Introduction
Dairy products account for almost 30 % of the average human diet (Stobiecka et al., 2022) and contain antioxidant compounds in varying proportions depending on the type of matrix (i.e. milk, yoghurt, fermented milk and cheese) and processing (i.e. mechanical, thermal and fermentative). These compounds include both, lipophilic and hydrophilic antioxidants like proteins (especially casein) (Fardet and Rock, 2018; Chen et al., 2019; Khan et al., 2019; Sik et al., 2023), antioxidant enzymes (i.e. superoxide dismutase (SOD), catalase and glutathione peroxidase), conjugated linoleic acid (CLA), coenzyme Q10, lactoferrin, vitamins (E, A and D3), carotenoids, some minerals and trace elements (Fardet and Rock, 2018) as well as bioactive peptides released during milk fermentation (Stobiecka et al., 2022) or digestion (Fardet and Rock, 2018). The antioxidant potential of dairy products is related to the quality of the raw material and bacterial cultures, as studies have shown that the overall antioxidant capacity of milk also varies depending on the animal species (Stobiecka et al., 2022), including its genetics (Fardet and Rock, 2018), which affects the chemical composition of the milk. The antioxidant activity of dairy products is also influenced by the natural plant additives used in animal feed or during the production (Stobiecka et al., 2022). The addition of plant extracts as a source of phenolic compounds and bio-flavonoids has attracted a lot of attention recently, as milk and dairy products are not an abundant source of these health-protecting components (Barukčić et al., 2022).
Phenolic compounds, known for their remarkable antioxidant activity (Rodríguez-López et al., 2020) are plant secondary metabolites and are widely studied due to the potential beneficial effects of polyphenol-containing foods (Jakobek, 2015; Khan et al., 2018; Kabaran, 2019). Eating foods rich in natural antioxidants, such as in Mediterranean diet (Klisović et al., 2022), slows down the aging process and reduces the risk of developing cardiovascular disease, cancer, or diabetes (Arts et al., 2002; Lamothe et al., 2014; Khan et al., 2018; Stobiecka et al., 2022).
Nowadays, functional foods are very popular as they can support consumers’ health (Sik et al., 2023), and their interest in goat cheese, especially the traditionally produced type, is steadily increasing, mainly because of its health benefits, nutritional value and special taste (Sepe and Argüello, 2019). For example, the fat in goat's milk contains more unsaturated fatty acids (UFA) than cow’s milk (Wang et al., 2016), which can improve various health issues (Patel et al., 2022; Badawy et al., 2023). A balanced ratio of omega-3 and omega-6 fatty acids is crucial for a healthy lifestyle as it can help reduce the risk of coronary heart disease (Patel et al., 2022), and conjugated linoleic acid (CLA) not only counteracts obesity, but also inflammation, carcinogenesis and atherogenicity, and has effects on immunomodulation and osteosynthesis (Badawy et al., 2023).
Extra virgin olive oil, one of the symbols of the highly valued Mediterranean diet, is considered a functional food because extensive research has provided convincing evidence that its consumption positively influences one or more target functions in the body, improves health and reduces the risk of chronic diseases. The health properties are associated with the major and minor fractions of extra virgin olive oil, with the phenolic fraction having received much attention due to its bioactivity (Barukčić et al., 2022) in various chronic diseases (Rodríguez-López et al., 2020). The most abundant antioxidants in olive oil are tocopherols, β-carotene, lutein, squalene, lipophilic and hydrophilic phenols. Phenolic acids and derivatives (vanillic acid, gallic acid), phenolic alcohols (tyrosol, hydroxytyrosol), secoiridoids (oleuropein, oleocanthal), lignans (pinoresinol) and flavones (luteolin) are phenolic compounds of olive oil. In addition to phenolic compounds, olive oil has a high content of monounsaturated fatty acids (MUFA), especially oleic acid, which is also associated with a positive effect on human health (Granado-Casas and Mauricio, 2019; Kabaran, 2019). Olive oil is mostly used fresh, but it is not uncommon to use it as a preservative for seasonal products - from vegetables to dairy products such as cheese (Klisović et al., 2022).
Due to the seasonal nature of goat cheese production and the great interest shown by consumers, artisanal cheesemakers have started to immerse the cheese in oil. This preservation method is a well-known practice of cheesemakers on the Croatian islands, such as Brač and Krk, where they use olive oil, while in the south of Croatia (Dubrovnik-Neretva County) they use a mixture of olive and sunflower oil. The time of cheese immersion in the oil is not standardized, but cheese from the islands of Brač and Krk is air-ripened for at least 2 months, while cheese from Dubrovnik and the island of Mljet is immersed into the oil after 1 week of air ripening (Caput et al., 2003; Barukčić and Tudor Kalit, 2019). The Spanish hard sheep's cheese Manchego is usually stored in olive oil after 3 months of maturing in the air and can be kept there until consumption (Ordoñez et al., 1978; Ordoñez and Burgos, 1980). The influence of oil as a ripening medium on different properties of cheese is almost unexplored, apart from two studies more than forty years ago (Ordoñez et al., 1978; Ordoñez and Burgos, 1980) and three recently published studies, one of which focuses on extra virgin olive oil used as a medium for cheese preservation (Klisović et al., 2022) and the others on the physicochemical composition and sensory properties of semi-hard goat cheese ripened in an oil mixture (Levak et al., 2023a; Levak et al., 2023b).
Considering the above-mentioned properties of olive oil, the aim of this study was to determine the nutritional value of semi-hard goat cheese ripened in an oil for different lengths of time, in terms of fatty acid profile and antioxidant potential (total polyphenol and flavonoid content and antioxidant activity).
Materials and methods
Cheese making and sampling
Five batches of semi-hard goat cheese were produced in the pilot plant of the Department of Dairy Science at the University of Zagreb Faculty of Agriculture (Croatia). Each batch was made from 100 L of raw goat milk (Saanen goat breed, family farm in Bjelovar-Bilogora County, Croatia). The milk was collected during evening and morning milking and stored in a cooling tank at 4 °C before cheese making.
The filtered milk was heated to 31 °C and then lysozyme (Lysozyme granular, Proquiga Biotech S.A., Spain - 5 g of lysozyme dissolved in 1.5 dL of distilled water at room temperature for 15 minutes), freeze-dried mixed starter cultures (Lyofast MT 096 FEN 5 UC, Sacco Srl, Italy - 1,352 g; 1/5 of the bag weight for 100 L of milk) and rennet powder (Caglificio Clerici Spa, Italy - 4 g of rennet powder dissolved in 1.5 dL of distilled water at room temperature for 10 minutes) were added according to the manufacturer's procedures and guidelines. After coagulation (setting time was between 50 and 60 minutes), the curd was cut into uniform walnut-sized grains with sharp edges using a cheese harp. The curd grains were heated to 39 °C with constant stirring. When the desired temperature was reached, the cheese grains were stirred for 30 minutes and then placed evenly in the plastic moulds and pressed with a cheese press, which produces a controlled and uniform pressure. The pressing process lasted 3 hours and started with light pressure (1 bar/1 h) that was increased to 2 bars for the next hour and finished with 3 bars for the last hour. Each time the weights were changed, the cheeses were turned over. Once a pH value of 5.4-5.5 was reached, the cheeses were dry salted, stored overnight in the refrigerator, washed on both sides in the morning under running drinking water, dried with paper towels and subjected to ripening.
A total of 65 cheeses were produced with an initial weight of about 800 g and a size of 12 cm x 4 cm. Each batch consisted of 13 cheeses, and the cheeses of the same batch were randomly divided into 3 groups according to the ripening treatment: (i) group 1 - ripening in air for 60 days (control group), (ii) group 2 - ripening in oil for 50 days after 10 days of ripening in air, (iii) group 3 ripening in oil for 40 days after 20 days of ripening in air. The temperature in the ripening chamber was 15-17 °C and the relative humidity was 70-80 %. The cheeses of groups 2 and 3 were placed in plastic vats (Figure 1) with lids filled with a mixture of extra virgin olive oil (EVOO) from the island of Mljet and commercial refined sunflower oil (50:50 ratio).
During ripening, whole cheeses were sampled: control group on days 0 (cheese curd), 10, 20, 30, 45, 60, group 2 on days 20, 30, 45, 60 and group 3 on days 30, 45 and 60. Each cheese represents a sample according to the ripening treatment and the day of sampling.
Before the samples were packaged, the surface of each cheese was blotted with a paper towel to remove any possible oil residue that could interfere with the results. To avoid dehydration, the samples were packed in plastic bags and transported to the laboratory with a mobile cooler at a temperature of 4 °C. The cheese samples were marked and stored at -80 °C until the chemical analyses were performed.

Figure 1. Ripening of semi-hard goat cheese in air and in a oil mixture
Total polyphenol and flavonoid content and antioxidant activity
The analyses of cheese curd (day 0) and cheese after 10, 20, 30, 45 and 60 days of ripening in different media (air, oil mixture) included the determination of total polyphenols, flavonoids and antioxidant activity and were carried out at the Department of Chemistry University of Zagreb Faculty of Agriculture (Croatia). The chemicals used for these analyses were sodium carbonate anhydrous, sodium nitrite, aluminum chloride hexahydrate and methanol, which were purchased from Kemika (Zagreb, Croatia). Folin-Ciocaulteu reagent, quercetin, gallic acid, ABTS (2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) and DPPH (2,2-diphenyl-1-picryl-hydrazyl hydrate) used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Prior to analyses, cheese samples were stored at room temperature and cheese slices (≈ 100 g) were cut from the core to the rind. The rind was cut off and the remainder was grated. All the analyses were performed in duplicate.
Extraction procedure
Extraction of phenolic compounds from cheese samples was performed according to the modified procedure (Lucera et al., 2018). Briefly, 2.5 g of the grated cheese without rind was mixed with 50 mL of acidified methanol (80 % MeOH containing 1 % HCl) and extracted for 30 minutes at 80 °C in a water bath (Julabo SW22, JULABO GmbH, Germany) at 0.06066 x g (200 rpm). The mixture was cooled to room temperature and filtered through the Whatman No. 4. The extracts obtained were analysed immediately. The extraction was performed in duplicate for each sample.
Determination of total polyphenol content
The total polyphenol content was determined by the modified Folin-Ciocalteau method (Lucera et al., 2018). The method is based on the colorimetric reaction of Folin-Ciocalteau reagent with a reducing reagent (polyphenolic compounds). In brief, 0.1 mL of the previously prepared extract of semi-hard goat cheese was mixed with 7.9 mL of distilled water and 0.5 mL of Folin-Ciocalteau reagent diluted with water in a 1:2 ratio, vortexed for 30 seconds and left for 10 minutes at room temperature. Then 1.5 mL of a 20 % sodium carbonate solution (Na2CO3) solution was added and vortexed again for 30 seconds. After 2-hour incubation at 20 °C, the absorbance of the samples was measured at 765 nm using a spectrophotometer (Shimadzu, UV-1900i, Shimadzu, Japan). The results were expressed as mg of gallic acid equivalent (GAE) per 100 g.
Determination of total flavonoid content
The total flavonoids in semi-hard goat cheese were determined according to the modified aluminium chloride colorimetric method by Lucera et al. (2018). Each extract (1 mL), prepared as previously described, was mixed with 6.4 mL of distilled water and 300 µL of a 5 % sodium nitrite (NaNO2) solution. After 5 minutes, 300 µL of a 10 % aluminium chloride (AlCl3) solution was added and the mixture was left to rest for 6 minutes. Finally, 2 mL of 1 M sodium hydroxide (NaOH) was added. Then, the solutions were mixed and for each sample the absorbance was read at 360 nm. The calibration curve was prepared using quercetin as standard and total amount of flavonoids was expressed in mg of quercetin equivalents (QEs) per g.
Antioxidant activity
The antioxidant activity in semi-hard goat cheese was evaluated using two methods: ABTS (2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) and DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) assays according to the modified methods by Qureshi et al. (2019). An aliquot of 40 µL extract (diluted as needed) is mixed with 4 mL ABTS+ solution in the cuvette and the absorbance is measured at 734 nm after exactly 6 minutes. The change in absorbance of the ABTS radicals after reaction with the sample is calculated by subtracting the absorbance of the sample from the absorbance of the blank. The results are expressed as mmol L-1 equivalent of Trolox (TE).
The DPPH method is based on the reduction of the violet-coloured stable DPPH radical to yellow 2,2-diphenyl-1-picrylhydrazine at 515 nm after reaction with the antioxidant. 100 µL of sample (diluted as needed) is placed in the test tube and 3.9 mL of a 0.094 mM DPPH solution is added and vortexed. The reaction takes place in the dark for 30 minutes (the time required to reach equilibrium). The absorbance at 515 nm is then measured in relation to the blank sample. The results are expressed as mmol L-1 equivalent of Trolox (TE).
Fatty acid profile
The preparation of fatty acid methyl esters (HRN ISO 15884:2003) (International Organization for Standardization, 2003a) and the determination of the fatty acid composition of semi-hard goat cheese by the gas chromatography method (HRN ISO 15885:2003) (International Organization for Standardization, 2003b) were performed in the Reference Laboratory for Milk and Dairy Products of the Department of Dairy Science at the University of Zagreb Faculty of Agriculture. The chemicals and consumables used for this analysis, purchased from Biovit (Varaždin, Croatia), were sodium methoxide, reagent grade, 95 % powder (Sigma), sodium hydrogen sulphate monohydrate (for EMSURE analysis) (Supelco), n-hexane for GC-MS (SupraSolv, Supelco) and reference fatty acid methyl esters (37 Component FAME Mix, Supelco). The FAME mixture was analysed according to HRN ISO 15884:2003, which means under the same working conditions as the samples. The normalisation method is used to estimate the percentage of a component represented by a peak in the chromatogram. The fatty acid profile was determined in cheese samples from the 60th day of ripening.
Statistical analysis
The statistical procedures were performed in SPSS version 21 (IBM Corporation, 2012). The effects of the different ripening treatments on total polyphenols and flavonoids, antioxidant properties and the fatty acid profile of the cheese after 60 days of ripening were analysed using a one-way ANOVA and the general linear model (GLM). The experimental data obtained during cheese ripening were also subjected to a one-way ANOVA with ripening time as a fixed factor and total polyphenols and flavonoids and antioxidant properties as dependent variables.
Mean comparisons were performed with the least significant difference test. Significance was indicated by p<0.05.
Results and discussion
The effect of ripening treatment on total polyphenols, flavonoids and antioxidant activity of 60-day ripened semi-hard goat cheese is shown in Table 1. The changes in polyphenol and flavonoid content and antioxidant activity during ripening of semi-hard goat cheese depending on the ripening treatment are shown in Figures 2-5. The fatty acid profiles (day 60) are shown in Tables 2 and 3 according to their saturation.
Table 1. Total polyphenol and flavonoid content and antioxidant properties of 60-day ripened semi-hard goat cheese (results are expressed as mean ± SE)
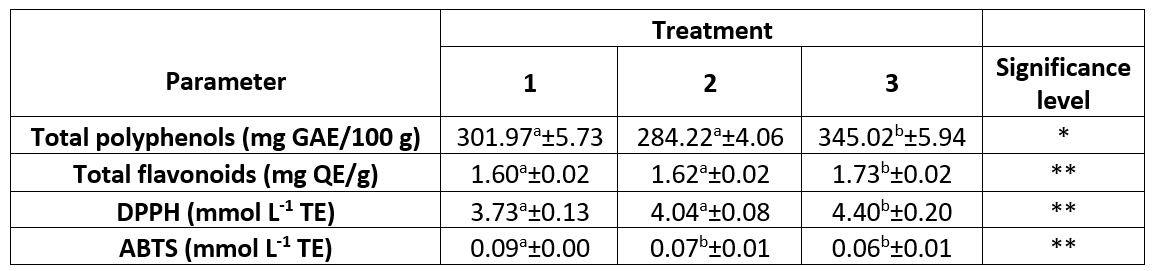
a,bMeans in the same row marked with different letters differ significantly, ** (p<0.01), * (p<0.05).
Treatment 1 (Control group) = cheese ripening in the air (60 days); Treatment 2 = cheese ripening in oil after 10 days of ripening in air; Treatment 3 = cheese ripening in oil after 20 days of ripening in air.
SE = standard error; GAE = gallic acid equivalent; QE = quercetin equivalent; TE = Trolox equivalent
The ripening treatment affected the total polyphenol and flavonoid content of the 60-day ripened semi-hard goat cheese. Cheeses from group 3 had the highest total polyphenol and flavonoid content (p<0.05 and p<0.01, respectively), while there was no significant difference between the control group and group 2 (Table 1). Most of the moisture loss in the cheese occurs at the beginning of ripening (Fresno and Álvarez, 2012; Álvarez and Fresno, 2021) and due to the prolonged ripening in air (20 days) before immersion in the oil, the cheeses from group 3 developed such a cheese structure through which the oil can penetrate (Levak et al., 2023a) and thus also polyphenols and flavonoids from the oil mixture. As shown in Figures 2 and 3, the total content of polyphenols and flavonoids increased significantly (p<0.05) during the ripening of group 3 cheeses, which was not the case for group 2 and control cheeses. The oil as a ripening medium caused water retention in the group 2 cheeses, which were immersed in oil after only 10 days of ripening in air, and thus prevented the oil from penetrating the cheese to a greater extent (Levak et al., 2023a), which meant that the total content of polyphenols and flavonoids did not change significantly (Figures 2 and 3). Consequently, the total content of polyphenols and flavonoids at the end of ripening was lowest in the cheeses of group 2 compared to the other two treatments (Table 1). The polyphenols in the control cheeses originate from animal feed (e.g. pasture), from where they naturally pass into the milk (Klisović et al., 2022; Stobiecka et al., 2022). Due to the exclusive air ripening, the control cheese has a higher dry matter content compared to the oil ripened cheeses (Levak et al., 2023a) and since the loss of water in the cheese has a concentrating effect, this resulted in a higher total polyphenol content (301.97 mg GAE/100 g) compared to group 2 (284.22 mg GAE/100 g).
Interestingly, the total content of polyphenols and flavonoids decreased in the control group after 45 days of ripening, which is not the case for the cheeses that ripen in oil (Figures 2 and 3). The reason for this could be a different course of catabolism and changes in the protein structure during ripening in air and ripening in oil, which can lead to the release of the bound phenolic compounds in the control cheese, which in turn can be oxidized and hydrolysed (Servili et al., 2011; Roila et al., 2019).
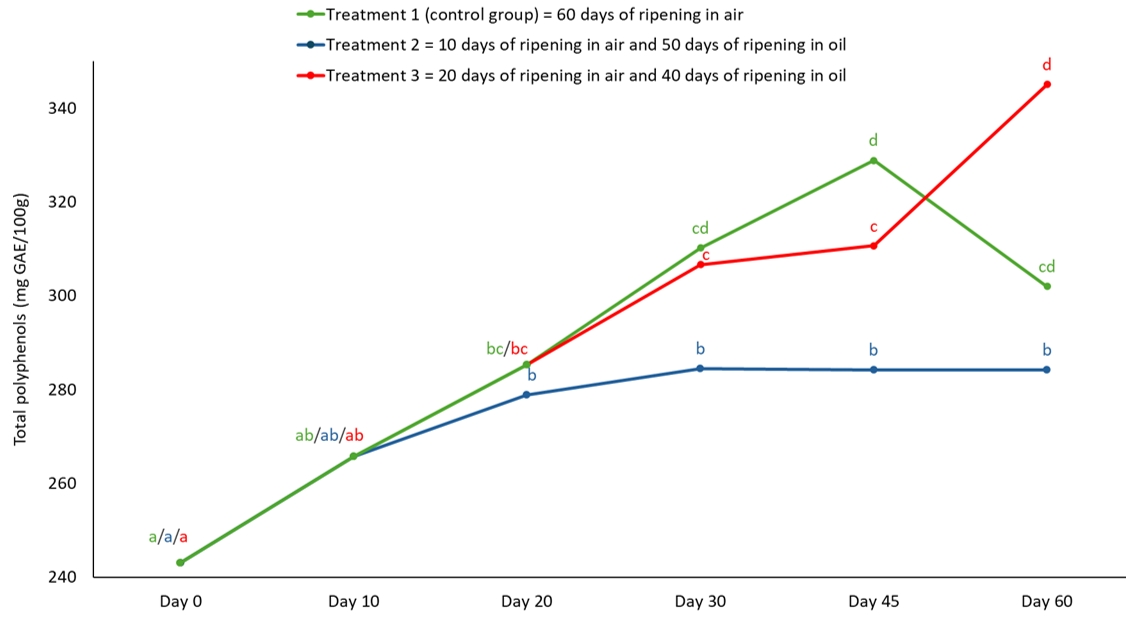
Figure 2. The content of total polyphenols in semi-hard goat cheese during ripening.
Means marked with different letters on each line of individual treatment differ significantly (a-d; p<0.05). Standard error mean: Treatment 1 = 11.17, Treatment 2 = 9.11, Treatment 3 = 10.53
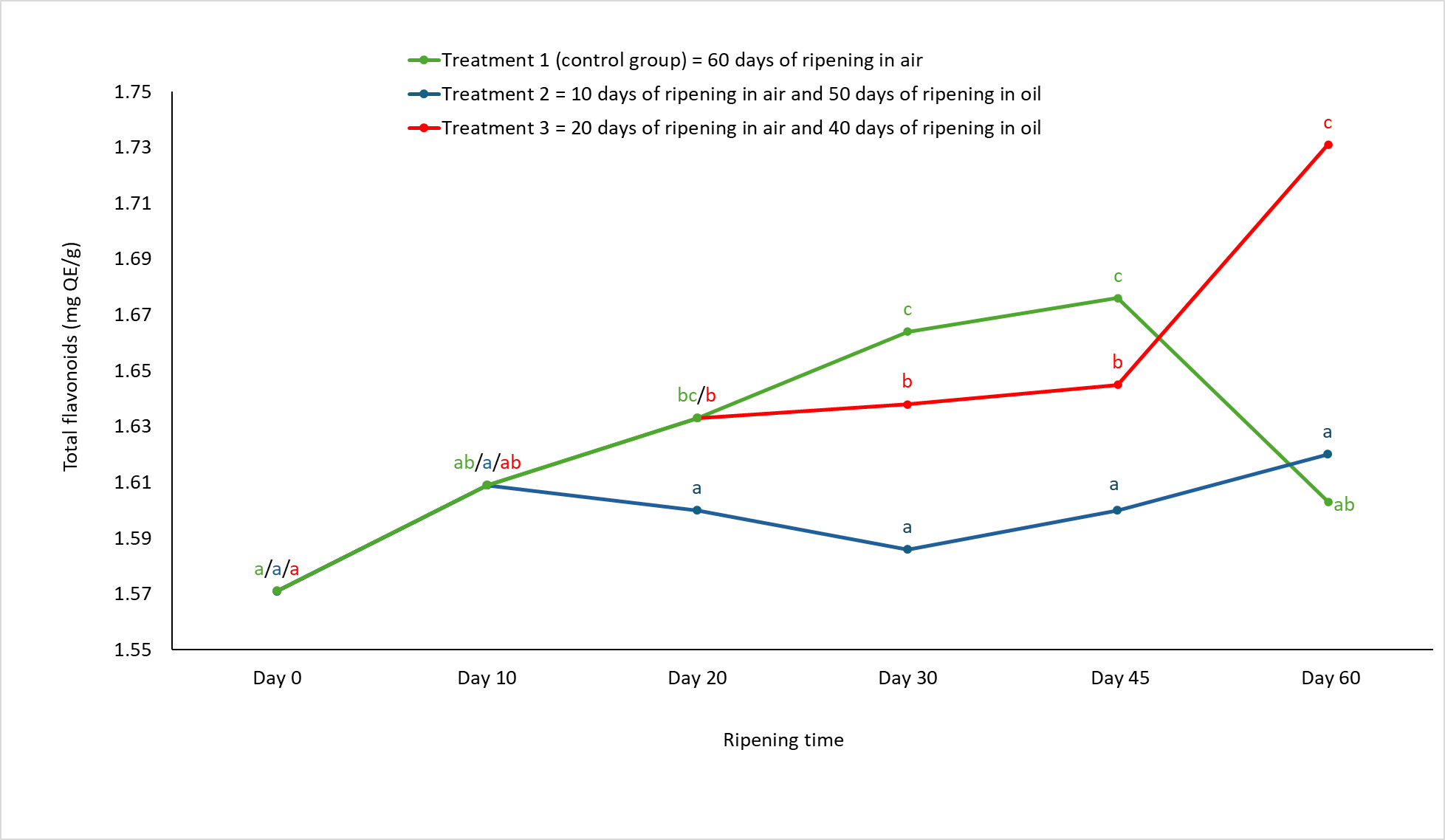
Figure 3. The content of total flavonoids in semi-hard goat cheese during ripening.
Means marked with different letters on each line of individual treatment differ significantly (a-c; p<0.05). Standard error mean: Treatment 1 = 0.018, Treatment 2 = 0.029, Treatment 3 = 0.017.
The ripening treatment influenced the antioxidant activity of 60-day ripened semi-hard goat cheese, which was determined using two spectrophotometric methods (DPPH and ABTS). On the last day of ripening, the cheeses in group 3 had the significantly (p<0.01) highest antioxidant activity as determined by DPPH (4.40 mmol L-1 TE), while there was no significant difference between the control group and group 2 (Table 1). Olive oil is an important source of phenolic compounds (Kabaran, 2019), which diffuse into the cheese to a greater (group 3) or lesser extent (group 2) when it is used as a ripening medium after different ripening times in air (Levak et al., 2023a). Phenolic compounds in olive oil can have lipophilic and hydrophilic properties (Kabaran, 2019), and the structure of group 3 cheeses after prolonged air ripening (Levak et al., 2023a) allowed more oil to diffuse into the cheese. This is also the reason why the antioxidant activity of group 3 cheeses continued to increase significantly (p<0.05) during ripening (Figure 4), while there were no significant changes in the antioxidant activity determined by DPPH in group 2 cheeses during ripening due to the higher moisture content (Levak et al., 2023a). As a result of the different immersion times and the differences in the physico-chemical composition of oil-ripened cheeses (Levak et al., 2023a), the cheeses in group 2 had significantly (p<0.01) lower antioxidant activity (4.04 mmol L-1 TE) on the last day of ripening than samples in group 3 (Table 1).
The cheeses in the control group had the lowest antioxidant activity, as determined by DPPH (3.73 mmol L-1 TE), compared to the oil-ripened cheeses (Table 1), where the oil diffused into the cheese structure during ripening (Levak et al., 2023a). The antioxidant activity also decreased significantly (p<0.05) in the cheeses of the control group during ripening until it reached its final value (Figure 4). This could be a consequence of catabolism (Servili et al., 2011; Roila et al., 2019) of naturally present antioxidants from animal feed (Klisović et al., 2022; Stobiecka et al., 2022). In the Cheddar cheese study (Gupta et al., 2009), scavenging activity increased significantly at the beginning of ripening (p<0.05), then decreased and maintained similar values until the end of ripening, which could indicate a possible role of the proteolytic enzymes of the adjunct cultures in the production of peptides with antioxidant activity. The decrease in antioxidant activity suggests that the antioxidant peptides were not resistant to further proteolysis (Gupta et al., 2009). The same observation was made in the study on the antioxidant potential of milk, yoghurt, fermented milk and cheese, in which the authors concluded that the antioxidant capacity of cheese increases to an optimum during ripening due to peptide release and then decreases due to peptide hydrolysis and that this effect and the optimum antioxidant capacity probably depend on the type of cheese and ripening time (Fardet and Rock, 2018).
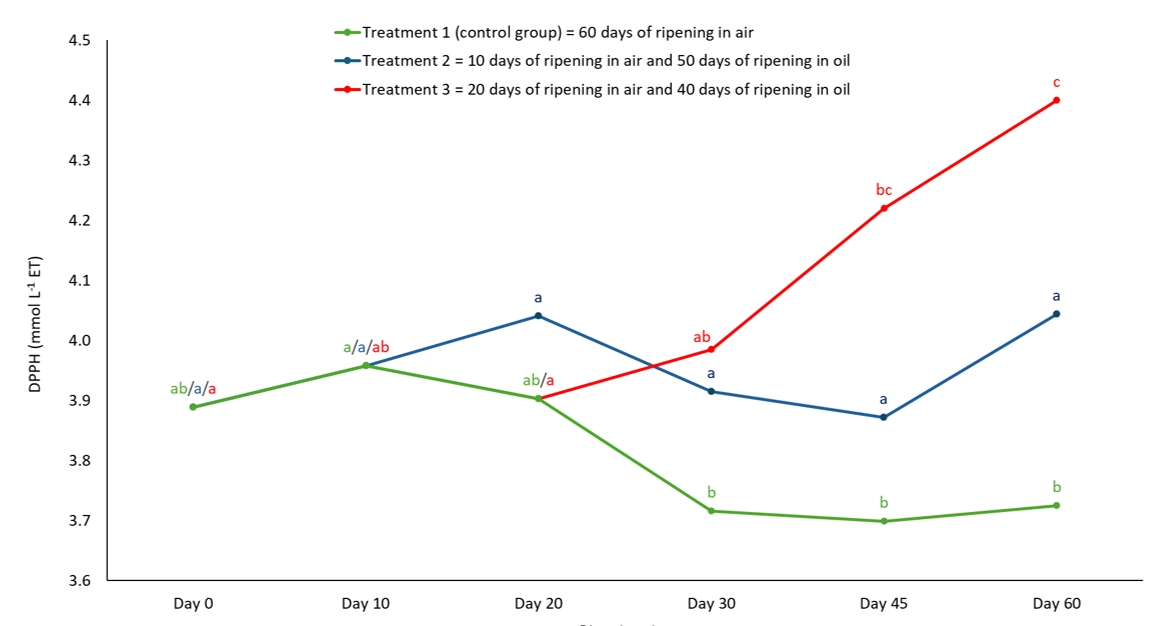
Figure 4. Antioxidant activity of semi-hard goat cheese during ripening determined by DPPH method. Means marked with different letters on each line of individual treatment differ significantly (a-c; p<0.05). Standard error mean: Treatment 1 = 0.073, Treatment 2 = 0.067, Treatment 3 = 0.094.
Antioxidant activity, as determined by ABTS, differed significantly (p<0.01) between the control group and the oil-ripened cheeses on the 60th day of ripening (Table 1). As can be seen in Figure 5, an inverse trend was observed in the antioxidant activity determined by ABTS compared to the DPPH spectrophotometric method, which was also found in another study in which the olive oils had higher values with the DPPH method than with the ABTS method (Christodouleas et al., 2015).
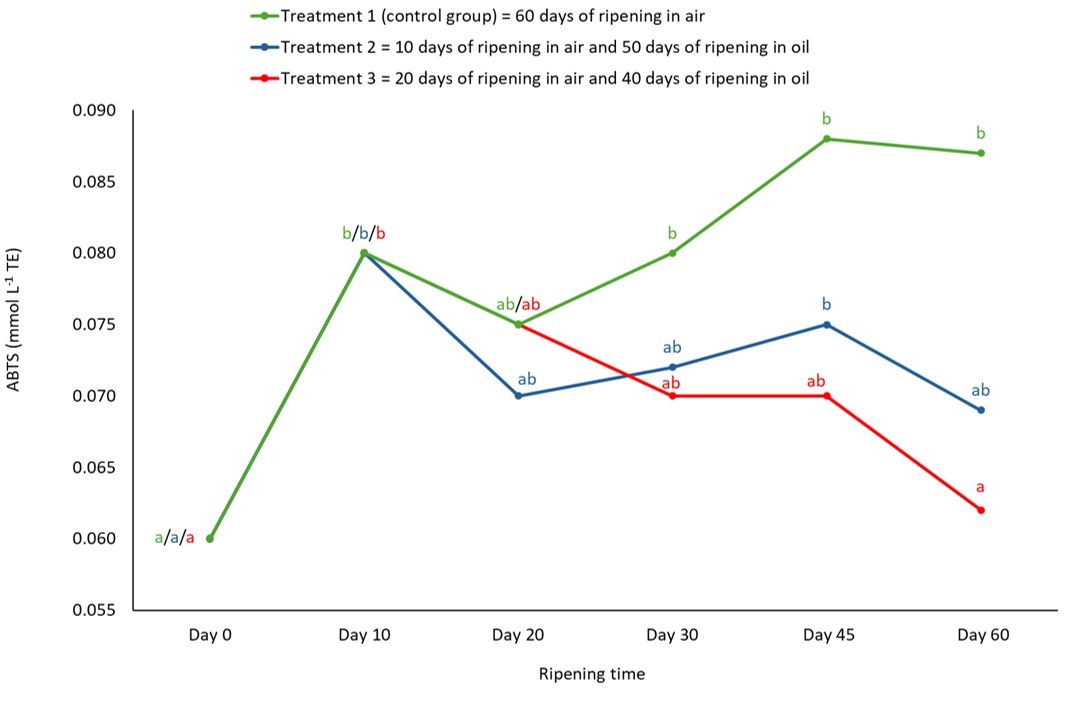
Figure 5. Antioxidant activity of semi-hard goat cheese during ripening determined by ABTS method. Means marked with different letters on each line of individual treatment differ significantly (a-b; p<0.05). Standard error mean: Treatment 1 = 0.007, Treatment 2 = 0.004, Treatment 3 = 0.008.
The DPPH and ABTS methods are the most commonly used spectrophotometric methods for assessing the antioxidant activity of milk and dairy products (Stobiecka et al., 2022) due to their simplicity, speed, sensitivity and the use of stable radicals (Olszowy and Dawidowicz, 2018). These methods have certain advantages and disadvantages compared to each other (Akan et al., 2021) and it is not uncommon for the results of the same material analysed by different methods to differ (Klisović et al., 2022), especially since ABTS can measure the antioxidant activity of both hydrophilic and hydrophobic substances and the affinity of the DPPH method depends on the solvent - hydrophilic (Christodouleas et al., 2015; Akan et al., 2021) for methanol and lipophilic for ethyl acetate. The DPPH method is particularly sensitive to the reaction conditions (e.g. higher moisture content) (Christodouleas et al., 2015).
It is also important to recognize that due to the great diversity in the structure of phenolic compounds, they have different properties, such as solubility and polarity, which allow them to interact with other molecules in different ways. They can interact with each other and with other molecules that surround them (Jakobek, 2015). For example, proteins are highly complex polymers that can form complexes with various dietary components, including polyphenols (Felix da Silva et al., 2015; Jakobek, 2015; Han et al., 2019) and flavonoids (Arts et al., 2001). Polyphenols can interact with milk proteins via both hydrophilic and hydrophobic interactions, especially with proline-rich proteins such as α- and β-caseins (Lamothe et al., 2014). Four types of protein-phenol interactions are known: hydrophobic, ionic, covalent and hydrogen bonds (Han et al., 2019; Klisović et al., 2022). However, not only proteins but also other nutrients such as carbohydrates and lipids have a very complex, porous structure that can intercept polyphenols and alter their availability. Due to these interactions, the antioxidant activity of polyphenols and flavonoids could be masked (Arts et al., 2001, 2002; Jakobek, 2015) and protein–phenol interactions can reduce the antioxidant activity of phenolic compounds (Klisović et al., 2022). In addition to the formation of complexes, many studies have also identified the proteins in milk as responsible for antioxidant activity. Casein can produce antioxidant substances under enzymatic hydrolysis, so the antioxidant property of cheese during ripening is related to the degradation of casein (Chen et al., 2019). In a study on Cheddar cheese, when comparing the extent of proteolysis with antioxidant activity, it was found that the changes in antioxidant activity were very similar to the rate of soluble peptide formation (proteolysis) (Gupta et al., 2009). Finally, in their study on the antioxidant activity of milk and dairy products, Stobiecka et al. (2022) concluded that it is difficult to compare the results obtained with different spectrophotometric methods, as neither method is considered a reference method. It would therefore be useful to select a method that is as objective as possible to assess antioxidant capacity and apply it in laboratories worldwide.
The fatty acid profile identified 16 out of 17 saturated fatty acids (SFA) (Table 2) and 14 out of 16 unsaturated fatty acids (UFA) (Table 3) in 60-day ripened semi-hard goat cheeses depending on the ripening treatment. As commonly observed among products of animal origin, SFA predominated (Barać et al., 2018) over UFA (Fernandes et al., 2018).
There was a significant difference (p<0.01) in the SFA content between the group 1 (control group) and group 2 and between the two groups of cheeses that continued their ripening in oil (groups 2 and 3). The cheese from group 2 had the significantly lowest (p<0.01) SFA content (18.30 g/100 g) on day 60 of ripening compared to the control group (21.28 g/100 g) and group 3 (21.55 g/100 g). As previously mentioned, the control group and group 3 ripened longer in air than group 2, and according to the literature, the greatest moisture loss occurs during the first 20 days of ripening (Fresno and Álvarez, 2012; Álvarez and Fresno, 2021; Levak et al., 2023a). Group 2 was immersed in the oil mixture after 10 days of air ripening, so no major water loss or concentration effect occurred, as in the control group and the cheeses in group 3.
In all treatments, the highest content of individual SFAs was observed for palmitic acid (C16:0), stearic acid (C:18:0), myristic acid (C14:0), capric acid (C10:0) and lauric acid (C12:0), which are influenced by the feeding system of the goats (Tejo Cavalcanti et al., 2023). Excluding stearic acid, the same results were obtained in the study on the influence of red wine marination on the Coalho semi-hard goat cheese (Tejo Cavalcanti et al., 2023). The most abundant SFAs in Serrana goat cheese subjected to particularly long ripening were palmitic acid, stearic acid and capric acid, the latter being responsible for the specific odor of the goat cheese (Fernandes et al., 2018).
Table 2. Saturated fatty acid profile of a 60-day ripened semi-hard goat cheese (results are expressed as mean ± SE)
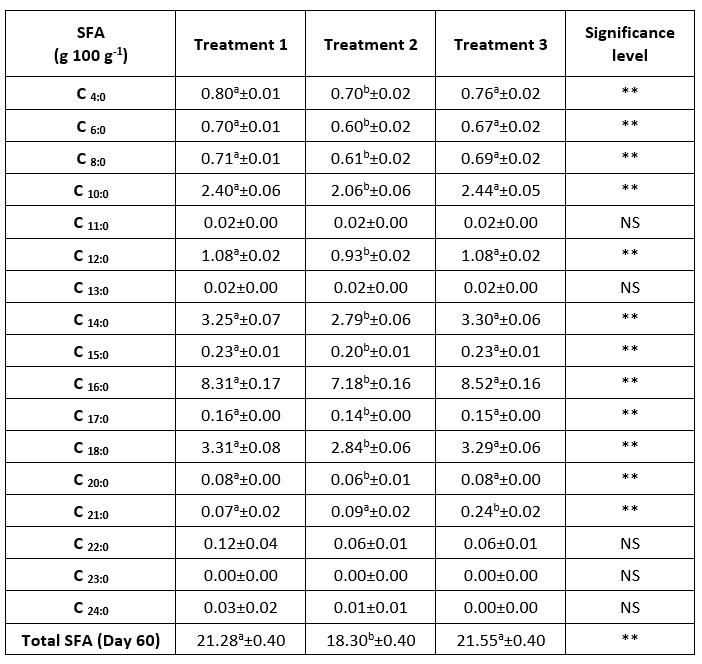
a,b Means in the same row marked with different letters differ significantly, ** (p<0.01), NS = no significant difference. SFA = Saturated fatty acids; Treatment 1 (Control group) = cheese ripening in the air (60 days); Treatment 2 = cheese ripening in oil after 10 days of ripening in air; Treatment 3 = cheese ripening in oil after 20 days of ripening in air. SE = standard error
A significant difference (p<0.01) in UFA content was found between groups 1 and 2, but also between groups 2 and 3 (Table 3). Oil-ripened cheeses (groups 2 and 3; 9.76 g/100g and 10.47 g/100g, respectively) had a lower UFA content than the control group (11.90 g/100g) on the 60th day of ripening. However, the longer air ripening increased the UFA content in the cheeses of group 3 due to the loss of moisture and the entry of oil into the cheese (Levak et al., 2023a), while group 2, in contrast, had a lower UFA content, which can be attributed to the moisture-protective role of the oil as a ripening medium (Levak et al., 2023a). All treatments showed that the most abundant MUFA was oleic acid (C18:1), and the predominant polyunsaturated fatty acid (PUFA) was linoleic acid (C18:2), which agrees with the results of the Coahlo cheese study (Tejo Cavalcanti et al., 2023). Group 3 had a significantly higher (p<0.01) oleic acid content than group 2, due to the prolonged air ripening, which leads to a higher dry matter content and the development of a cheese structure that allows oil to penetrate the cheese (Levak et al., 2023a), as well as oleic acid, the highest levels of which are found in olive oil (Granado-Casas and Mauricio, 2019; Kabaran, 2019).
Table 3. Unsaturated fatty acid profile of a 60-day ripened semi-hard goat cheese (results are expressed as mean ± SE)
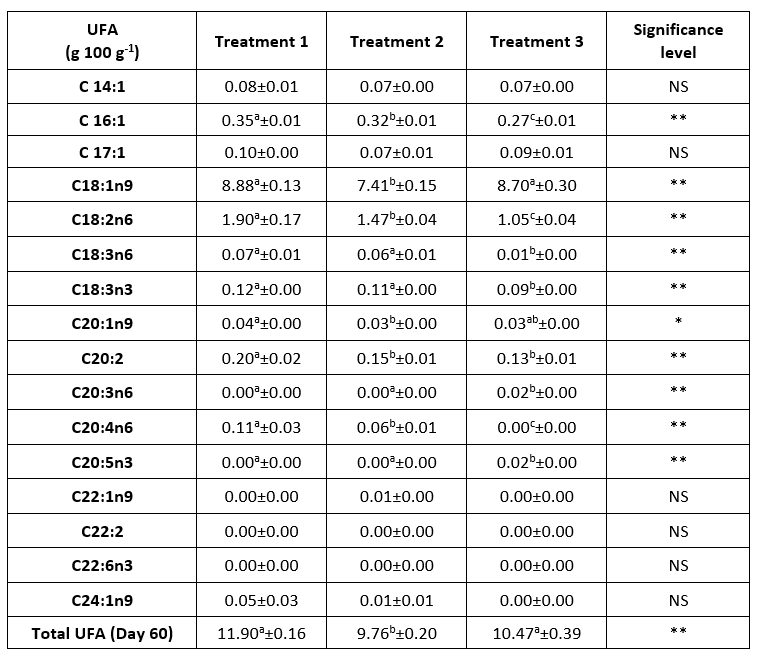
a,b,c Means in the same row marked with different letters differ significantly. * (p<0.05) ** (p<0.01). NS = no significant difference. UFA = Unsaturated fatty acids; Treatment 1 (Control group) = cheese ripening in the air (60 days); Treatment 2 = cheese ripening in oil after 10 days of ripening in air; Treatment 3 = cheese ripening in oil after 20 days of ripening in air. SE = standard error
The migration of fatty acids between cheese and oil matrix was confirmed by other authors (Klisović et al., 2022) and was supported by the increase in total fat, which is consistent with our results (Levak et al., 2023a). The mechanism of migration implies a diffusion between the oil and fat-rich phase (cheese) driven by triacylglycerol molecules. Since diffusion proceeds until equilibrium is established between the two phases, the above-mentioned peculiarity (Klisović et al., 2022) of the basic chemical composition of group 2 cheeses (high moisture content, lowest total fats) (Levak et al., 2023a) could contribute to a much faster establishment of thermodynamic equilibrium than in other groups. This could be the reason why the migration of fats between oil and matrix is limited in cheeses with shorter ripening in air (group 2) and faster in semi-hard cheeses with longer ripening in air (group 3). It therefore appears that the prolonged air ripening of the cheese in group 3 before immersion in the oil leads to a better nutritional value, which is reflected in a higher content of UFA and oleic acid compared to group 2.
Conclusions
The total polyphenols, flavonoids and antioxidant properties of semi-hard goat cheese were influenced by the ripening treatment. Our previous results showed that prolonged ripening in air before immersion in oil leads to a greater loss of moisture and the formation of a cheese structure that allows the oil to penetrate the cheese. As a result, these cheeses have a higher content of polyphenols and flavonoids and a higher antioxidant activity (DPPH) as well as a higher UFA content (including oleic acid and linoleic acid) at the end of the ripening. These results suggest that a longer maturation of the cheese in air before immersion in oil has a positive effect on the nutritional value of the cheese.
Acknowledgements and funding
This study was partially funded by the Support for basic financing of scientific and artistic activities of the University of Zagreb, Croatia.
References
https://doi.org/10.1016/j.foodres.2023.113158
https://doi.org/10.3390/foods11050701
Rodríguez-López, P., Lozano-Sanchez, J., Borrás-Linares, I., Emanuelli, T., Menéndez, J.A., Segura-Carretero, A. (2020): Structure–biological activity relationships of extra-virgin olive oil phenolic compounds: health properties and bioavailability. Antioxidants 9 (8), 1-17. https://doi.org/10.3390/antiox9080685
