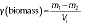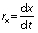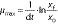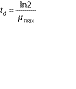INTRODUCTION
One of the biggest changes in the modern world diet has been in the quality of the consumed food. For that, much focus has been placed on the sources of ’green biomolecules’ such as terrestrial plants and algae. They are considered as highly efficient ’biofactories’ that produce mainly primary metabolites (lipids, proteins, carbohydrates) and secondary metabolites (carotenoids, polyphenols, terpenes, etc.). Plant-derived secondary metabolites, including antioxidants, have been widely studied for their potential to reduce a risk of illness and enhance the strength of the defense of the human body against pathologies such as neurodegenerative and cardiovascular diseases (1). Antioxidants from green sources including polyphenols, vitamins and carotenoids are becoming of great importance to replace the synthetic ones such as butylated hydroxytoluene (BHT).
Microalgae are gaining considerable interest worldwide due to their unique biomass composition extremely rich in functional ingredients, especially carotenoids, vitamins, minerals, proteins and long-chain polyunsaturated fatty acids. Microalgae do not need arable land, and can be cultured massively in controlled ponds or photobioreactors, which makes them advantageous over terrestrial plants (2). Although most microalgae are photoautotrophic, several strains are capable of using different carbon sources to grow heterotrophically, which may improve growth performance and biomass concentrations (3).
Since several essential molecules must be provided through food, microalgae have been reported to represent an excellent choice for consumers who are looking for tasty foods without harmful effects (4). This should replace the conventional forms of bioactive phytocompounds marketed as tablets, capsules or powders (5,6).
In the last years, several researchers have incorporated microalgal biomass in conventional food recipes to improve their basic nutritional value. Furthermore, microalgal supplementation has become economically promising for the food industry considering first the low environmental impact, and secondly the fact that consumers give importance to the relationship between diet and health (7). The main microalgal enrichment includes products like gluten-free bread (8), cookies (9,10), bread (11), yogurt (12), pasta (13) and biscuits (14). This increasing application of microalgae in foods takes advantages from their diversity and variable biochemical composition influenced by the production mode and culture medium (15). It has been demonstrated that nutrients (macro- and micronutrients) strongly affect the biochemical composition of the microalgae (16). The composition of culture medium is among the main factors affecting the bioactive compound accumulation in the algal biomass (17). Thus, science is still trying to domesticate novel microalgal strains to enhance their growth and improve the overall biochemical composition, which is exclusively assessed at the cultivation level. The best alternative to evaluate the effect of nutrient availability on microalgal growth and biochemical composition, on laboratory scale, is variation of the culture medium formulations.
In this context, the aim of this work is to evaluate the enrichment of a traditional dairy product, ricotta cheese, with microalgae using a commercial sample of Arthrospira platensis (syn. Spirulina platensis) as reference. It was proposed to firstly optimise the antioxidant ability and the nutritional properties of two microalgae, namely Chlorella sp. and Nannochloropsis gaditana, with potential use in food production by varying the growth media, and secondly to assess their addition to the cheese to enhance the functional properties and estimate the acceptability of the new product designated ‘Ricottalgue’.
MATERIALS AND METHODS
Algal strains and culture conditions
The used strains were previously isolated from two different saline sites situated in the northern part of Tunisia (North Lake lagoon: 36°49'25.6"N 10°12'36.4"E, salinity: 33.8 g/L, and Monastir lagoon: 35°46'18.0"N 10°46'34.4"E, salinity: 44.4 g/L), maintained in LIP-MB laboratory (Laboratoire d'Ingénierie des Protéines et des Molécules Bioactives, Tunis, Tunisia) and identified microscopically and molecularly as Chlorella sp. SM1 (GenBank accession number KM401849, NCBI, Bethesda, MD, USA) and Nannochloropsis gaditana L2 (GenBank accession number KT932831, NCBI) (18,19). The commercial Spirulina sample used in the sensory assay was provided by Bio-Gatrana Laboratories (Gatrana Sidi Bouzid, Tunisia), referenced as TN BIO 001, N° SP01018 and identified as Arthrospira platensis.
The strains were cultured in four different media enriched with artificial seawater: algal (20), BG-11 (21), f/2 (22) and Conway medium (23). The nutrient composition of each medium is detailed inTable S1 and all the chemicals were purchased from Sigma-Aldrich S.a.r.l, Merck (Saint-Quentin Fallavier, France). All cultures were first grown in 500-mL Erlenmeyer flasks, then transferred to 2-litre glass reactors (0.07 m diameter, 0.5 m length) with the respective growth media. All experiments were conducted in batch mode and under controlled conditions of light intensity (200 μmol/(m2·s)) with a photoperiod 14:10 (light/dark), temperature 23 °C and continuous aeration (0.2 L/(L·min)). The absorbance was determined colorimetrically at 750 nm (spectrophotometer model 1240 UV-Vis; Shimadzu, Kyoto, Japan). Cell counts were performed every 3rd day under optical microscope (model Pégase; Nachet, Paris, France) using Malassez counting chamber (depth 0.200 mm; Hecht Assistent®, Sondheim vor der Rhön, Germany). Algal biomass concentration (γ/(g/L)) was measured gravimetrically after drying a centrifuged (13 000×g for 5 min, model MPW-350R; MPW Med. Instruments, Warsaw, Poland) culture sample at 105 °C for 24 h.
where m1 is the initial mass, m2 is the mass of the biomass after drying, and Vi is the initial volume.
Different growth parameters were determined as follows (24):
where rx is biomass productivity, x is biomass concentration in mg/L and t is time in days.
where μmax is specific growth rate and x0 and xf are the mean dry biomass concentrations at the times t0 and tf, respectively, and:
where td is biomass generation time.
Preparation of microalgal extracts
The biomass was harvested at the beginning of the stationary phase of culture and washed twice by centrifugation at 13 000×g for 5 min (MPW-350R; MPW Med. Instruments, Warsaw, Poland). After harvesting, the microalgal biomass was dried at 60 °C in a regular oven (ED115UL; Binder GmbH, Tuttlingen, Germany) for 24 h until there was no change in the mass. Microalgal biomass was then macerated in ethanol (1:15 m/V) for 3 h at room temperature in darkness under agitation (130 rpm, Precision 2871 reciprocating water bath; Thermo Scientific, Waltham, MA, USA). The extraction was repeated several times until the supernatant was colourless. The extraction method was selected and adjusted on the basis of the results obtained by Maadane et al. (25) on different microalgal biomass, which permitted to obtain an efficient extraction of biomolecules. The extracts were filtered (membrane filters 0.2 µm; Whatman®, Merck) and the solvent was removed by rotary evaporation (IKA RV-10; IKA®-Werke GmbH & Co. KG, Staufen, Germany) at 40 °C. All concentrated extracts were weighed and stored at −20 °C until use.
Biochemical composition
Total lipids were measured according to a modified version of Kochert’s method (26). Briefly, the dried biomass was ground with an equivalent mass of alumina for 5 min. A methanol/chloroform (2:1 V/V) solution was added to the biomass and alumina mixture. The mixture was then centrifuged (2054×g for 5 min, MPW-350R; MPW Med. Instruments). The pellet underwent three additional extractions. To the final supernatant, 158 mM HCl (Sigma-Aldrich S.a.r.l, Merck) and 0.015% MgCl2 (Sigma-Aldrich S.a.r.l, Merck) were added. The lower phase containing the lipid fraction was extracted using a Pasteur pipette and transferred to a new pre-weighed tube.
Lipid productivity was determined as follows:
where rp and rx are lipid and biomass productivity.
Fatty acids were transesterified according to ISO 12966-2:2017 (27). The resulting fatty acid methyl ester (FAME) composition was determined by gas chromatography (CP-3800 GC; Varian, Walnut Creek, CA, USA) equipped with 30 m SUPELCOWAX 10 capillary column (0.32 mm internal diameter and 0.25 μm film thickness). The injector (split 1:50) and detector (flame ionization) temperatures were kept constant at 250 °C. The oven temperature program started at 200 °C for 8 min, then increased up to 230 °C at 5 °C/min and maintained constant at that temperature for 16 min. Helium was used as the carrier gas, and kept at a constant flow rate of 1.3 mL/min. The content of FAMEs was calculated as the mass fractions of the total fatty acids present in the sample, determined from the peak areas. The 1, 2-diheptadecanoyl-sn-glycero-3-phosphocholine (17:0) was used as an internal standard. Fatty acid content was calculated according to the UNE EN 14103:2020 (28).
Mass fractions of carbohydrates were determined using the conventional phenol-sulfuric acid method developed by DuBois et al. (29) with glucose as a standard. The sample was mixed with 72% sulfuric acid (Merck) at 30 °C for 1 h. The mixture was autoclaved (BKM-Z12N; Biobase, Jinan, Shandong, PR China) for 1 h at 120 °C after the dilution of the sulfuric acid to 4%. An equivalent volume of 5% phenol solution (Merck) and 1 mL of concentrated sulfuric acid where added quickly to the lipid fraction. Then the mixture was heated to 100 °C for 5 min. After 30 min at room temperature, the absorbance was read at 480 nm (1240 UV-Vis spectrophotometer; Shimadzu).
Relative proteins were estimated from the difference between the total dry ash-free biomass and the sum of the lipid and carbohydrate contents (19).
Phenolic content
Phenolic content was determined using Folin-Ciocalteu reagent based on the slightly modified method of Singleton and Rossi (30), using gallic acid as a standard. Briefly, 125 µL of extracts (1 mg/mL) were mixed with an equal volume of Folin-Ciocalteu reagent (Sigma-Aldrich, Merck), 1 mL of 7% sodium carbonate and the volume was made up to 3 mL by adding distilled water. The mixture was mixed and incubated for 90 min in the dark. After that, the absorbance was measured at 760 nm (1240 UV-Vis spectrophotometer; Shimadzu) and the results were expressed in mg gallic acid equivalents (GAE) per g dry extract.
Carotenoid content
Carotenoid content of algal extracts was estimated spectrophotometrically according to Lichtenthaler and Buschmann (31) method. Aliquots of the extracts were prepared at concentration of 1 mg/mL in ethanol. Absorbances were measured at 470, 648 and 664 nm (1240 UV-Vis spectrophotometer; Shimadzu), and carotenoid content was calculated using the Lichtenthaler equations (25,31).
where Chla and Chlb are chlorophyll a and b respectively. The numbers in the equations are the specific absorbance coefficients.
DPPH radical scavenging assay
The antioxidant capacity of the samples was evaluated by their ability to scavenge the DPPH (2,2-diphenyl-picryl-hydrazyl-hydrate; Sigma-Aldrich) radical at various concentrations. The method of Kumar et al. (32) was used with slight modifications. A volume of 1 mL of 0.2 mM (in absolute ethanol) DPPH solution was added to 0.2 mL of different methanolic extracts (1, 2, 3, 4 and 5 mg/mL). Absorbance was measured at 517 nm (1240 UV-Vis spectrophotometer; Shimadzu) after 30 min of incubation in the dark against methanol as blank. DPPH radical scavenging capacity (%) was calculated as follows:
where Acontrol is the absorbance of the control (DPPH), and Asample is the absorbance of the tested sample (with DPPH). The absorbance of extracts in methanol (without DPPH) was determined in order to subtract the absorbance of coloured extracts.
The IC50 was calculated by linear regression, and expressed in milligram per millilitre. Butylated hydroxytoluene (BHT) was used as reference standard for both strains.
Ricotta cheese production
The control ricotta cheese sample was prepared by heating whey (FarmCheese, Manouba, Tunisia) obtained from cow’s milk, which was previously coagulated with rennet, at 45 °C by adding salt and continuing heating in large open kettles until the temperature reached 80 to 85 °C. At that point, a suitable food grade acidulant (citric acid) was added to reduce the pH to 6.0 and induce coagulation of the proteins. The curd particles float to the surface of the hot liquid, are scooped off and placed in a perforated recipient. Then, to coagulated whey the microalgal biomass was added at 0.2, 1 and 1.5% mass fraction between different layers to obtain homogeneous product. The samples were left to drain and cool overnight.
Sensory analysis
Hedonic evaluation of the ricotta supplemented with the biomass of Chlorella sp. SM1 obtained from BG-11 medium and commercial Arthrospira platensis biomass, as well as the control sample was performed based on the protocol previously described by Batista et al. (9). Chlorella sp. SM1 was selected for this analysis instead of Nannochloropsis gaditana L2 because of its interesting biochemical profile and a good biomass quantity to be used in the ricotta. Sensory evaluation was carried out in an appropriate room at 25 °C with adequate lighting respecting the international standard (ISO 8589:2007) (33). The main purpose of the study was clearly explained to the panellists (INSAT researchers) who had to sign an informed consent in order to express their agreement to participate in this research program. Each panel member was trained how to score different characteristics. Ricotta samples were served a day after being cooked in random order to 30 individuals, 26 female and 4 male aged between 23 and 45, who were asked to evaluate the following attributes of the samples: colour, odour, taste, texture and global appreciation (5 levels from ‘very pleasant’ to ‘very unpleasant’). Panellists were also asked whether they would buy the ricotta they tested (from ’would certainly buy’ to ’certainly would not buy’).
Statistical analysis
The results of three replicates from each sample were used for statistical analysis and the values were expressed on dry mass basis as mean value±standard deviation. Origin Pro v. 8.0 software (34) was used. Results were compared among culture conditions and strains by one-way ANOVA in conjunction with Tukey’s test at a significance level of 95% (p<0.05).
RESULTS AND DISCUSSION
Growth performance of microalgae
Effects of different medium compositions on growth parameters of Chlorella sp. SM1 and Nannochloropsis gaditana L2 are shown inTable 1 andFig. S1. The two strains followed conventional growth curve (lag, exponential and stationary phase). It can be observed that Chlorella sp. SM1 grew better in BG-11 medium, yielding 1.97·108 cells/mL after 17 days. The highest number of N. gaditana L2 cells was obtained in the algal medium (1.42·108 cell/mL). Results inTable 1 confirm that Chlorella sp. SM1 and N. gaditana L2 achieved higher growth rates and biomass productivities in BG-11 and algal media. The biomass productivity of the two strains was significantly different when growing in the same culture medium (Table 1). However, among the selected growth media, f/2 gave the highest lipid productivities. Biomass productivities found in this work were higher than those reported by George et al. (35) for Ankistrodesmus falcatus cultivated in BG-11 and Bold’s basal medium medium (6.14 and 1.6 mg/(L·day) respectively). Xia et al. (36) mentioned comparable biomass and lipid productivities (75.4 and 21 mg/(L·day), respectively) for Desmodesmus sp. cultivated in modified BG-11 medium.
Mean values and standard deviations followed by the same lower-case letter in the column did not differ significantly among culture media for each strain, and mean values followed by the same capital letter in the column did not differ significantly between the strains in the same culture medium based on Tukey’s test (p>0.05), N=3
The richness in macronutrients (nitrogen and phosphorous) significantly influences microalgal cultivation (Table S2). Thus, it is obvious that the presence of higher concentrations of N and P in algal and BG-11 media is important for the cell growth, while their limitation leads to poor growth ability and higher lipid productivity. These results are similar to those of Jazzar et al. (19), who suggested that N and P are important for cell division and protein accumulation. N is converted inside algal cells into a useable form, i.e. nitrite that is reduced to ammonium, which in turn produces glutamine responsible for protein production. This explains the lower protein contents obtained in N-deficient f/2 medium (Fig. 1). N. gaditana L2 reached its highest biomass productivity and cell growth in algal medium, which had the highest P concentration, fivefold higher than BG-11 medium. Indeed, excess phosphorus concentration results in excess of ATP and NADPH synthesis, carrying energy for cell functions, which in turn, enhance growth and biomass production (37).
Accordingly, previous studies have demonstrated that lipid and biomass production is affected by N:P ratios in culture media (19). The N:P ratios in f/2 and Conway media used here were 29:1 and 7:1, respectively, which seems suitable for higher lipid production, while N:P ratios 16:1 and 98:1 (algal and BG-11 media respectively) induce optimal cell growth. This was also reported by Chia et al. (38), who mentioned a higher lipid production in Chlorella vulgaris at N:P ratio 10:1 but not 100:1.
Micronutrients such as magnesium, sulfur and iron play a significant role in the growth of microalgae (39). Previous studies have reported the contribution of iron to microalgal growth as one of the most important trace metals involved in the oxidation–reduction of photosynthesis pathway. Magnesium is a constituent of chlorophyll, playing a key role in O2/CO2 utilization in photosynthesis. Sulfur is involved in cell division and lipid accumulation. Wong et al. (40) reported a lower growth rate of Chlorella vulgaris when cultivated under iron-deficiency coupled with lower amount of Mg and S. The lower Mg and Fe concentrations are observable in f/2 and Conway media, while S is totally absent from Conway medium.
Biochemical characterization
Primary metabolites
Fig. 1 shows the impact of varying the growth media (algal, BG-11, f/2 and Conway) on Chlorella sp. SM1 and Nannochloropsis gaditana L2 biomass composition. The biochemical composition of the two strains varied significantly in response to nutrient availability in the growth media. Chlorella sp. SM1 accumulated the highest mass fractions of lipids and carbohydrates in f/2 medium (Fig. 1a). The same behaviour was reported for N. gaditana L2 (Fig. 1b). The highest mass fraction of protein was measured in Chlorella sp. SM1 (67.0%) cultivated in BG-11, while N. gaditana L2 accumulated the highest protein mass fraction in algal medium (58.7%). Proteins from microalgae are well appreciated for human consumption due to the high content of essential amino acids. Matos et al. (41) mentioned an average protein mass fraction of 40% in six different types of microalgal biomass determined by Kjeldahl method using a nitrogen-to-protein conversion factor of N×4.78. It should be noted that the estimates for the crude protein include other nitrogen compounds, which in general are expected to account for around 10% of the total nitrogen found in microalgae. The same team (41) registered a lower lipid (8.1 and 15.6%) and carbohydrate mass fractions (18.6 and 16.7%) in N. gaditana and N. oculata cultivated in f/2 medium. Chlorella sp. SM1 recorded twofold increase in lipids compared to Chlorella vulgaris cultivated in several growth media (40) and an interesting carbohydrate content compared to Chlorella sp. cultivated under nitrogen-limited condition (41).
Our findings indicated that N-deficient medium, particularly f/2, is suitable for enhancing lipid and carbohydrate accumulation, while at higher nitrate and phosphate concentrations (in algal and BG-11 media), higher protein content was obtained (19,40).
Carotenoids and phenolic compounds
Microalgae are known to be an interesting source of antioxidants, including skeleton carbon compounds (as carotenoids and phenolic compounds), that play an important role in scavenging reactive oxygen species generated during photosynthesis. In order to investigate the effect of nutrient availability on the accumulation of antioxidants in the studied microalgae, total carotenoid and phenolic contents of N. gaditana L2 and Chlorella sp. SM1 obtained in different culture media (algal, BG-11, f/2 and Conway) were determined. Total carotenoid and phenolic mass fractions in both strains differed significantly among the tested media (Table 2 (15,42,43)). Briefly, carotenoid and phenolic mass fractions were enhanced in f/2 and Conway media. The lowest antioxidant mass fraction; however, was observed in BG-11 and algal media. This suggests that both strains accumulated larger amount of antioxidants when cultivated under nitrate limitation (f/2 and Conway media). Previous works have reported the effect of modulating nitrate availability on the accumulation of carotenoids in some microalgal species such as Dunaliella and Haematococcus, well known for exploiting significant percentage of secondary metabolites under nutrient stress (44). Moreover, it is important to note that a limited number of papers have described the phenolic content in microalgae, especially when it is associated with nutrient stress. For a better discussion of our results,Table 2 further summarizes the comparison of carotenoid and phenolic contents reported in some Chlorophycae strains. Regardless of the effect of the composition of different growth media, both phenolic and carotenoid mass fractions of the analysed extracts were comparable to those reported earlier (15,25,45). Nevertheless, when comparing the results reported here with other studies, it should be taken into consideration that the content and composition of carotenoids and phenolic compounds are typically influenced by other stress factors such as extracting solvents (25), UV stress (46) or metal stress (47). Taken altogether, it would be interesting in the future to cultivate the strains under investigation in two stages. In the first stage, microalgae could be cultivated in N- and P-rich algal or BG-11 medium to ensure a good biomass productivity. In the second stage, the cells should be transferred, at the end of their exponential growth phase, to N- and P-deficient f/2 medium for better secondary metabolite accumulation.
| Strain | Nutrient medium | Other culture conditions | w(carotenoids)/(mg/g) | w(phenolics as GAE)/(mg/g) | Reference |
|---|---|---|---|---|---|
| Chlorella sp. SM1 | |||||
| Algal | Air flow rate 0.2 L/min, luminosity 200 μmol/(m2·s), photoperiod light/dark=14:10 t=23 °C | (0.9±0.1)aA | (27.8±2.3)aA | Current study | |
| BG-11 | (0.76±0.04)aA | (10.2±0.5)bA | |||
| f/2 | (2.2±0.3)bA | (41.4±2.7)cA | |||
| Conway | (2.17±0.04)bA | (48.8±4.5)cA | |||
| Nannochloropsis gaditana L2 | |||||
| Algal | Air flow rate 0.2 L/min, luminosity 200 μmol/(m2·s), photoperiod light/dark=14:10 t=23 °C | (0.61±0.06)aB | (20.8±3.5)aB | Current study | |
| BG-11 | (0.76±0.05)aA | (15.0±1.1)aB | |||
| f/2 | (12.0±0.2)bA | (52.7±7.6)bA | |||
| Conway | (1.6±0.4)bB | (35.1±3.4)cB | |||
| Chlorella vulgaris | |||||
| Control: c(N)=5 mM | Air flow rate 0.25 L/min, luminosity 125 µmol/(m2·s), photoperiod light/dark=12:12 | 3.8 | 3.3 | (15) | |
| c(P)=0.25mM | |||||
| c(P)limitation=0.01 mM | 0.9 | 1.8 | |||
| c(N)limitation=0.2 mM | 0.4 | 1.3 | |||
| Tetraselmis suecica | |||||
| Control: c(N)=5 mM | Air flow rate 0.25 L/min, luminosity 125 µmol/(m2·s), photoperiod light/dark=12:12 | 2.5 | 3.3 | (15) | |
| c(P)=0.25 mM | |||||
| c(P)limitation=0.01 mM | 1.3 | 2.8 | |||
| c(N)limitation=0.2 mM | 0.5 | 1.5 | |||
| Chlorella elipsoida | |||||
| Control: c(N)=10 mM | Flow rate (0.03 % CO2 in air), luminosity 919 or 1839 µmol/(m2·s) under c(N)limitation t=(25±3) °C | 6.5 | 0.9 | (42) | |
| c(N)limitation=0.7 mM | 30.4 | 4.31 | |||
| Haematococcus pluvialis | |||||
| Control: c(N)=6.3 mM | Air flow rate 0.05 L/min, luminosity 850 μmol/(m2·s) t=25 °C | 0.7* | n.d. | (43) | |
| c(N)limitation=1.5 mM | 10* | n.d. | |||
Mean values and standard deviations followed by the same lower-case letter in the same column did not differ significantly among culture media for each strain, and mean values followed by the same capital letter in the same column did not differ significantly between the strains in the same culture medium based on Tukey’s test (p>0.05), N=3. n.d.=not determined, *astaxanthin
DPPH radical scavenging activity
The antioxidant activity of the samples was assessed using the DPPH as a stable free radical. The different inhibition capabilities of the methanolic extracts obtained from N. gaditana L2 and Chlorella sp. SM1 at different concentrations in different growth media (algal, BG-11, f/2 and Conway) are shown inFig. 2. The IC50 values of the extracts were calculated and compared with the standard antioxidant BHT (Table S2).
Fig. 2 shows the ability of both strains to reduce the DPPH, which considerably increased with increasing concentration of the strains. Methanolic extract of 5 mg/mL Chlorella sp. SM1 in f/2 medium had the highest scavenging ability (80%), while that of Nannochloropsis gaditana L2 at the same concentration was 58%. Previous works emphasized an interesting DPPH radical scavenging activity of Chlorella species (45,48,49).
The IC50 values ranged from (4.49±0.05) to (2.5±0.3) mg/mL for N. gaditana L2 and from (1.97±0.07) to (1.2±0.3) mg/mL for Chlorella sp. SM1 (Table S2). The lowest IC50 values were obtained in f/2 medium. The tested extracts possessed a radical scavenging activity lower than the BHT that was used as positive control, exhibiting an IC50 of 0.56 mg/mL.
The antiradical activity of the extracts of both strains did not differ significantly in algal, BG-11 and Conway media. However, it was significantly higher in N-deficient f/2 medium. Thus, it is clear that when reducing the nitrate content of the medium to a moderate level (from 1.5 g/L in BG-11 medium to 0.99 and 0.074 g/L in algal and Conway media respectively), the inhibition of Chlorella sp. SM1 and N. gaditana L2 methanolic extracts remained apparently unaffected (Fig. 2). The nitrate concentration in f/2 medium presented a stressed condition for the evaluated strains, which has been translated in a significant increase in the ability of scavenging DPPH at different concentrations. Elevated antioxidant activity under low nitrate concentration was previously recorded in nine microalgal species (50).
Fatty acid profiles
The quality of lipids obtained from each strain cultivated in a different medium (algal, BG-11, f/2 and Conway) was assessed by gas chromatography after transesterification of all extracts. The variation in the mass fraction of PUFA (polyunsaturated fatty acids), MUFA (monounsaturated fatty acids) and SFA (saturated fatty acids) is summarized inTable 3.
The same lower-case letter in a row indicates not significant differences between culture media of each strain, and the same capital letter in a row indicates significant differences between the strains in the same culture medium based on Tukey’s test (p>0.05). n.d.=not detected, SFA=saturated fatty acids, MUFA=monounsaturated fatty acids, PUFA=polyunsaturated fatty acids, ω3=omega-3 fatty acids, ω6=omega-6 fatty acids. Results are expressed as mean value±standard deviation (N=3)
Regardless of the used medium, palmitic (C16:0), oleic (C18:1), linoleic (C18:2) and linolenic (C18:3) acids were detected in both strains. An exception was N. gaditana L2 in algal medium, where linolenic acid (C18:3) was not detected. It is also notable that nutrient variation influences strongly the fatty acid composition. Higher mass fractions of SFAs and MUFAs were recorded in Conway and f/2 medium (p<0.05), whereas mass fractions of PUFAs were significantly higher in BG-11 and algal medium.
Elevated MUFA mass fractions were noticed under N-deficient conditions, and the highest MUFA accumulated is the oleic acid (C18:1) observed in Chlorella sp. SM1 (33.9%) in f/2 medium. Moreover, the sum of all identified fatty acids ranged from 71.5% in N. gaditana L2 in algal medium to the highest in Chlorella sp. SM1 in f/2 medium (93.5%). The overall fatty acid profiles found in this work corroborate those previously published by Mendes et al. (51) in Chlorella sp., and those reported by Jazzar et al. (19) in Chlorella sorokoniana and Neochloris sp. Fatty acid variations reported in this work were also in agreement with previous studies that suggested an increase in fatty acid content in response to nitrogen deficiency (52,53) accompanied by an increase in oleic acid content.
Ricotta enrichment and sensory evaluation
Making a good quality fresh ricotta cheese is still an art because of the crucial requirements for satisfactory texture and flavour. Adjusting the manufacturing procedure steps is considered critical, and even more if an additional ingredient is to be added to the original recipe. For this purpose, several assays were made at laboratory scale to adjust the manufacturing procedure and then move to small-scale assays. It was finally decided to add microalgal biomass equally between different layers of the ricotta when filling the recipients. The obtained ricotta samples with microalgal biomass had an original appearance with attractive colour in different green tonalities of the used microalgae (Fig. S2).
The assay of ricotta cheese formulations with Chlorella sp. SM1 biomass obtained in BG-11 medium showed a good compromise between the biomass productivity and bioactivity results. This enrichment of cheese with Chlorella was compared to that of cheese prepared with a commercial Arthrospira platensis, which is a reference strain in food industry to better ascertain the impact of the pigment on the sensory assays. These two strains are generally recognized as safe (GRAS) and are already widely used in food formulations (6,9). Ricotta samples with mass fractions of 0.2, 1 and 1.5% Chlorella sp. SM1, 1.5% A. platensis and a mixture of 1.5% Chlorella sp. SM1 and 1.5% A. platensis were therefore evaluated by a sensory panel of INSAT researchers (Fig. 3).
Fig. 3 shows the average scores for the main sensory attributes (colour, odour, flavour and texture) as evaluated by the panel. The least appreciated sample was the one containing 1.5% Spirulina platensis. In fact, the panel showed more preference for the sample with 0.2% Chlorella sp. SM1 and the control one, in terms of global appreciation. Concerning the colour, the tasters classified positively the samples with microalgal biomass (0.2 and 1% Chlorella sp. SM1) and the 1.5% mixture as the least appreciated one.
Consumers have always been sensitive to the taste and odour when evaluating a product even before the health benefit consideration. Higher concentration of microalgae had a negative impact on the odour and flavour of the samples, which were the least appreciated.
Sensory properties of several products enriched with microalgae have already been tested and globally appreciated (6). However, in some cases, the addition of high mass fractions of microalgae (2%) negatively influenced the flavour parameter and led to a negative global appreciation (13).
In the comments section, the tasters mentioned that the ricotta with 1.5% Arthrospira platensis had a very unpleasant fishy odour and aftertaste. In fact, the ricotta with 0.2% Chlorella sp. SM1 was considered the most balanced in terms of flavour, followed by the sample with 1% Chlorella sp. SM1.
The texture was estimated manually by stirring the sample with a spoon. As seen inFig. 3, the samples show a significant change in the texture with the addition of microalgae (>0.2%). This change in the texture was positively received by the panellists and reached 4 on the scale, corresponding to ’pleasant’ texture. Actually, this change could be related to the fact that standard ricotta usually has a very soft texture with a tendency to crumble very easily. Microalgal biomass brought an overall positive structural effect.
Fig. 4 gives the answers by the tasters regarding the buying intention. Sixty-three and 53% of the tasters ’would probably buy’ the ricotta with 0.2 and 1% Chlorella sp. SM1, respectively, while 26% of the tasters ’would certainly buy’ and 6% ’would probably buy’ the ricotta with 1.5% Chlorella sp. SM1. The ricotta with 1.5% Arthrospira platensis was the least appreciated with 26% of the tasters stating that they ’certainly would not buy’ it and 34% ’probably would not buy’. This simulation is of great importance because it helps to predict a possible future commercialization of the product since it gives a perspective of the potential consumer acceptance.
CONCLUSIONS
This work investigated the feasibility of using microalgae to enrich and enhance the nutritional properties of ricotta, a Mediterranean dairy product made from cheese whey. This approach can be considered as a new application that allowed obtaining an innovative product. The variation of growth media confirmed the role of nitrogen-deficient medium like f/2 or Conway to boost the antioxidant activity of microalgae while keeping basal nutritional benefits. The cultivation of Chlorella sp. SM1 and Nannochloropsis gaditana L2 in f/2 and Conway media enabled high-quality PUFA accumulation. The highest antioxidant activity, carotenoid and phenolic contents were also obtained in these two media. The formulation of the enriched ricotta was successfully made without altering either the flow diagram, or the sensorial acceptability of the product, especially at low microalgal mass fraction (0.2%). The addition of microalgal biomass to fresh ricotta had a favourable impact on the sensory attributes of the final product designated ’ricottalgue’. Based on the obtained results, the attractiveness of a cheap artisanal dairy product could be enhanced using microalgae as natural and healthy ingredient.