Introduction
With over 3000 species in more than 90 genera, the rose family (Rosaceae) is one of the most diverse angiosperm families (Zhang et al. 2017). The family includes many ecologically and economically important species that contain the whole spectrum of beneficial properties for biodiversity, as well as for human nutrition and healthcare. Phylogenetic relationships within Rosaceae are complicated and have not been fully clarified, as homoplasy of morphological characters, frequent hybridization and apomixis complicate their classification and phylogenetic reconstruction (Zhang et al. 2017). Within the family, some genera engage in interspecific hybridization more easily than others, like Malus Mill. (Larsen et al. 2008), Sorbus L. (Németh et al. 2020) and Pyrus L. (Bell and Hough 1986). However, hybridization in Rosaceae is not limited only to that between species within the same genus, but crosses between species from different genera are also possible (Postman 2011). Intergeneric hybridization in Rosaceae often results in highly fertile individuals that appear repeatedly in nature (Campbell et al. 2007).
Among the genera with the largest number of intergeneric hybrids are Pyrus and Crataegus L. Successful hybridization has been reported between Pyrus species and Sorbus (Postman 2011), Cydonia L. (Shimura et al. 1983) and Malus (Pasqualetto et al. 2022), resulting in new hybrid genera like ×Sorbopyrus C.K.Schneid. and ×Pyronia Veitch ex. Trab. In addition, the best-known intergeneric hybrid of Crataegus is Crataemespilus Camus, a sexual hybrid between Crataegus and Mespilus L. (Phipps 2016). These hybrid individuals usually display new, intermediate forms of vegetative and generative traits (Pasqualetto et al. 2022). However, most of these hybrids have been obtained artificially in attempts to obtain individuals with superior morphological, sensory or physiological characteristics, as hybridization is recognized as the most important source of genetic variation in fruit breeding (Van Tuyl and de Jeu 1997). When successful, intergeneric hybridization allows the introduction of chromosomal genomic regions of one taxon into that of another taxon through subsequent backcrossing, enabling the introduction of favourable traits to improve flavour, texture or disease resistance (Fischer et al. 2014).
In addition to the aforementioned intergeneric hybrids, sporadic mentions of a hybrid between Pyrus and Crataegus, named × Pyrocrataegus Rehder (Rehder 1949, McNeill et al. 2016) can be found in the literature. This proposed hybrid was described as the result of hybridization between Crataegus oxyacantha and Pyrus communis L., as well as between C. monogyna Jacq. and P. pollveria Lej (Rehder 1949). It is important to note that the author of C. oxyacantha was not noted, and therefore the exact species is not clear, as by present taxonomy it could be synonymous with any of the following accepted taxa, depending on the author: C. × polyacantha Jan, C. laevigata (Poir.) DC. or C. marshallii Eggl. Furthermore, according to the World Flora Database (WFO 2024), the taxonomic classification of P. pollveria is still unclear. Unfortunately, no further investigations were conducted on this hybrid, nor was it described in more detail in the available literature. Therefore, an intergeneric hybrid between Pyrus and Crataegus remains a botanical curiosity and is yet to be confirmed by modern taxonomical methods.
During field research in 2021, we observed almond-leaved pear (Pyrus spinosa Forssk.) individuals with peculiar, hawthorn-like leaves on a few branches, which aroused our interest in the long-described hybrid between these two genera. In this particular area, along the eastern Adriatic coast, both the almond-leaved pear and the one-seed hawthorn (Crataegus monogyna Jacq.) can be found. These are deciduous shrubs or small trees that reach up to 10 m in height (Zamani et al. 2012, Nabavi et al. 2015). However, they differ significantly in leaf morphology. Almond-leaved pear leaves are narrowly lanceolate or elliptic in shape, up to 7 cm long and 3 cm wide. The leaf edge is entire, sometimes moderately crenate. Leaves are shiny and vary in colour, from green to dark green, greyish- to bluish-green from below, initially hairy on both sides, later glabrous or only hairy below (Zamani et al. 2012). On the other hand, one-seed hawthorn leaves are 3-5 cm wide and long, broadly ovate to rhombic, deeply lobed, with pointed tips of the lobes. Lobes sometimes reach up almost to the midrib. Colour-wise, the leaves of common hawthorn are dark green, glabrous and shiny, lighter from below, hirsute only in the vein corners (Idžojtić 2009). Both species have up to 2 cm-long petioles. The almond-leaved pear is native to xerophytic habitats of Southern and South-eastern Europe and of Asia Minor, where it grows in discontinuous bush associations and open spaces, on a wide range of soil and habitat conditions (Vidaković et al. 2021). On the other hand, the one-seed hawthorn is widely spread across most of Europe and western Asia (Nabavi et al. 2015).
Considering their overlapping natural distribution and occasional reproductive compatibility described in the literature (Rehder 1949, McNeill et al. 2016), in this study we aimed to investigate the possibility of the presence of a long-described yet uninvestigated hybrid between Crataegus and Pyrus called ×Pyrocrataegus along the eastern Adriatic coast. In addition, the variability of their respective leaf morphologies is set to be studied, along with the population variability of both species. These data would provide valuable insight into the diversity of leaf sizes and shapes of these species, which can provide additional knowledge about their plasticity and adaptation processes.
Plant material and morphometric analysis
The plant material for morphometric analysis was collected in three C. monogyna (P1-P3) and four P. spinosa (P4-P7) populations (Tab. 1, Fig. 1).
Tab. 1. Populations, sampling sites, taxa, geographic coordinates, and multivariate diversity index (MDI) for eight studied populations. The significance level of differences in the average values of MDI between groups according to the Kruskal-Wallis test is marked by asterisk (*).
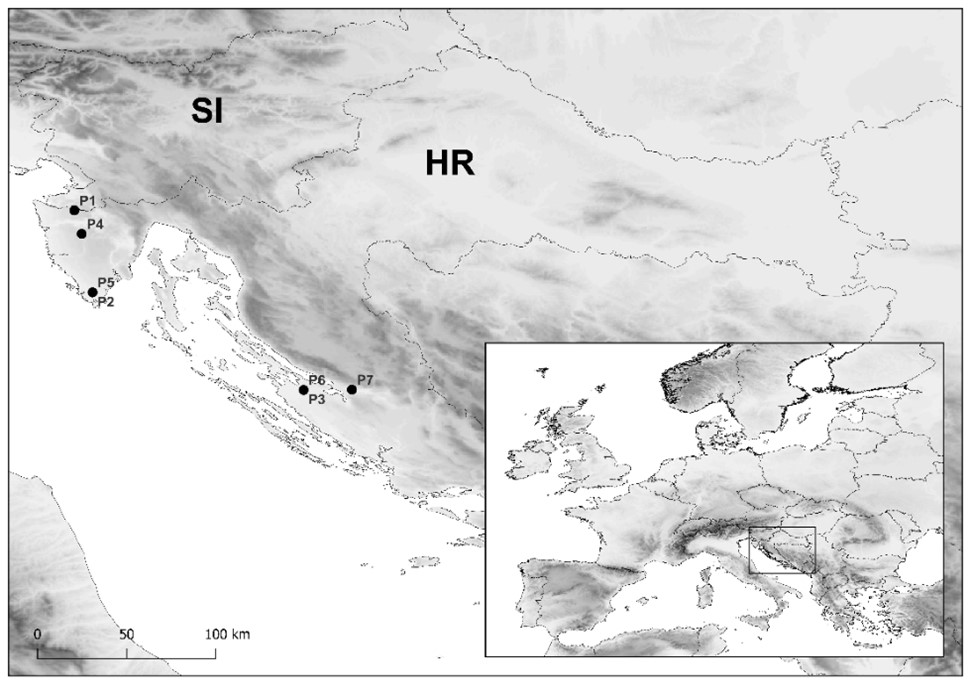
Fig. 1. Geographic locations of the studied Crataegus monogyna (P1-P3) and Pyrus spinosa (P4-P7) populations. Populations: P1 – Buje; P2 – Pula; P3 – Nin; P4 – Škropeti; P5 – Pula; P6 – Nin; P7 – Obrovac. The rectangle on the map in the lower right corner indicates the research area in the SE European context. Abbreviations: SI – Slovenia, HR – Croatia.
It is important to note that P. spinosa samples were subsampled from a larger study oriented towards research into genetic diversity and population genetics (Vidaković et al. 2024). The collection area encompasses the regions of Istria and Northern Dalmatia, where these two species have overlapping natural distribution areas, and where dimorphic P. spinosa individuals were observed. At each location, 10 shrubs/trees were selected for the analysis. From each shrub/tree, 20 fully developed leaves with no signs of disease or damage were collected from the short shoots in the sunlit part of the canopy. The leaves were collected during the vegetation period of 2022. Upon collection, leaves were herbarized, scanned using Microtek ScanMaker 9800XL scanner, measured using WinFolia software (WinFoliaTM 2001) and stored at the Faculty of Forestry and Wood Technology of the University of Zagreb.
In total, 10 leaf morphological traits were measured: leaf area (LA), perimeter (P), form coefficient (FC), leaf length (LL), maximum leaf width (MLW), leaf length, measured from the leaf base to the point of maximum leaf width (PMLW), leaf blade width at 90% of leaf blade length (LW90); angle closed by the main leaf vein and the line defined by the leaf blade base and a point on the leaf margin, at 10% (LA10) and 25% (LA25) of leaf blade length and petiole length (PL). In total, 1400 leaves were measured, 800 of P. spinosa and 600 of C. monogyna.
Statistical analysis
Following the procedure described in Sokal and Rohlf (2012), descriptive statistical parameters for all of the studied traits were calculated, including arithmetic mean (M), standard deviation (SD) and coefficient of variability (CV). These parameters were calculated at individual population level and in total and gave insights into morphological characteristics and range of variation for each population and trait.
In addition, principal component analysis (PCA) was conducted in order to assess population structure and to reveal interactions between individuals and studied morphometric traits. To enhance the analysis, a biplot was constructed by first two principal components. The principal component analysis was conducted using the “MorphoTools” R scripts in R v.3.2.2 (R Core Team 2016).
The Euclidean distance matrix was calculated between all pairs of individuals based on the scores of the first two principal components (PC) considering 10 leaf traits. The average Euclidean distances were calculated for each population and species and used as the multivariate diversity index (MDI) of a population (or species) (Poljak et al. 2024). The Kruskal-Wallis test between species was performed using the STATISTICA version 13 software package (STATISTICA version 13, 2018).
In addition, the Euclidean distance matrix was also used in the analysis of molecular variance (AMOVA; Excoffier et al. 1992) to partition the total morphological variance between species, among populations within species and within populations (two-way AMOVA) and to partition the total morphological variance among and within populations of each species (one-way AMOVA). The significance levels of the variance components were determined after 10.000 permutations. The calculations were performed in Arlequin ver. 3.5.2.2 (Excoffier and Lischer 2010).
Results
The results of descriptive statistics for both species are shown in Tab. 2, individually per population and in total.
Tab. 2. Results of the descriptive statistical analysis for the studied populations and morphometric traits. Morphometric traits analysed: LA – leaf area (cm2); P – leaf perimeter (cm); FC – form coefficient; LL – leaf blade length (cm); MLW – maximum leaf width (cm); PMLW – leaf blade length measured from the leaf base to the point of maximum leaf width (cm); LW90 – leaf blade width at 90% of the leaf blade length (cm); LA10 – angle closed by the main leaf vein and the line defined by the leaf blade base and the point on the leaf margin, at 10% (˚); LA25 – angle closed by the main leaf vein and the line defined by the leaf blade base and the point on the leaf margin, at 25% (˚); PL – petiole length (cm). Descriptive parameters: M – arithmetic mean, SD – standard deviation and CV – coefficient of variation (%). Populations: P1-P7 as in Tab. 1.
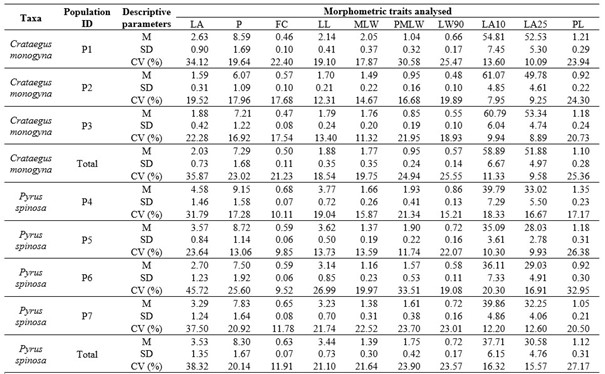
On average, one-seed hawthorn leaves were 1.88 cm long, 1.77 wide, with 1.10 cm long petioles. The morphometric trait that refers to leaf shape, i.e., form coefficient (FC) had an average value of 0.50. The most variable trait was leaf area (LA), with CV value of 35.87%, followed by petiole length (PL) with CV value of 25.36%. On the other hand, the least variable traits were the angles closed by the main leaf vein and the line defined by the leaf blade base and a point on the leaf margin, at 10% (LA10) and 25% (LA25) of leaf blade length, with CV values of 11.33 and 9.58%, respectively. On an individual population level, population P1 (Buje) was characterized by on average the largest leaves, with seven out of 10 maximal values (LA, P, LL, MLW, PMLW, LW90, PL). In contrast, the largest number of minimal values was found in P2 (Pula) (LA, P, LL, MLW, LW90, LA25, PL), which characterizes this population as the one with the smallest leaves. By far the most variable population was P1 (Buje), with the highest CV values for all of the measured leaf traits except petiole length (PL), ranging from 10.09 (LA25) to 34.12 (LA). On the other hand, leaf traits were the least variable in P3 (Nin), with six minimal CV values (P, FC, MLW, LW90, LA25, PL).
The average leaf of the almond-leaved pear was 3.44 cm in length, 1.39 cm in width and had a 1.12 cm-long petiole. Form coefficient has an average value of 0.63. Coefficients of variability between the studied leaf traits ranged from 15.57 (LA25) to 38.32% (LA). The second most variable trait, as for C. monogyna, was petiole length, with CV value of 27.17%. When observing individual populations, P4 (Škropeti) had the highest values in all of the measured leaf traits except LA10. In contrast, population P6 (Nin) had eight out of 10 the lowest average values of leaf morphometric traits (LA, P, FC, LL, MLW, PMLW, LW90, PL). In addition, P6 (Nin) had also the most variable leaf morphology, with seven maximal CV values (LA, P, LL, PMLW, LA10, L25, PL). On the other hand, population P5 (Pula) was the least variable, with seven minimal CV values (LA, P, LL, MLW, PMLW, LA10, LA25).
The multivariate diversity index (MDI) values, based on leaf morphological traits, ranged from 1.639 to 2.474 in C. monogyna, and from 1.727 to 2.712 in P. spinosa (Tab. 1). On overall individual species level, P. spinosa had a significantly larger MDI (2.632) than C. monogyna (2.326), as demonstrated by the Kruskal-Wallis test (P = 0.0023). The results of two-way AMOVA conducted for both species showed statistically significant differences between the two species, among populations within species and within populations (Tab. 3). The analysis also revealed that within-population and between-species variabilities contributed almost equally to the total variability, with 46.21% and 44.89%, respectively. One-way AMOVA conducted on individual species showed significant differences among populations within both species. In addition, within-population variability accounted for most of the total variability in both species.
Tab. 3. The results of two-way (between species) and one-way (within species) analysis of molecular variance (AMOVA). df – degrees of freedom; fST – measure of differentiation among populations; *** significant at P < 0.001; ** significant at 0.001 < P < 0.01.
Principal component analysis (PCA) was conducted, based on 10 morphological leaf traits. The two first principal components explained 83.52% of the total variability, with additional 9.95 and 3.36% explained by the third and fourth principal components, respectively. Five traits were in a high negative correlation with the first principal component (LA, P, LL, PMLW, LW90) and two traits were in a high positive correlation with the same component (LA10, LA25). The second principal component was in a high negative correlation with MLW, while the third principal component was highly positively correlated with FC (Tab. 4).
Tab. 4. Pearson’s correlation coefficients between morphometric traits and scores of the first four principal components. Morphometric traits’ acronyms as in Tab. 2.
The biplot constructed by the first two principal components is shown in Fig. 2. Clear separation of the two species can be observed along the first axis, where barycentres of P. spinosa are separated on the left, and those of C. monogyna on the right side of the first axis. Crataegus monogyna was characterized by generally wider leaf base angles (LA10, LA25), while P. spinosa was characterized by longer and wider leaves.
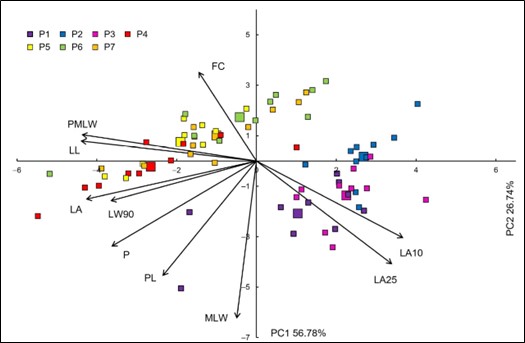
Fig. 2. Biplot of the principal component analysis (PCA) based on ten leaf morphometric traits in the studied Pyrus spinosa and Crataegus monogyna populations. Each individual shrub/tree is indicated by a small sign, while the population barycenters are represented by larger ones. Morphometric traits’ acronyms: LA – leaf area; P – leaf perimeter; FC – form coefficient; LL – leaf blade length; MLW – maximum leaf width; PMLW – leaf blade length measured from the leaf base to the point of maximum leaf width; LW90 – leaf blade width at 90% of the leaf blade length; LA10 – angle closed by the main leaf vein and the line defined by the leaf blade base and the point on the leaf margin, at 10%; LA25 – angle closed by the main leaf vein and the line defined by the leaf blade base and the point on the leaf margin, at 25%; PL – petiole length. Populations acronyms: P1 – Buje; P2 – Pula; P3 – Nin; P4 – Škropeti; P5 – Pula; P6 – Nin; P7 – Obrovac.
However, a few individuals of both species ended up on the opposite side of the axis. For instance, two individuals in P1 were separated on the left side of the first axis, and they were characterized by long petioles and high perimeter value, while a few individuals in P4, P6 and P7 were separated on the right side of the first axis. These few individuals from P. spinosa populations, with unusual, hawthorn-like leaves indicate possible hybridization between these two genera (Fig. 3).
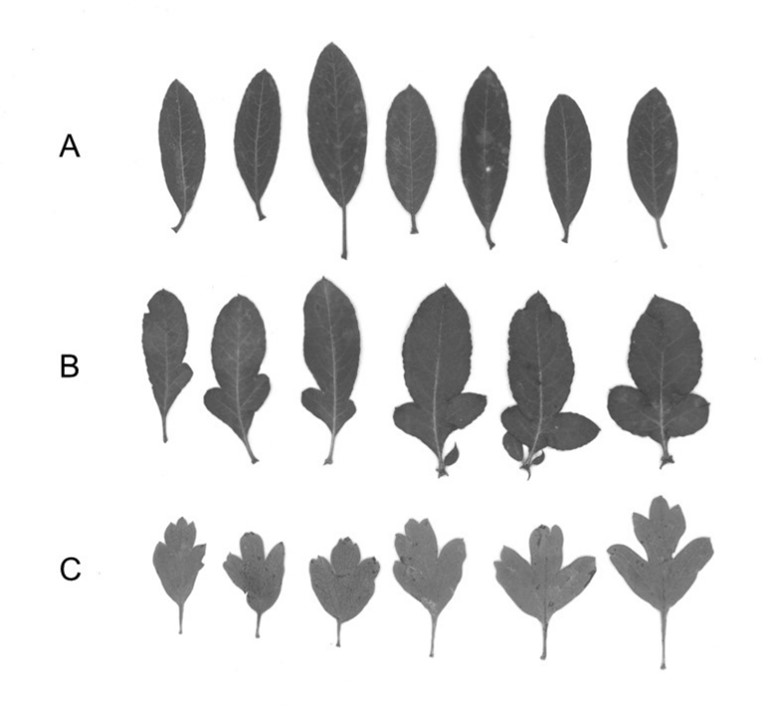
Fig. 3. Leaf variability of Pyrus spinosa (A), possible hybrid between P. spinosa and Crataegus monogyna (B) and C. monogyna (C) from the Nin in the eastern Adriatic.
Discussion
Leaf dimensions of C. monogyna obtained in this research were slightly low compared to the length and width of 3-5 cm listed by Schuck (2008), and within the range of 1-6 cm stated by Fichtner and Wissemann (2021) and Khadivi et al. (2019). The petiole length of 1.10 cm fits within the ranges listed by all the above mentioned authors (1-3 cm). On the other hand, leaf dimensions of P. spinosa were in accordance with previous descriptions of 2.5-7 cm long and 1-3 cm wide leaves with a petiole of 1-2 cm (Idžojtić 2009, Zamani et al. 2012, Vidaković et al. 2021). In both species, the leaf area (LA) and petiole length (PL) were the most variable traits, with CV values above 30% in LA and 25% in PL. Such a pattern of variability is very common among woody species (Khadivi-Khub et al. 2015, Kumar et al. 2018). In C. monoygna, the coefficients of variability in the majority of the measured leaf traits were very similar to that obtained by Khadivi et al. (2019), but significantly higher than in Khadivi-Khub et al. (2015). However, their respective studies did not include leaf area. Coefficients of variability in P. spinosa traits ranged from 11.91 to 38.32%, which is lower than the range of 18.02-45.62% obtained by Vidaković et al. (2021).
According to AMOVA, the majority of total variability in both species could be attributed to within-population variability, while a much smaller percentage was associated with among-population variability. Such a distribution of variability is expected, as it was previously confirmed in many other insect-pollinated and animal-dispersed species (Vidaković et al. 2021, 2022). However, C. monogyna populations were somewhat better differentiated than those of P. spinosa, which is also supported by weak genetic differentiation of P. spinosa populations in the area (Vidaković et al. 2024). Furthermore, significant differences in morphological variability between the two species were confirmed by MDI values, which demonstrated greater morphological variability of P. spinosa. This result is supported by the greater overall variability of leaf morphological traits in P. spinosa (Vidaković et al. 2021) than in C. monogyna (Khadivi-Khub et al. 2015, Khadivi et al. 2019). This could be the result of adaptation to microhabitat conditions, but also of phylogenetic and evolutionary processes, for P. spinosa exhibits greater morphological variability than the phylogenetically older P. pyraster (L.) Burgsd. (Korotkova et al. 2018, Vidaković et al. 2021, 2022). This may indicate still ongoing evolutionary speciation and morphological differentiation, resulting in more diverse leaf morphology.
As mentioned in the Introduction section, during field research in 2021, conducted along the eastern Adriatic coast, we observed a few P. spinosa individuals with unusual, hawthorn-like leaves on numerous branches. Hybridization between Pyrus and Crataegus could indeed be possible, as they both belong to the tribe Maleae (Sun et al. 2024), which indicates their close taxonomic relationship. Furthermore, both genera possess the basal chromosome number of 17 (Evans and Campbell 2002), which is thought to have originated from aneuploidization events approximately 50 million years ago (Considine et al. 2012), with Gillenia Moench as a probable common ancestor (Sun et al. 2024). A common chromosome number, along with coordinated flowering phenology, reproductive compatibility and common pollinators, is one of the main prerequisites for successful hybridization (Rieseberg and Carney 1998).
Our results based on the morphological analysis of the leaves did indeed show a few intermediate individuals, indicating possible hybridization between the two species. Alternatively, the dimorphic leaves of P. spinosa individuals could be explained by the sporadic appearance of juvenile leaves in the adult stage. Namely, almond-leaved pear seedlings were reported to have lobed, hawthorn-like leaves (Dostálek 1980), which was also observed by personal observation of young plants in an ongoing outdoor seed germination experiment carried out on the Faculty of Forestry and Wood Technology in Zagreb. Such substantial differences between juvenile and subsequent or adult forms in plant species are known as heteroblasty (Zotz et al. 2011). This botanical phenomenon of distinct morphological phenotypes in juvenile, transitional and adult stages is present in many agricultural species, as well as in some woody species like Acacia confusa Merr., A. colei Maslin et L.A.J.Thomson, Eucalyptus globulus Labill., Hedera helix L., Quercus acutissima Carruth. and Populus spp. (Manuela and Xu 2020).
The reappearance of juvenile leaves in the adult stage could be explained by the process of rejuvenation. This process enables plants to reverse the adult phase characteristics and recover some juvenile traits (Zhang et al. 2020). Small RNA profiling revealed an increase in microRNA156 (miR156) during plant rejuvenation (Chen et al. 2013), which maintains juvenile traits by repressing a group of SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) transcription factors (Ye et al. 2019). Additionally, miR156 is subject to epigenetic regulation (Manuela and Xu 2020), which makes epigenetics one of the main factors controlling plant development and rejuvenation (Zhang et al. 2020). For instance, new sprouts from the adult tree collar or water sprouts, which are very common in pears, are considered to be ontogenetically juvenile, compared to their parent tree (del Tredici 2001). Among other juvenile traits that occur on such sprouts are dimorphic leaves, usually larger and more variable in shape (del Tredici 2017). In our case, this could be an alternative explanation for the occurrence of dimorphic leaves in this P. spinosa. However, further genetic and morphometric studies should be conducted in order to draw a definite conclusion about hybridization between P. spinosa and C. monogyna.
Conclusions
Both the almond-leaved pear and the one-seed hawthorn are widespread in the coastal areas of Southern Europe and play a vital role in local ecosystems and the maintenance of biodiversity. This study was aimed at supplementing knowledge on the morphological variability of these two sympatric species. The results showed great variability of leaf morphological traits within and between studied populations, as well as a clear differentiation between the two species. However, hawthorn populations were better differentiated than those of the almond-leaved pear, but the latter had generally more diverse leaf morphology. High variability of almond-leaved pear leaves was also manifested through the presence of dimorphic, hawthorn-like leaves on some individuals, which raised the suspicion of the presence of a long-described but under-investigated hybrid between the two genera. Although the results showed several intermediate individuals, a possible explanation for dimorphic leaves on almond-leaved pear individuals, apart from hybridization, could be the reappearance of juvenile leaves on adult trees by means of rejuvenation. In order to draw a definitive conclusion about the existence of hybrid individuals, in the next study, DNA markers and a much larger sample, especially of morphologically intermediate individuals per population, should be included.
Acknowledgements
The authors have contributed to the special issue of Acta Botanica Croatica on the occasion of its 100th anniversary issue.
This research was funded by the University of Zagreb financial support.
Author contribution statement
A.V., Z.Š., Z.L., I.P – conceptualization and study design; A.V., I.P. – sampling; M.J. – morphometric analysis; Z.Š., I.P. – data analysis; A.V., I.P – writing original draft preparation; Z.Š., Z.L., M.J. – review and editing. All authors have read and agreed to the published version of the manuscript.
