Introduction
Nowadays, sheep and goat milk are highly used in the production of different dairy products, especially cheese and yoghurt, because of their high contents in fat and protein. Therefore, focusing on its safety and quality is highly recommended. Any changes in the milk composition have a detrimental effect on the quality of milk and its products. Therefore, the health status of the small ruminant udder is a serious issue (Novotna et al., 2018; Paskaš et al., 2020). Subclinical mastitis (SCM) is an intramammary infection, which is considered a significant health problem in small ruminants farming, with a financial loss accompanied by a decrease in milk production, a lowering in milk quality, and a high cost of therapy (Puggioni et al., 2020). Therefore, effective dealing with the SCM is essential to high-quality milk production. Constant management tactics depend upon an early and precise diagnosis of mastitis, particularly SCM (Zhong et al., 2018).
The determination of udder health is mainly performed by the apparent evaluation of selected udder morphological features such as udder depth, udder width, symmetry of the udder, degree of separation, degree of suspension, teat length, and teat angle (Akdag et al., 2018; Margatho et al., 2020). In addition, determination of protein, fat, lactose and solids not fat contents, and milk somatic cell count (MSCC) are used to assess the quality of the produced milk. All the previous parameters can be used as indicators of the status of an animal udder to detect the SCM (Akdag et al., 2018). The measurement of lactate dehydrogenase (LDH) enzyme is also used as a biomarker for intramammary inflammation (Viguier et al., 2009). LDH was reported to increase with an increase in SCC concerning SCM, as one of the fastest diagnostic methods (Seligsohn et al., 2021). However, Bagnicka et al. (2011), concluded that SCC could not be the definite, measurable test for SCM in goats and that more indicators are needed, as this is related to the apocrine secretion process.
S. aureus or coagulase-negative staphylococci (CNS) are the common pathogens distinguished as the causative agent of intramammary infection (IMI) in small ruminants (Gocmen et al., 2019). The early detection of SCM caused by staphylococci is a concern to prevent its progress into a clinical form that develops a tissue barrier that lowers the response to antibiotic therapy (Mahlangu et al., 2018; Zigo and Ondrasovicova, 2020). In addition, E. coli and streptococci are the most frequent microbial items included in the etiology of mastitis in small ruminants (McDougall et al., 2014). The rapid diagnosis of SCM is mainly performed with the California Mastitis Test (Tanni et al., 2021). Also, somatic cell count (SCC) can be quantitatively determined by a laboratory milk scan (Youssif et al., 2020; Darbaz et al., 2023). The legal limit established for the United States by the Food and Drug Administration (2011); for ewe and goat milk is 7.5×105 and 106 cells/ mL, respectively, and not more than 7.5×105 cells/mL according to Egyptian standard (ES: 154-1/2005).
The udder traits are an uncomplicated and a low-cost indicator for possible animal milk yield, which is known to have suitable ecological and genetic variations. These traits also appeared with the resistance of an animal to IMI (Szymanowska et al., 2010). A few studies proceeded to determine the relationship between SCM and the systemic response of animal bodies by assessing haematological parameters in goats (Hristov et al., 2018; Garba et al., 2019) and ewes (AL-Hadithy and Suleiman, 2014; Swiderek et al., 2016). There was a considerable difference in defensive leukocytic cells between animals with mastitis and those without was observed in haematological measurements (Alba et al., 2019).
Another fast diagnostic method for early diagnosis of SCM is the C-reactive protein (CRP) as a specific inflammatory biomarker in blood. It was reported that any affected inflamed tissue begins an acute phase reaction, which causes the synthesis of many proteins by the liver; that are released in the bloodstream are considered a positive acute-phase proteins (Iliev and Georgieva, 2018). These acute-phase proteins can be used as an indicator of the inflammatory progression in the mammary glands (Dimitrov et al., 2018). Several studies concluded that acute-phase proteins, including CRP in animals infected with mastitis, are reliable diagnostic tools in distinguishing between subclinical and clinical mastitis regardless of the etiological pathogens (Thomas et al., 2018).
The main aim of this study was to evaluate the prevalence of SCM and assess the correlation between the morphological udder traits, milk chemical composition with SCM, and its causative agents of intramammary infections. The measurement of haematological parameters and the C-reactive protein was used to evaluate their values in SCM small ruminant's diagnosis.
Materials and methods
Illustration of the risk factors associated with the investigated farm
The study was conducted on fifteen apparently healthy adult Egyptian Shami goats and twenty-four sheep, Rahmani breed from Damanhur, northeast of Behera Governorate. They were fed with a commercially balanced ration and potable water (common trough) and kept in a standing position on the slope-free ground to evaluate udder conformation. The environmental temperature and humidity were determined by a digital thermometer at the measurement time. The mean air temperature and humidity during January 2022 were 17 °C and 70.0 %, respectively.
The information was collated with the aid of farm leaders and workers as follows: breed of both species as mentioned before. The land type was sand. The average age was 2.5 years for sheep and 3 years for goats. The average body weight was 35-40 kg (does) and 50-55 kg (ewes). The animals were in the mid-lactation period with no automatic milking. In addition, pest control was applied. There was no application of artificial insemination or oxytocin. During the study, standard sanitary operating procedures were applied. Approval of the ethical committee was done with certificate number (Vet-CU 03162023680), Cairo University, Faculty of Veterinary Medicine.
Measurements of the udder and teat parameters
The physical udder dimensions were evaluated on a 5-score scale based on Novotna et al. (2018); Margatho et al. (2020). The rear udder attachment was evaluated by its width and the degree of udder occupied by the area supplied by the posterior legs. The assessment was made from the rear using a five-score scale: 1. very wide attachment, the room provided by the legs filled; 2. wide attachment, the room provided by the legs nearly filled; 3. intermediate attachment, room enough for the udder; 4. weaker attachment, droopy udder; 5. very weak attachment, baggy udder, skin folds. Udder symmetry (US) was estimated (symmetrical, moderate, and asymmetric), degree separation (slight, moderate, and severe), and a degree of suspension (extremely, intermediate, pendulous, and extremely loose). The udder cleft was evaluated by the inter-mammary channel depth. It was evaluated from the rear on a 5-score scale: 1. clear udder cleft and suspensory ligament; 2. less pronounced udder cleft, pronounced suspensory ligament; 3. perceptible suspensory ligament, clear udder cleft; 4. unclear udder cleft; 5. baggy suspensory ligament, udder portion is under the teat level.
Udder depth (UD) and udder width (UW) were evaluated from the rear with a ruler to the closer 1 cm. UD was measured from the upper edge of the udder to the lowest point of the udder. The UW was measured at the widest part of the udder. The teat length (TL) was measured from the rear with a ruler to the closer 1 cm. TL was measured from the base to the tip of the teat. The teat placement was evaluated from the rear on a 5-score scale: 1-nearly perpendicular teat location, teats placed on the udder bottom, 2-teats pointing moderately sideways, placed on the lower edge of the udder, 3-the teat angle is approximately 45° from the intermammary groove, 4-teats are placed on the udder sides, 5-teats point horizontally located over the udder flanks. In addition, the evaluation of the teat shape was as; a funnel, bottle, and teat angle (orientation) as; 160⁰-180⁰, 120⁰-160⁰, and 90⁰-120⁰ degrees and assessed with MSCC, while the teat tip end shape was categorized as follows: pointed, round, and flat.
Determination of compositional parameters, somatic cells, and LDH in the investigated milk samples
After the first stream of milk was discarded, each goat and sheep half-milk sample was collected aseptically, after the end of the teats was sterilized with a swab of alcohol 70.0 %. About 20 mL of milk from each half were collected in sterile test tubes for further examination. 30 -goat milk and 48 sheep milk samples were immediately placed into the ice box for transport to the laboratory. The percentage of fat, protein, lactose, solids not fat, and salts were determined using a milk analyser (Lactoscan SH, Milkotronics, Bulgaria). The SCM was preferably determined using the California Mastitis Test (CMT) for each sample from both halves separately based on Nesma et al. (2020), then MSCC was measured automatically using Bentley 150 milk scan as the SCM was recorded on both udder halves and animal levels based on CMT scores as well as MSCC. The +2 and +3 CMT results are only considered an SCM case based on Kandeel et al. (2018). At the animal level, the udder was determined as infected with SCM if at least one-half was affected. The milk from all samples was centrifuged at 12000 RPM/ 30 min to separate the milk serum. The activity of LDH in all half samples of milk serum was determined by the colorimetric method based on Babaei et al. (2007).
Bacteriological examination of milk samples
For bacterial isolation and identification, each milk sample was cultivated on blood agar (Oxoid, CM0055), which was prepared with 5.0 % sheep blood, Baird-Parker (Oxoid, CM1127), Eosin Methylene Blue agar (Oxoid, CM0069) and Edwards media (Oxoid, CM0027). Then all plates were incubated aerobically at 37°C and examined after 48 h. All colonies were purified and identified biochemically using several tests such as Gram’s stain, coagulase, catalase, thermonuclease (TNase), mannitol fermentation, indole, Vogues Proskauer, urease test, citrate, methyl red, triple sugar iron, and CAMP test (Da Silva et al., 2018).
Measurements of hemogram, leukogram, and C-reactive protein
Three mL of whole blood samples were collected on an ethylenediaminetetraacetic acid (EDTA) tube (Greiner Bio-One, North America, Inc.) from the jugular vein for estimation of haematological parameters, including packed cell volume (PCV), haemoglobin (Hb), red blood corpuscles (RBCs) count, total leucocyte count (TLC), differential leucocyte count, and platelets using the Diatron haematology analyser, USA. For CRP estimation, 3 mL of blood samples were collected in plain tubes to separate clear non-haemolysed serum. Sera were analysed calorimetrically using a specific ELISA kit produced by Sigma-Aldrich. The kit was used according to the manufacturer’s instructions.
Statistical analysis
The estimated parameters for each milk species were demonstrated as mean±standard error. Data were analysed using the SPSS program v. 25 at a 95.0 % Confidence Interval. The T-test applied to calculate the p-value for significant differences between positive and negative SCM for measured parameters of each animal species. The one-way ANOVA was used to calculate the p-value for significant differences between different CMT degrees of each milk species. The correlation coefficient (r) number was calculated between the measured variables with each other in the SCM animals to investigate the possibility of a significant association between them. The significance statistical p-value was less than 0.05.
Results and discussion
The study was performed on 39 animals; 15 goats and 24 sheep. According to the CMT represented eight halves of goats with +2 CMT and SCC mean of 1243.63±96.30×103/ mL, while thirteen sheep udder halves were in a percentage of 16.66 and 10.42 for +2 and +3 CMT with SCC mean of 852.88±11.99 and 1342.80± 79.01×103/mL, respectively. Only 3 ewes were infected with SCM in both udder halves. Concurrently, the SCM was determined at the animal and half levels (Tables 1 and 2).
Table 1. Incidence of SCM in dairy small ruminants according to results of CMT scores
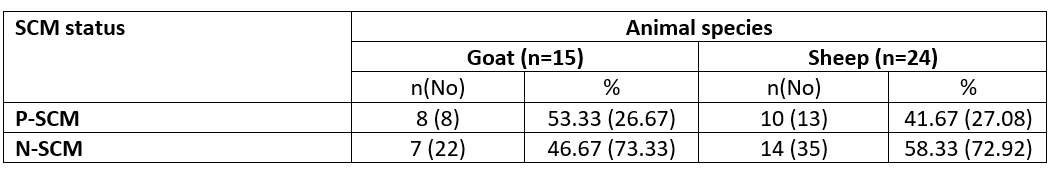
n= number of examined animals; No= number of udder half samples; P= Positive SCM samples; N= Negative SCM samples
Table 2. Categorization of animals' milk according to results of CMT and in relation to their MSCC based on half-udder infection
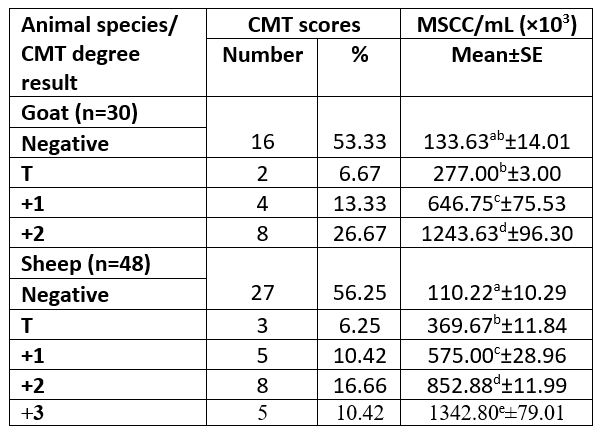
n= number of examined samples; T= traces; MSCC= milk somatic cell count; ±SE= standard error.
For goat milk, ab, b - b, c only superscript letters in the same column indicate non-significant difference (p>0.05).
For sheep milk, different superscript letters in the same column indicate significant difference (p<0.05).
As presented in Table 3, there was a significant difference (p<0.05) for each species between their udder depth, teat length, and teat tip shape with positive and negative SCM. In addition to udder symmetry, udder cleft, teat angle, degree of suspension, and a degree of separation in goats only. In Table 4, goat udder traits showed a correlation to SCC, such as the asymmetrical udder, high depth, very weak attachment, pendulous, moderate, and severe degree of separation for SCM. All these features indicated the goat SCM with high SCC. In addition, SCC was correlated to a low udder width, teat length with flat teat end, and wide angle 180 degrees. There was a significantly positive relation between the SCC and the asymmetrical udder and high udder depth in sheep. The chemical parameters showed a significant difference (p<0.05) between animal udders with SCM for protein concentration, SNF%, and lactate dehydrogenase, in addition to a percentage of fat and salt in sheep and lactose in goats (Table 5).
The high SCC was correlated with the LDH enzyme in both species. However, sheep milk samples were positively correlated to salt content, but negatively correlated to fat and lactose content (Tables 6 and 7).
Table 3. Descriptive statistics of the udder morphological traits of examined animals according to SCM status
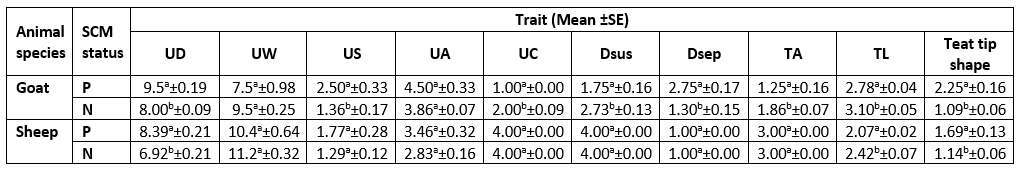
UD= Udder Depth; UW= Udder Width; US= Udder Symmetry; UA= Udder Attachment; UC= Udder Cleft; Dsus= Degree of Suspension; Dsep= Degree of Separation; TA= Teat Angle; TL= Teat Length.
P= Positive SCM samples; N= Negative SCM samples
For each milk species, different superscript letters in the same column indicate significant difference (p<0.05).
Table 4. Correlation between SCC and mammary anatomical features in SCM milk samples
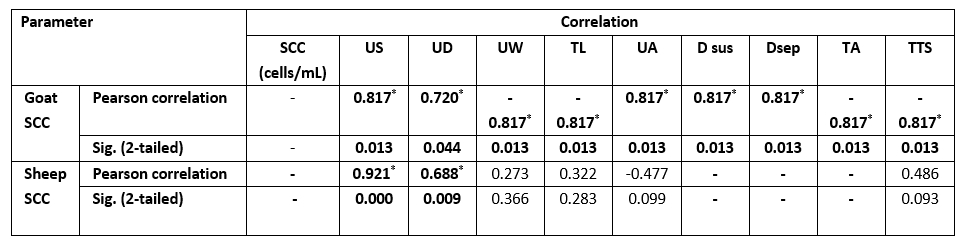
Values equal to correlation coefficient (r); - = non-detected correlation; *Correlation with asterisk is only significant at the 0.05 level.
Table 5. Descriptive statistics of milk chemical properties and LDH concentration according to SCM status
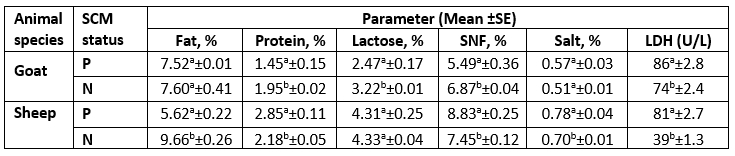
LDH= Lactate Dehydrogenase; Units per Liter (U/L); SNF= Solids Not Fat; P= Positive SCM samples; N= Negative SCM samples.
For each milk species, different superscript letters in the same column indicate significant difference (p<0.05).
Table 6. Correlation between SCC and chemical parameters in the examined SCM goat milk samples
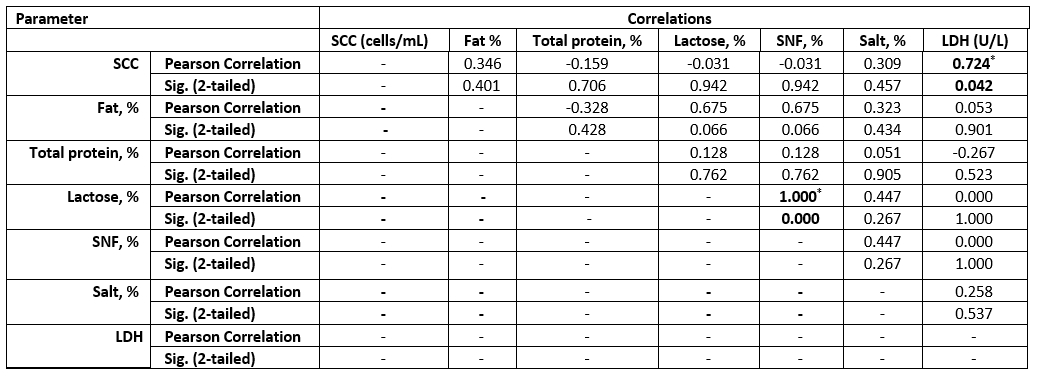
Values equal to Correlation Coefficient (r). *Correlation with asterisk is only significant at the 0.05 level (2-tailed).
Table 7. Correlation between SCC and chemical parameters in the examined SCM sheep milk samples
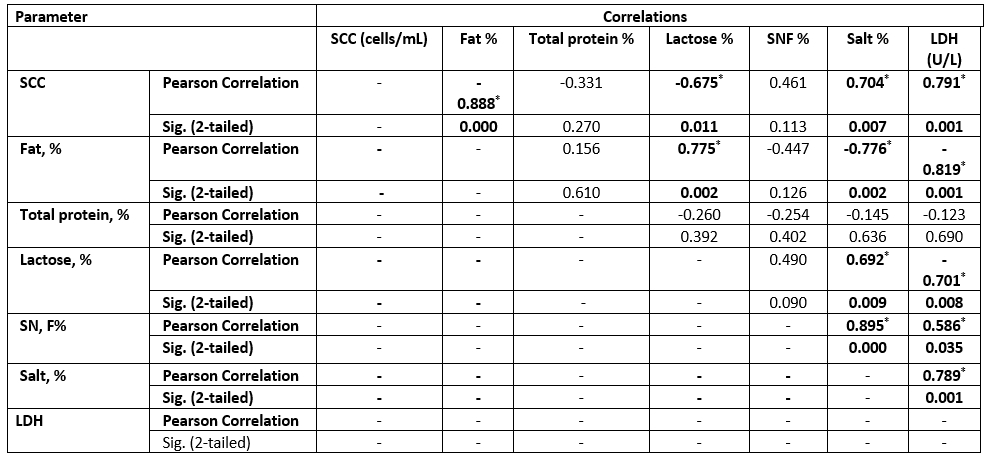
Values equal to Correlation Coefficient (r); *Correlation with asterisk is only significant at the 0.05 level (2-tailed).
In Table 8, 12 udder halves of goats and 18 sheep were infected with bacterial pathogens resulting from CMT grades (+1, +2, and +3); the samples were classified based on bacterial infection. Dairy goats were classified into two groups; S. aureus + Streptococcus spp. (n=4) and S. aureus (n=8). The mammary gland infection with S. aureus was correlated to low fat, lactose, and SNF content, low udder width, while it was correlated positively with high LDH enzyme and very weak udder attachment. However, dairy ewes were divided into five groups CNS (n=7), S. aureus + E. coli (n=3), S. aureus (n=3), CNS + Streptococcus spp. (n=3), and Streptococcus spp. (n=2).
Table 8. Correlation between SCC and LDH, chemical parameters, and mammary anatomical features based on isolated bacteria from each milk species
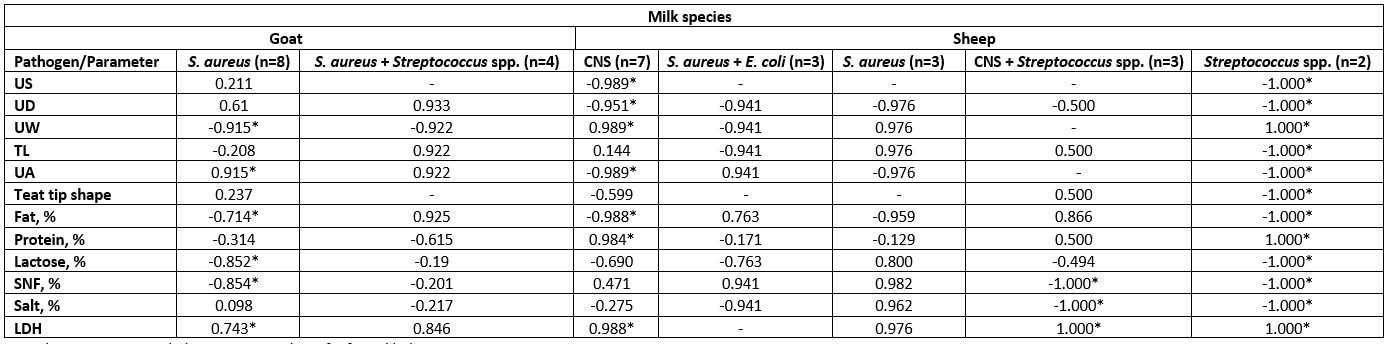
CNS= Coagulase Negative Staphylococci; n= number of infected halves.
Values equal to Correlation Coefficient (r); - = non-detected correlation; *Asterisk only significant difference (p< 0.05).
Table 9. Descriptive statistics of the hemogram, leukogram, and C-reactive protein in examined sheep and goats according to SCM status
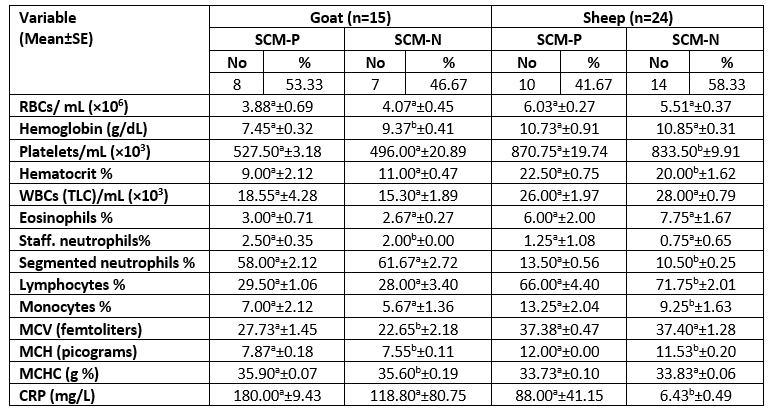
P=Positive; N=Negative; No=number of examined animals; g/dL=grams per deciliter; RBCs=Red Blood Cells; WBCs=White Blood Cells; TLC=Total Leucocytic Count; MCV=Mean Corpuscular Volume; MCH=Mean Corpuscular Hemoglobin; MCHC=Mean Corpuscular Hemoglobin Concentration; CRP=C-reactive protein.
For each milk species, different superscript letters in the same row indicate significant difference (p<0.05).
The means of RBCs, WBCs, and platelet count in SCM and healthy ewes were 6.03±0.27×106, 26.00±1.97×103, 870.75±19.74×103 and 5.51±0.37×106, 28.00±0.79×103, 833.50±9.91×103, respectively. While in goats with SCM and healthy were as follows 3.88±0.69×106, 18.55±4.28×103, 527.50±3.18×103 and 4.07±0.45×106, 15.30±1.89×103, 496.00±20.89×103, respectively (Table 9).
The total number of animals infected with SCM was 10 ewes and 8 goats with a percentage of 41.67 and 53.33, respectively. These results indicated a higher incidence of SCM in goats than in sheep, as previously recorded by El-Zamkan and Mohamed (2021).
The MSCC exhibited a significant difference (p<0.05) between all samples categorized based on CMT scores in sheep milk samples. The +2 showed a significant difference with other CMT degrees and +1 with negative CMT in goat milk samples. The occurrence of high somatic cells is known to be unfavourable as it's associated with inflammation of the mammary gland, lowering milk production and causing alteration in milk constituents, which concerning to changes in final dairy product characteristics (Sharma et al., 2011).
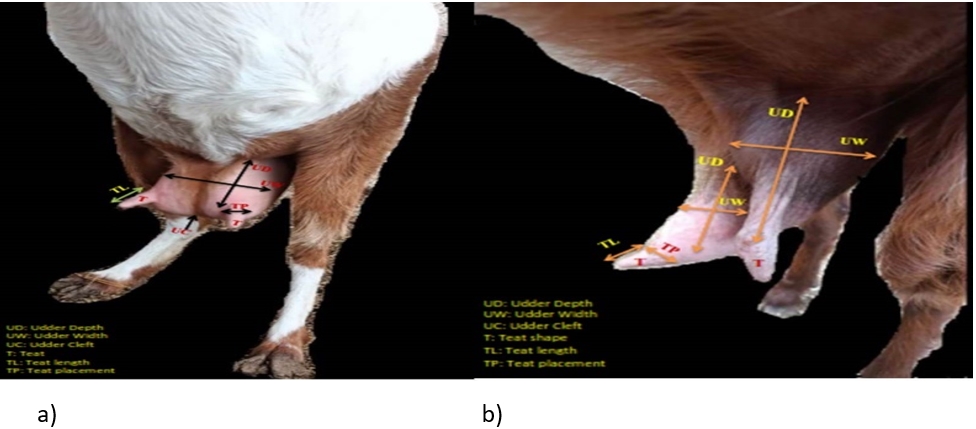
Figure 1. A photograph showing: a) symmetrical udder, b) asymmetrical udder with vertical teat
The difference between udder symmetry is presented in Fig. 1. According to the obtained results, the gross anatomical investigation revealed that the udder of sheep and goats was located in the inguinal region. It was divided into two halves by a well-defined median inter-mammary groove and suspended in the ventral abdominal wall and pelvic floor through the medial and lateral suspensory ligaments. Following Margatho et al. (2020), the degree of suspension, separation degree of the udder, and presence of a pendulous udder are IMI indicators due to their tendencies for injury and contamination. The greater depth of the udder is related to an increased risk of SCM with more probability of udder injury or contamination (Singh et al., 2014; Bharti et al., 2015).
The measurements of the udder and teat were done to help in the rapid diagnosis of SCM. The study of mammary gland traits and their relation to SCM with SCC may help in the incorporation of breeding programs to choose herds more resistant to SCM (Margatho et al., 2020). As Margatho et al. (2020) previously noted, the higher SCC in caprine milk is associated with severe halves separation, extremely loose, and very weak attachment. These proved that the previous parameters are a newly beneficial tool for IMI diagnosis. Another study by Novotna et al. (2018) confirmed that the animal with a deeper udder has an elevated SCC, as high udder depth is combined with high SCC. In sheep, the SCC was significantly (p<0.05) positively related to the asymmetrical udder and high udder depth only.
The SCM was mainly associated with alteration in milk chemical composition that might be attributed to destruction in the mammary gland or migration of milk constituents into perivascular space out from the lumen of the alveoli (Paixão et al., 2017). The primary factors influencing the SNF content were lactose and protein levels, which were shown to be lower in does with SCM because of udder injury, but higher in ewes because of high whey protein content, which made up for the casein loss. The increase in salt is mainly attributed to the inflammation that influenced the damage in epithelial tissue, causing an increase in permeability with alteration in electrolyte percentages and the migration of sodium ions into the udder (Youssif et al., 2021).
Gelasakis et al. (2018) reported a significant difference (p<0.001) in milk fat, protein, and lactose among milk samples of healthy goats and others with SCM. The modification in milk composition is related to high levels of SCC due to a reduction in the ability of nutrient synthesis in the mammary gland tissue (Lindmark-Månsson et al., 2006). More studies reported that fat and protein contents varied among mastitis and healthy udders with a decrease in lactose content (Akdag et al., 2018). The milk protein content in ewes with SCM was higher than in healthy animals. The increase in total protein is mainly influenced by increasing whey proteins due to the possible passage of serum proteins into milk (Sutera et al., 2018).
The LDH (U/L) was high in SCM animals with a mean of 86±2.8 in does and 81±2.7 in ewes and a significant correlation in both species for MSCC. As presented in Table 7, the LDH was positively correlated to the SCC and salt content but negatively correlated to the fat and lactose content. Following our results, the LDH is a trusted method for the detection of SCM, as mentioned by Sani et al. (2018). As the LDH is suggested to be produced from high leukocyte cells and the destructed epithelial cells of the infected udder half with exhibited a positive correlation to SCC, this parameter may be helpful for early detection of SCM (Klein et al., 2020).
The MSCC and the pathogenic bacteria are considered the main standards for indicating the udder health of small ruminants (Klein et al., 2020). However, dairy ewes were divided into 5 groups: CNS (n=7), S. aureus+ E. coli (n=3), S. aureus (n=3), CNS+ Streptococcus spp. (n=3) and Streptococcus spp. (n=2). The infection with coagulase-negative staphylococci and Streptococcus spp. showed a positive correlation (p<0.05) to the asymmetrical udder, udder depth, udder width, baggy udder (very weak attachment), protein content, and LDH level, but a negative correlation (p<0.05) to fat content. Although infection with Streptococcus spp. exhibited the highest significant (p<0.05) correlations, it was related to low teat length, low lactose, SNF, and salt percentage. When the sheep udder was infected with both CNS and Streptococcus spp., it was found to be significantly (p<0.05) correlated with high LDH, low SNF, and salt percentage. The teat tip shape was significantly negatively correlated in the Streptococcus spp. group (Table 8). In addition, the LDH showed a significant (p<0.05) positive correlation with CNS, Streptococcus spp., and S. aureus-identified bacteria in dairy goats and ewes. In agreement with Klein et al. (2020), LDH could be used for the diagnosis of SCM, even in the absence of SCC elevation.
The most prevalent identified pathogen was S. aureus in goats and CNS in sheep, with a higher frequency of mixed infection in sheep than in goats. The results were similar to data reported by Abdallah et al. (2018) and Udoh et al. (2019), who recorded that S. aureus was the maximum isolated bacteria from infected does, while Zigo and Ondrasovicova (2020) declared that the most isolated microorganism from mastitis ewes’ milk was CNS. Staphylococci are usually found inside the mammary glands, the skin of the udder, in the teat canal, and are conveyed via low hygienic and improper milking procedures (Mahlangu et al., 2018). The increased incidence of S. aureus in goats was most probably related to the use of hand milking that was attributed to the re-infection from teat injuries, as reported by Khaled et al. (2015).
In contrast to findings of Margatho et al. (2020), the teat shape and angle were not significantly correlated with the infected bacteria. The decrease in milk fat associated with pathogen infection and IMI is mainly linked to impairment of excretion and functionality of cells in the mammary gland due to elevation of plasmin content. Several studies proved the reduction in milk lactose content was mainly related not only to the SCM status of the animal but to the type of bacteria, which is responsible for IMI by the ability to ferment the lactose (Gelasakis et al., 2018).
The routine analysis of blood and serum parameters is pivotal for the observation and control of small ruminant health, which is used as an indicator for SCM (Siddiqe et al., 2015). In the current study, the complete blood picture and CRP were determined in order to investigate whether these diagnostic parameters could be helpful in the early diagnosis of SCM. Only platelet count/ mL in SCM-free ewes was lower than in SCM ewes with a statistically significant difference (p<0.05) similar to results reported by AL-Hadithy and Suleiman (2014).
The haematological results demonstrated a non-significant difference (p>0.05) for RBCs, platelets, WBCs, and lymphocytes percentage in does with or without IMI, similar to results obtained by Garba et al. (2019). While the total leukocyte count in dairy does show an increase along with the high SCC in SCM that was also related to a slight increase in staff neutrophils, lymphocytes, monocytes, and eosinophils percentage. In agreement with Bagnicka et al. (2011), who reported that depending on SCC alone couldn’t be effective, as it is known to increase also related to the stage of lactation. However, WBCs associated, especially with neutrophils, monocytes, and eosinophils percentage was elevated in SCM related to bacterial pathogens.
The mean value of Mean Corpuscular Haemoglobin (MCH) was 12.00 ±0.00 in SCM ewes; and 7.87±0.18 in SCM does, as both species exhibited values higher than those of SCM-free animals, with a statistically significant (p<0.05) similar to result obtained by Hristov et al. (2018); Garba et al. (2019). In addition, the Hb was minimum in SCM does than in SCM-free animals, with a mean value of 7.45±0.32 and 9.37±0.41 (g/dL), respectively, as the difference was statistically significant (p<0.05) almost similar to AL-Hadithy and Suleiman (2014), Hristov et al. (2018). The reduction in goats’ blood Hb and a slight decrease in RBCs were probably related to haemolysin produced by S. aureus, which was responsible for the SCM content (66.67 %), as reported by AL-Hadithy and Suleiman (2014).
There was a significant difference (p<0.05) in amounts (%) of lymphocytes, segmented neutrophils and monocytes between the SCM-free ewes and others with SCM, which was specified with lower lymphocytes (%) and higher neutrophils and monocytes (%) compared to SCM-free animal. Swiderek et al. (2016) reported similar findings, demonstrating that the SCM and high SCC were associated to high neutrophils and low lymphocytes (%). The increase in neutrophils and monocytes percentage with a slight decrease in the concentration of lymphocytes could be attributed to an early response to inflammation, as stated by Swiderek et al. (2016). The diversity in parameters might refer to a type of feeding program, variable physiological conditions, and genetic factors (Badawi and AL-Hadithy, 2014), as well as the present study, which suggested that depending on the type of pathogen.
Serum CRP is one of the most rapidly reacting acute phase proteins (Zeng et al., 2021). It is primarily induced by interleukin-1, and the CRP concentration increases within four hours after stimulation (Kuzi et al., 2020; Xia et al., 2021). The C-reactive protein (mg/L) was significantly (p<0.05) higher in SCM ewes than in SCM-free ones in mean values of 88.00 ±41.15 and 6.43 ±0.49, respectively. It has been reported that CRP is a biomarker for assessing the health status of a herd and should be considered a criterion to assess the stress levels within dairy ewes herds (Lee et al., 2003).
Conclusions
Besides using the traditional methods (CMT and SCC) for the SCM detection in small ruminants, the determination of milk composition adds value, as SCM is significantly correlated to low fat and lactose contents and high salt contents and LDH. The does with an asymmetrical pendulous udder with weak attachment, high depth, and severe separation indicated an increased probability of SCM. This finding may help in choosing the appropriate new dairy goat or early excluding the diseased one. However, ewes are only identified by their asymmetrical udder and high depth. Serum CRP can be used as a helpful diagnostic biomarker for the early detection of SCM in sheep. All of the mentioned tools may be used to rapidly indicate the suspicion of an infection in dairy animals. In addition, the udder traits are considered a cheap method, which is highly successful for does. Finally, a preliminary diagnosis of SCM may reduce the possibility of clinical mastitis occurrence, reduce the cost of treatment, and prevent farm damage.
References
https://doi.org/10.56093/ijans.v88i10.84160
AL-Hadithy, H.A.H., Suleiman, J.M. (2014): The hematological parameters in clinically normal lactating and ewes affected with mastitis. Kufa Journal for Veterinary Medical Sciences 5(2), 46-54.https://doi.org/10.36326/kjvs/2014/v5i24201
https://doi.org/10.1016/j.smallrumres.2011.04.014
Margatho, G., Quintas, H., Rodríguez-Estévez, V., Simões, J. (2020): Udder morphometry and its relationship with intramammary infections and somatic cell count in Serrana goats. Animals 10 (9), 1534.http:// doi.org/10.3390/ani10091534
McDougall, S., Malcolm, D., Prosser, C. (2014): Prevalence and incidence of intramammary infections in lactating dairy goats. New Zealand Veterinary Journal 62 (3), 136-145.http://doi.org/10.1080/00480169.2013.865294
