differentiation. These results, derived from vegetation data, are in accordance with those found by Walas et al. (2019), concerning the genetic diversity of Ae. hippocastanum in the main part of its distribution range (Greece). According to them, species genetic diversity is, to a great extent, the result of the lack of connectivity among its populations, leading to unique genetic signatures even among spatially adjacent populations, but also of ecological differentiation, thus potentially highlighting the importance of the concept of microhabitat differentiation.
The taxonomic, functional and phylogenetic diversity of all relevés (except one) included in the communities that represent the main current habitat of Ae. hippocastanum (2nd and 3rd cluster) has been previously investigated and compared to the rest of the forest community types (broadleaved deciduous and coniferous) occurring within the main native distribution area of the species, namely central and northern Greece (Mastrogianni et al. 2019, Mastrogianni 2020). According to these studies, the ravine forest types to which the communities of Ae. hippocastanum investigated belong, had a particularly unique profile of species diversity, mainly characterized by the occurrence of a great number of woody taxa in the forest overstorey layer, in agreement with the observation also of Raus (1980). Moreover, these ravine forest communities were found to have a distinct functional signature, defined mostly by the presence of several species with particular seed (large, heavy and recalcitrant seeds) and dispersal-related characteristics, indicative of a potential refugial role of these habitats (Keppel et al. 2018). Finally, the ravine forests were found to preserve high levels of phylogenetic diversity, which has been attributed mostly to the persistence of species with evolutionary distinctiveness in these communities due to long-term environmental stability (Mastrogianni et al. 2019). The aforementioned characteristics of ravine forests constitute significant arguments for the great conservational value of the relict Ae. hippocastanum and its habitat, in addition to the already recognized significance due to their rarity and distinct evolutionary history. In particular, from their distinct patterns of diversity it can be inferred that these habitats have played a significant role as shelters for several species during past climatic variations, therefore constituting potentially crucial localities for biodiversity conservation under the forthcoming effects of current climate change (Hampe and Petit 2005).
Conclusions
Despite the relict and endemic character of Ae. hippocastanum, as well as its narrow distribution range and relatively rare occurrence throughout this range, the habitat diversity of the species has not been thoroughly studied. The present study contributes to the understanding of the habitat diversity of Ae. hippocastanum, by identifying five floristically and ecologically well-defined syntaxonomical units as the main habitats of the species. These units are the associations Rusco hypoglossi-Aesculetum hippocastani, Juglando-Aesculetum hippocastani, the newly defined association Staphyleo pinnatae-Aesculetum hippocastani, but there are also two communities (Aesculus hippocastanum-Tilia platyphyllos, and Aesculus hippocastanum-Abies borisii-regis). The geographic distribution and isolation of these distinct habitats across its overall distribution range are in agreement with the paleo-distribution of Ae. hippocastanum and the historical events that led to its restriction and survival in only a few spatially restricted refugial areas. The biological characteristics of the species, such as its seed weight and storage behavior, as well as its ecological requirements for high levels of humidity and special soil characteristics, constitute the main attributes that explain the floristic diversity of A. hippocastanum communities, which, according to historical evidence, have included taxa such as Ulmus, Platanus, Juglans, Fagus and Carpinus since the Pleistocene. Preexisting knowledge regarding the taxonomic, functional and phylogenetic diversity of its communities has highlighted the great conservational value of Ae. hippocastanum and its habitats. Therefore, the contribution of the present study towards the understanding of Ae. hippocastanum habitat diversity, can be particularly useful for the development and prioritization of the necessary conservation actions.
Acknowledgements
The second author (AM) was financially supported by The Hellenic General Secretariat of Research and Technology (GSRT) and the Hellenic Foundation for Research and Innovation (HFRI; Scholarship Code: 18). The Bulgarian team expresses its special thanks to the Regional Inspectorate of Environment and Waters in Shumen town and especially to Mrs. Krasimira Borisova for the opportunity to undertake a field study in “Dervisha” Reserve.
