Introduction
2-hydroxybenzoic acid, also known as salicylic acid (SA) [1], has played a very important role in daily human life from the past to the present. Due to its antibacterial properties, SA has been used in many fields, such as in the food industries and agriculture. SA is used to control plant diseases and prolong the spoilage of agricultural products. It is also used as a preservative in fermented food [2]. Additionally, in cosmetic and pharmaceutical industries, SA is an ingredient in creams or serums for treating acne or wart, including various skin medicines because of its antiseptic and anti-inflammatory properties [3,4]. Although SA is enormously useful, it is also dangerous for humans. Given high doses of SA, fever, difficulty breathing, cancer, tinnitus and possibly death are detected [2].
Consequently, the analysis of SA content has attracted great interest from scientists. The literature has shown that there are several popular techniques for SA analysis, such as high-performance liquid chromatography, UV-visible spectrophotometry and fluorescence spectroscopy [5-7]. Nonetheless, the above-mentioned techniques, even with high accuracy and precision, have limitations on the size of the tool, high cost and require experts to analyse. One of the most popular techniques used for SA analysis is various modes of electrochemical techniques. They are quick techniques with the potential to fabricate a small-size device. The improvement of these analytical electrochemical techniques is mostly a modification of the working electrodes to improve sensitivity and selectivity.
Currently, nanoparticles have received a lot of attention for their good catalytic properties. These can conduct electricity and improve the surface of the working electrode, causing to increase the analytical efficiency even higher [8,9]. One of the nanoparticles that are very popularly used in analytical electrochemical techniques is magnetite nanoparticles (Fe3O4) [10,13]. Particularly, the magnetite nanoparticles were combined with materials such as gold nanoparticles, carbon nanotubes and reduced graphene oxide, including chitosan [14-16]. The surface of the magnetite nanoparticles was enhanced with chitosan to enhance the analytical efficiency due to adsorption between positively charged chitosan and negatively charged species [17], like SA in this work. Hence, the notable aim of this work is to use the magnetite nanoparticles coated with chitosan (Chitosan@Fe3O4/CPE) as the working electrode to enhance the analytical efficiency [19] for SA determination in wart treatment pharmaceuticals.
Experimental
Reagents and materials
Distilled water (DI water) was used throughout all experiments from a Milli-Q Plus System (Millipore). All chemicals used in this work were analytical-grade reagents (AR-grade). Salicylic acid (C7H6O3) was purchased from Rankem (India). Potassium dihydrogen phosphate (KH2PO4), acetone (C3H6O) and 99 % ethanol (C2H5OH) from QRËC™ (New Zealand) were obtained. Graphite powder (≤20 μm powder) and Whatman™ qualitative filter paper, grade 1 from Sigma-Aldrich (Island) and paraffin oil from Fisher Scientific (USA) were used for the preparation of the proposed electrode.
Fabrication of the electrode
The fabrication procedure of the carbon paste electrode modified with chitosan@Fe3O4 in this work was adopted from [18]. Briefly, it was prepared by weighing 0.0800 g of the graphite powder and 0.0100 g of chitosan@Fe3O4. Then 1.0 mL acetone was added into the mixture and sonicated using an ultrasonic homogenizer (ZEALWAY/China) for 30 min. It became the carbon paste modified with chitosan@Fe3O4. After that, 50 μL paraffin oil was added to the paste and mixed up. The paste was compressed into an electrode mole (BAS/Japan) until tight. The carbon paste electrode modified with chitosan@Fe3O4 was polished using WhatmanTM qualitative filter paper grade 1 to smooth the electrode surface.
Apparatus
The potentiostat (Autolab PGSTAT204, Metrohm, Netherlands) were used for all electrochemical experiment. A conventional three-electrode system: chitosan@Fe3O4 modified CPE (chitosan@Fe3O4/CPE), Ag/AgCl (in 3 M KCl), platinum wire as a working electrode, a reference electrode and an auxiliary electrode were utilized. For the standard method, a specord 210 plus UV-Visible spectrophotometer (Analytica Jana/ Germany) was used.
Electrochemical methods
The square wave voltammetry (SWV) was carried out in a 0.1 M phosphate buffer solution containing pH 5. Scan potential 0.2 to 1.4 V, frequency 50 Hz, amplitude 50 mV/s, step potential 10 mV/s, and pre-concentration step at potential 0.2 V for 30 s were used as voltammetric parameters. The results of the study of the optimum conditions for SWV parameters are shown inFigure S1 in the Supplementary document. The electrochemical impedance spectra (EIS) were created using a frequency range of 0.1 Hz to 10 kHz and an amplitude of 10 mV/s at a voltage of 0.2 V.
Sample analysis
Generally, SA is one of the important ingredients in medicine for treating warts. Then, wart medicine was selected as a sample in this study. These samples are usually paste-like. Thus, all these experiments were treated the same way as in SA solutions. In this work, the standard addition was selected to determine SA in all samples. Briefly, the samples were diluted 200 times with 70 %v/v ethanol to determine the amount of SA using the square wave voltammetry technique.
Results and discussion
Electrochemical response of SA on chitosan@Fe3O4/CPE
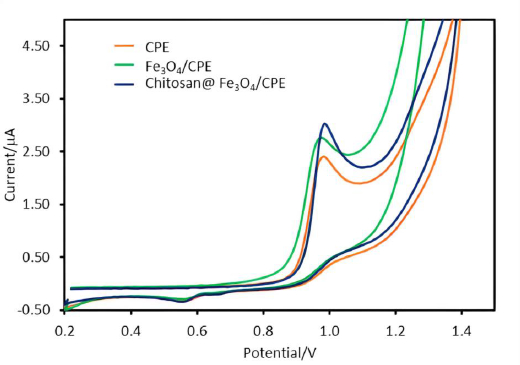
The responses of SA on various electrodes: bare CPE, Fe3O4 modified carbon paste electrode (Fe3O4/CPE) and chitosan-coated on Fe3O4 modified CPE (chitosan@Fe3O4/CPE) were studied. The results demonstrate that the oxidation current of SA detected by chitosan@Fe3O4/CPE (I = 3.0 μA) gave higher currents than on the bare CPE (I = 2.4 μA) and Fe3O4/CPE (I = 2.7 μA) as shown inFigure 1. It was observed that the nanoparticles-modified CPE showed higher currents compared to bared CPE due to the high surface area from the nanoparticles [10]. The surface area of the modified (chitosan@Fe3O4/CPE) and unmodified electrodes (bare CPE) was calculated according to the Randles-Ševčík equation [20]. It was found that the electroactive surface area of 30.11 mm2 and 24.09 mm2 for chitosan@Fe3O4/CPE and bared CPE, respectively. Additionally, another reason for the higher observed current from chitosan@Fe3O4/CPE may be the attraction between the protonated amine group of chitosan coating on the Fe3O4 and SA, which leads to the enhancement of response currents according to our previous work [18]. The proposed mechanism between SA and electrode surface is shown inFigure S2 (supplementary).
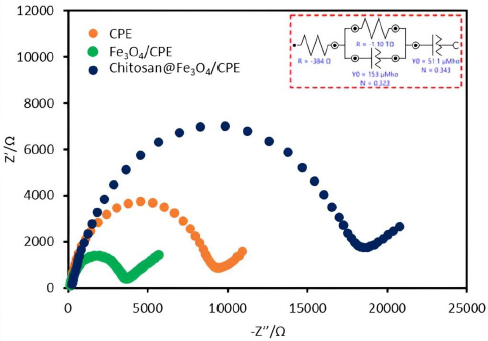
Additionally, the electrochemical impedance spectroscopy (EIS) was used for the investigation of the proposed electrode surface. EIS data can be represented by a Nyquist diagram from which some conclusions about the interface properties of the electrode can be drawn. Generally, the frequency dependence of EIS can be divided into two regions, semi-circular and straight-line. The semi-circular sections show the resistances of the electrodes. In the case of a narrow semicircle, the resistance is small or there is good electrical conductivity. On the other hand, a wide semicircle indicates a large electrical resistance or lower conductivity. The straight-line section depicted the diffusion process of the electrodes [21,22]. EIS data of 0.5 mM Fe(II) in 0.1 M KNO3 were obtained for three various types of electrodes: bare CPE (orange line), Fe3O4/CPE (green line) and chitosan@Fe3O4/CPE (blue line) (Figure 2). From the results, it can be observed that the smallest semicircle was obtained for Fe3O4/CPE electrode. This is because of the good electrical conductivity and fast electron transfer rates at Fe3O4 nanoparticles. Although the chitosan@Fe3O4/CPE similarly contains Fe3O4 nanoparticles, the surface of these particles is encapsulated with chitosan. Therefore, the chitosan@Fe3O4/CPE shows the wider semicircle part in Nyquist diagrams compared to the Fe3O4/CPE. However the oxidation current of SA detected with chitosan@Fe3O4/CPE illustrated the highest response (Figure 1), which may help confirm the attraction between the protonated amine group of chitosan favours the electrochemical reaction.
Electrochemical behaviour
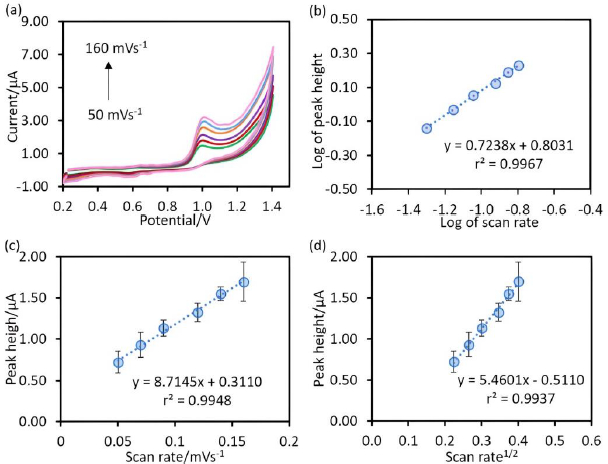
The behaviour of the chitosan@Fe3O4/CPE as the working electrode was studied using cyclic voltammetry at various scan rates (50-160 mV/s) (Figure 3(a)). The results shown inFigure 3 were plotted as follows:Figure 3(b) is the plot between the logarithm of peak current (y-axis) and logarithm of scan rate (x-axis) to study the mixed behaviour (adsorption and diffusion),Figure 3(c) is the plot between the peak current (y-axis) and the scan rate (x-axis) to study adsorption process andFigure 3(d) is the plot between the peak current (y-axis) and square root of scan rate (x-axis) to study diffusion process [23]. As considering the slope value of the plot inFigure 3(b), this value (0.7238) was greater than 0.5 but less than 1.0, indicating that charge transfer is under mixed control according to Wyantuti, Hartati, Panatarani & Tjokronegoro [24]. Additionally, to confirm the behaviour of the electrode, the relative coefficients ofFigure 3(c) andFigure 3(d) were compared. It was found that the relative coefficients of bothFigure 3(c) andFigure 3(d) were close to 1, indicating that the electron transfer at the developed electrodes is under mixed control, which is consistent with the above [24].
Effect of pH
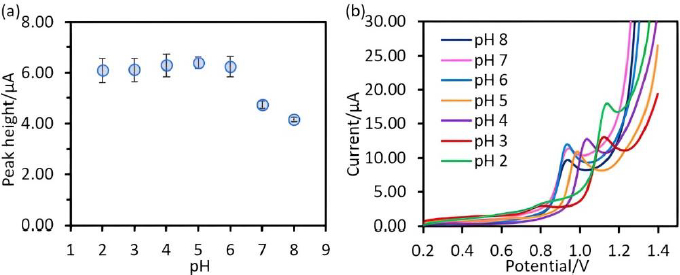
In this work, the study of the electrochemical response of SA in dependence on the solution pH was done in a phosphate buffer in the pH range between 2 to 8 because of pKa1 of SA is 2.9 [25]. The results show that the oxidation current signals of SA detected by Chitosan@Fe3O4/CPE in the pH range between 2-6 exhibit no differrence, while the signals decreased at pH values above 7, as shown inFigure 4(a). Due to the deprotonated amine group of chitosan (pH 7-8), the attraction between chitosan and SA was diminished. However, in a highly acidic medium, losing of the cross-linkage between chitosan and glutaraldehyde occurred, as referred to in the report of Freire et al. [11]. Additionally, it was found that SA responses at pH 2-4 were slightly lower than the SA response at pH 5 as shown inFigure 4(b). Therefore, pH 5 was selected as the optimum medium for further work.
Interference study
Electrochemical techniques can detect the following sample interferences: resorcinol, phenol, lactic acid, and fluorouracil, each to a different extent. In this study, the measurements were conducted by adding interference to 50 μM SA under optimum conditions. As shown inTable 1, the tolerance limit is set to have a relative error of not more than 10 % [26]. In addition, for sample analysis, the standard addition method was used, the determination should not also be affected by the interfering agents.
Analytical performance
Linear range
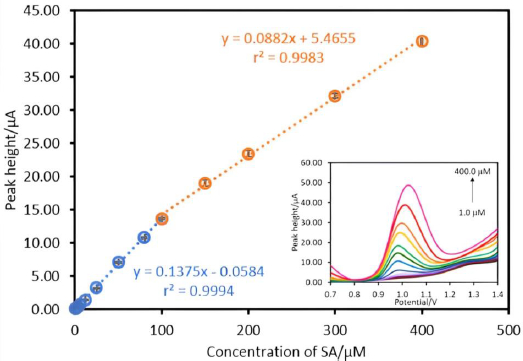
The various concentrations of SA (1.0-400.0 μM) were measured by the proposed electrode under optimal conditions using square wave stripping voltammetry (SWV). The calibration plots between the various concentrations of SA and oxidation peak current are indicated inFigure 5. It was found that the linearity was in the range of 1.0-100.0 μM (r2=0.9994) and 100.0-400.0 μM (r2=0.9983).
Limit of detection (LOD) and limit of quantitation (LOQ)
The detection limit can be calculated from 3s.d./slope and the limit of quantitation is calculated from 10SD/slope in which s.d. is the standard deviation and slope from the calibration plot. The measured detection and the quantitation limits were 0.57 and 0.90 μM, respectively, as shown inTable 2.
Application for real samples
As mentioned above, the standard addition was used for SA analysis in all samples. The obtained results were compared with those of UV-Visible spectrophotometry as the standard method, as shown inTable 3. It was found that the two methods were not significantly different at the 95% confidence level (tstat = 0.69, tcrit = 2.78). In addition, the recovery percentages of samples adding with 3 concentrations of 10.00-30.00 mM SA were in the range of 94.40-113.51 that remained within the acceptable range (85-115 %) in response to the AOAC standard (Table 4) [27].
Comparison with previously published research
The comparison of the proposed method using Chitosan@Fe3O4/CPE with some studies in the literature is shown inTable 5. The procedure of all reports shown in this table consisted of various voltammetric modes and different working electrode types. It was discovered that most of the electrodes modified with nanoparticles showed high sensitivity, including this work [2,4,28-31]. Although techniques for electrode preparation using nanoparticles require complicated electrode fabrication methods, a suitable wide working range for determining SA in the pharmaceutical samples is found especially in this work.
Conclusions
In this work, the carbon paste electrodes modified with chitosan-coated magnetite nanoparticles (Chitosan@Fe3O4/CPE) were developed for SA analysis. At acidic conditions, the amine proton of chitosan at magnetite nanoparticle as the positively charged surface attracted the negatively charged SA increasing the sensitivity and selectivity of SA analysis. Under the optimum conditions, the linearity ranges of 1.0-100.0 and 100.0-400.0 μM with r2 of 0.9994 and 0.9983) were observed. The proposed method gave high sensitivity (LOD = 0.57 μM). The steadiness of the proposed electrodes as precision showed actual stability with 1.17 %RSD (n = 35). Furthermore, this method was also successfully applied for the determination of SA in wart treatment pharmaceutical samples.