Introduction
Cow’s milk and dairy products have long traditions in human nutrition (Jianqin et al., 2016). The consumption of milk and milk products vary considerably among regions - from about 180 kg of drinking milk yearly per capita in Island and Finland to less than 50 kg in Japan and China (Singh et al., 2015). Milk is a complex food made up of components, which per se may have negative or positive health effects, respectively (Puvača et al., 2020). Milk composition can be altered by the feeding regime. Cow milk contains the nutrients needed for the growth and development of the calf and is a source of lipids, proteins, amino acids, vitamins, and minerals. It contains immunoglobulins, hormones, growth factors, cytokines, nucleotides, peptides, polyamines, enzymes, and other bioactive peptides (Haug et al., 2007). Milk composition has a dynamic nature, and the composition varies with the stage of lactation, age, breed, nutrition, energy balance, and health status of the udder (de Vries and Veerkamp, 2000).
Generally speaking, milk represents a significant source of bacteria (Puvača et al., 2020). When milked from the healthy udder of cows, milk contains a variety of microorganism who enters the teat canal through its opening (Bhutto et al., 2010). The number of these microorganisms ranges between a hundred and thousand in 1mL (Roberts et al., 2005).
Milk and milk products are the prime habitats to complex microbial ecosystems which are responsible for the broad variations in taste, aroma, and texture of milk and milk products. Contamination of milk and milk products with pathogenic bacteria is mainly due to processing, handling, and unhygienic environment (te Giffel & Wells-Bennik, 2010). The occurrence of these pathogenic bacteria in milk and milk products can cause severe health hazards to people and cause food borne diseases.
Food-borne diseases (FBD) are defined by the World Health Organization as diseases of infectious or toxic nature caused by or thought to be caused by the consumption of food or water (Kadariya et al., 2014). The pathogenesis of bacteria causing food-borne poisoning depends on their capacity to produce toxins after ingestion or intoxication (Sergelidis and Angelidis, 2017). Among the bacteria predominantly involved in these diseases, S. aureus is a leading cause of gastroenteritis resulting from the consumption of contaminated food. Staphylococcal food poisoning is due to the absorption of staphylococcal enterotoxins contained in the food (Puvača et al., 2021).
S. aureus mastitis is a serious problem in dairy production and infected cows may contaminate raw milk. S. aureus is a very important cause of FBD worldwide (Rainard et al., 2018). The ability of S. aureus to grow and produce staphylococcal enterotoxins under a wide range of conditions is evident from the variety of foods implicated in staphylococcal food poisoning.
Antibiotics are used to treat animal diseases, but their indiscriminate use has led to the development of multiple antibiotic resistances thereby rendering the antibiotic treatment ineffective. Resistant bacteria occur in soil, water, plants, and animals. The resistant bacteria present in environments are in contact with human beings and animals. It has been estimated that nearly equal ton of antimicrobial agents is used in the human population and agriculture worldwide (Iwu et al., 2020). Antimicrobial resistance is a major public health concern in many countries due to the persistent circulation of resistant strains of bacteria in the environment and the possible contamination of water and food. S. aureus has been reported to frequently show multiple antimicrobial resistance patterns (Kovačević et al., 2021; Puvača and de Llanos Frutos, 2021).
This paper aimed to determine the prevalence rate of antimicrobial resistance of S. aureus isolated from raw cow milk samples in Western Balkan countries Albania and Serbia.
Materials and methods
Sample collection
A total of 100 samples of raw milk, 50 from Albania, and 50 from Serbia was randomly collected from dairy cows’ productions farms between September 2020 and February 2021. Collected samples were immediately transported to the laboratory in a cooler with ice packs and were processed within one hour of collection.
Isolation of S. aureus
The samples were processed immediately upon arrival using aseptic techniques. To detect S. aureus, 1mL of each milk sample was inoculated on Baird - Parker agar (Difco, Detroit, Michigan, USA). After 24 - 48 h of incubation at 37 °C, suspected colonies were sub-cultured on a blood agar plate (Difco, Detroit, Michigan, USA) and incubated for 24 h at 37 °C. To identify S. aureus, Gram stain, catalase, coagulase, and Voges-Proskauer (VP) tests were conducted on suspected colonies. One strain from each S. aureus-positive sample was further tested for antimicrobial susceptibility and minimum inhibitory concentrations (MICs) of antibiotics (total of 22 for Albania and 29 for Serbia).
Antimicrobial susceptibility testing
One strain from each S. aureus-positive sample was selected for susceptibility tests. Antimicrobial susceptibility testing was performed by the Kirby-Bauer disc diffusion method using Mueller-Hinton agar supplemented with 5 % defibrinated sheep blood, according to the Clinical Laboratory Standards Institute. The following antimicrobial impregnated disks were used: tetracycline (30 μg), cephalothin (30 μg), vancomycin (30 μg), chloramphenicol (30 μg), ciprofloxacin (30 μg), sulfatrim (25 μg), oxacillin (15 μg), erythromycin (15 μg), ampicillin (10 μg), and methicillin (5 μg). After incubation at 37 °C for 48 h, the susceptibility of the S. aureus isolates to each antimicrobial agent was measured and the results were interpreted following interpretive criteria provided by Clinical Laboratory Standards Institute (CLSI, 2018).
Minimum inhibitory concentrations (MICs) of antibiotics
The MICs of then representative antibiotics were determined according to ISO Standard 10932:2010 using VetMIC plates for LAB (National Veterinary Institute, Uppsala, Sweden). Individual colonies grown on Mueller-Hinton agar plates were suspended in 2 mL sterile saline solution (Oxoid) to obtain a density corresponding to McFarland standard 1 (3 × 10 8 cfu/mL). This suspension was further diluted 1:1000 in Mueller-Hinton broth to a final concentration of about 3 × 10 5 cfu/mL. One hundred microliter of this inoculum were then added to each well of the VetMIC plate. The VetMIC plates contained serial 2-fold dilutions of the antibiotic’s tetracycline, cephalothin, vancomycin, chloramphenicol, ciprofloxacin, sulfatrim, oxacillin, erythromycin, ampicillin, and methicillin. MICs were visually read after 48 h of incubation at 37 °C.
Determination of antibiotic residues in milk samples
A total of 10 ± 0.1 g of the milk samples were weighed into a 50 mL polypropylene centrifuge tube, after adding 2 mL of Na 2EDTA-McIlvaine buffer solution (0.1 M, pH 4.0) and vortexing for 1 min, 10 mL of acetonitrile for protein precipitation was added, vortexing again for 1 min, followed by centrifugation at 4000 rpm/min for 5 min. The supernatant was transferred to a 15 mL polypropylene centrifuge tube, containing MgSO 4, PSA, and C18 sorbent. For sample clean-up, the supernatant was vortexed for 2 min and shaken for 2 min, and put in a fridge for one hour, followed by centrifugation at 4000 rpm/min for 5 min. After centrifugation, 5 mL of the clean supernatant is transferred to a vial after filtration (0.45 μm, PTFE).
The limit of quantification (LOQ) was established to 0.005 µg/L for all antibiotics. The recoveries ranged from 76 % (erythromycin) to 92% (vancomycin) using the standard addition approach for the calibration and internal standard trimetophrim-D9. The linearity: The R 2 in the concentration range from 0.005 to 0.05 ng/mL of the calibration curves for the method was higher than 0.99 for all investigated antibiotics (Table 1).
Table 1. Multiple reaction monitoring transitions, retention time, collision energies, and fragmentation as acquisition parameters
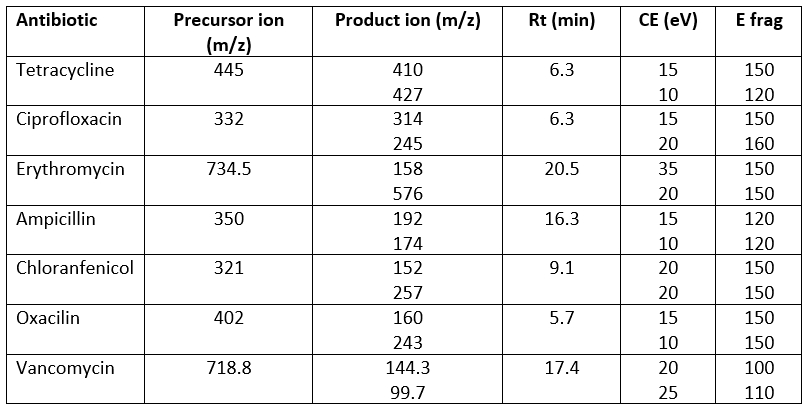
HPLC Agilent 1290 Infinity II chromatograph equipped with a quaternary pump, multi sampler, and column compartment thermostat was used for the detection of 81 pesticides. The HPLC system was coupled to an Agilent 6495 LC/TQ triple quadrupole mass spectrometer with AJS ESI (Jet Stream Technology Ion Source). A Zorbax Eclipse Plus C18 column Rapid Resolution HD (50×2.1mm, 1.8 µm particle size) was used for the chromatographic separation. The flow rate (0.25 mL/min) of the mobile phase was used and the injection volume for the LC system was 2 µL. Also, the column temperature was hold on at 35 ºC. The chromatographic separation of pesticides was carried out with a mobile phase consisted of water (A) and methanol (B) both contained formic acid (0.1 %, v/v) in a gradient mode. A gradient elution started at 5 % of B and held 1 min. This composition was increased to 40 % B at 7 min, 90 % B at 8 min, and held for 2 min. The composition of the mobile phase returned to the initial conditions in 1 min and the system was equilibrated for 2 min. The total running time was 11 min. The ESI source was used with the following settings: drying gas (nitrogen) temperature 200 °C, drying gas flow rate 16 L/min, nebulizer pressure 30 psi, sheath gas temperature 300 °C, sheath gas flow 12 L/min, and capillary voltage 3000 V. The detection was performed using the dynamic multiple reactions monitoring mode (dMRM). The Agilent MassHunter software (version B.10.0 SR1 Agilent Technologies, 2006-2019) was used for the optimization and quantification.
Results and discussion
In this study, we described the isolation and antibiotic susceptibility characterization of S. aureus strains isolated from cow milk obtained from two Western Balkan countries, Albania and Serbia. Twelve of 100 samples (12 %) were positive for S. aureus. Five raw cow milk samples from Albania (41.66 %), and seven raw milk samples from Serbia (58.33 %) were contaminated with S. aureus. This contamination rate is similar to that observed in the surveys previously conducted in other countries on several kinds of raw milk (Ertas et al., 2010; Normanno, La Salandra, et al., 2007). The resistance pattern of S. aureus isolates to 6 antimicrobial agents tested in this study is presented in Table 2. Most of the isolates were resistant to one or more antimicrobial agents. MDR which is defined as resistance to 3 or more antimicrobial agents was found in S. aureus raw milk isolates. Resistance to ampicillin of S. aureus isolates from both milk samples, Albania (36.7 %) and Serbia (34.1 %), was the most common. Isolates from milk samples from Albania were most resistant to tetracycline (16.9 %), compared to isolates from Serbian milk samples which showed higher resistance towards oxacillin (18 %). Recorded resistance towards erythromycin (13.2 %; 13.1 %), and sulfatrim (7.6 %; 6.9 %) was similar between isolates from samples of milk of both countries. Obtained results have shown higher resistance of isolates towards cephalothin in milk samples from Serbia (3.9 %), compared to milk samples from Albania (1.6 %), respectively. All isolates tested for antibiotic sensitivity were susceptible to methicillin, vancomycin, chloramphenicol, and ciprofloxacin.
The antimicrobial resistance profile of the tested S. aureus strains shows susceptibility to at least one of tested antibiotics (Figure 1).
Table 2. Overall antimicrobial resistance of S. aureus strains isolated from raw milk samples, 5 from Albania and 7 from Serbia, (%)
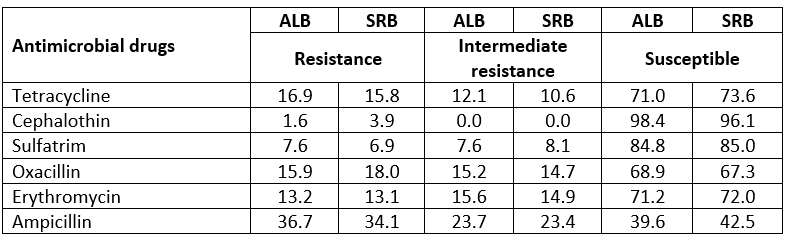
ALB - Albania; SRB - Serbia
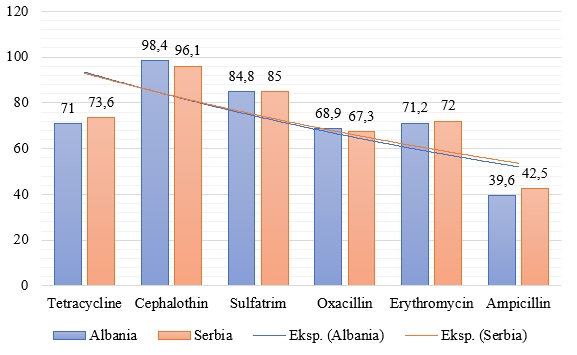
Figure 1.Antibiotics' susceptibility of S. aureus strains isolated from raw milk samples, 5 from Albania and 7 from Serbia, (%)
As reported by some previous studies, the resistance of S. aureus isolates to β-lactams such as ampicillin, penicillin, tetracycline, and oxacillin was evident (Peles et al., 2007; Pereira et al., 2009; Yucel et al., 2010). The finding that a large number of S. aureus were resistant to ampicillin, penicillin, tetracycline, and oxacillin are, however, a cause for concern and should be further investigated. These drugs are commonly used in veterinary medicine in Albania and Serbia.
Table 3 shows the MICs of six antibiotics to S. aureus strains of this study. Analysis of these MICs and comparison with data in the literature indicated that isolates were resistant to tetracycline, oxacillin, erythromycin, and ampicillin, respectively.
Table 3. Distribution of MICs of antibiotics to S. aureus isolated from raw milk samples. One strain from each S. aureus-positive sample was tested, total of 22 for Albania and 29 for Serbia
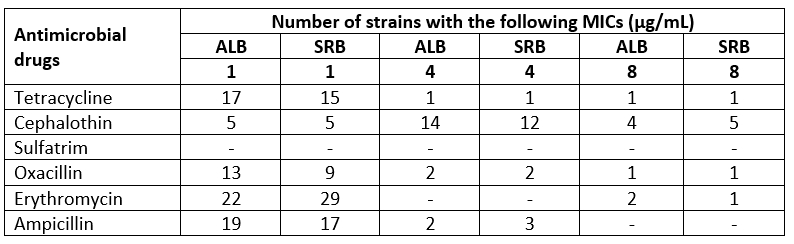
Variations in the MIC values in S. aureus isolates similar to those obtained in the present work have been reported elsewhere for other starter strains (Alegría et al., 2013; Maxson et al., 2017; Titouche et al., 2019). In almost all analysed milk samples originated both from Albania and Serbia, resistance to antibiotics cephalothin and erythromycin was absent. This absence of AMR indicates that these antibiotics are not in frequent use in cows’ therapy on farms. A similar tendency is observed regarding the sulfatrim, with a recorded small percentage of AMR of isolates. Previously, several studies have shown that S. aureus isolated from meat, and milk products express resistance towards sulfatrim (Hachemi et al., 2019; Normanno et al., 2007; Pekana and Green, 2018).
Earlier reports have shown that Gram-positive bacteria are susceptible to methicillin, vancomycin, chloramphenicol, and ciprofloxacin (Watson et al., 2019). Due to methicillin resistance of S. aureus (MRSA) these antibiotics have been excluded from animal treatments at the farms, regardless of the fact that MRSA has been related mostly to mastitis (Luini et al., 2015; Vanderhaeghen et al., 2010).
The occurrence of antibiotic-resistant bacteria in food commodities that can be transmitted to the human population led to a ban of antibiotic usage as a growth promoter in food animals’ daily diet (Capita and Alonso-Calleja, 2013; Khachatourians, 1998; Kostadinović and Lević, 2018). Results of our research have shown that resistant bacteria can be transferred to cow milk, which can be one of the main reasons for infection occurrence and resistance to certain antibiotics in humans.
To overcome the very important rising problem in dairy farm production, biosecurity levels should be increase as well as hygienic conditions on the farm, and appropriate handling of the cow during the milking.
S. aureus was isolated from twelve milk samples in total from Albania and Serbia region. A significant proportion of the isolates obtained were resistant to three or more antibiotics. One of the aspects that need to be investigated is the cause of the observed resistance phenotypes. Furthermore, impacts and dynamics of genetic antibiotic determinants should also be investigated using molecular methods. Therefore, antibiotic residues in examined milk samples were determined. The obtained results of antibiotic residues in milk samples are presented in Table 4.
Table 4. Residues of antibiotics in milk samples from Albania and Serbia, (%)
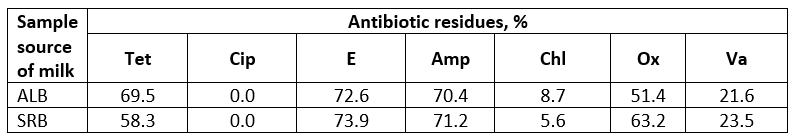
Tet - Tetracycline; Cip - Ciprofloxacin; E - Erythromycin; Amp - Ampicillin; Chl - Chloranfenicol; Ox - Oxacilin; Va - Vancomycin
The prevalence of S. aureus has been reported to vary with the size and geographic region of the area sampled, a high proportion of these bacteria in milk relates to poor hygiene practices (Ateba et al., 2010; Mahlangu et al., 2018; Reta et al., 2016). Results of our investigation have shown that the highest antibiotics residues are related to the usage of erythromycin (72.6 % and 73.9 %), followed by ampicillin (70.4 % and 71.2 %) while residues of ciprofloxacin in investigated milk samples was not registered. In our study, we have used seven antimicrobial agents, from different antibiotic classes, and the selection has been made because some studies have shown that large numbers of bacteria were resistant to them (Ateba and Bezuidenhout, 2008).
The analyses of 12 milk samples were done with this highly sensitive and selective LC-MS/MS method. The total ion chromatogram (TIC) of 12 milk samples in total for erythromycin antibiotic is given in Figure 2.
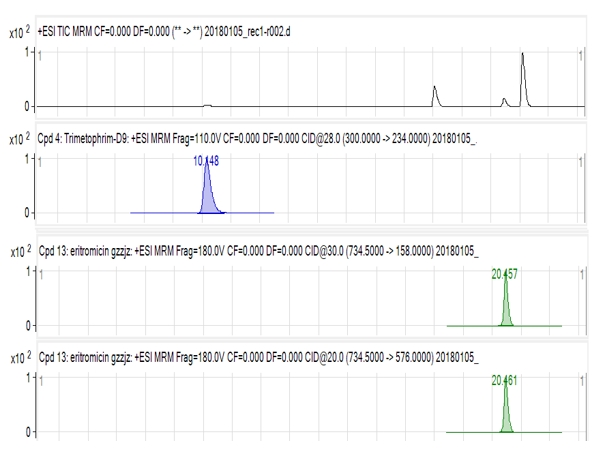
Figure 2. The total ion chromatogram (TIC) of milk samples for erythromycin
S. aureus is normally resident in humans; therefore the S. aureus present in cow milk may have resulted from transmission from humans, which raises questions regarding the hygiene practices followed. The inadequate hygiene and poor farm management practices contribute to the presence of S. aureus in the milk (Abebe et al., 2016). Therefore, an investigation of the antibiotic-resistance profiles of isolates could serve as a tool in assessing both the sanitary conditions employed during milking and the health risks that humans may encounter when infected by antibiotic-resistant strains.
Conclusion
S. aureus was successfully isolated from milk samples and a large proportion of S. aureus isolates exhibited resistance towards the different antibiotics tested. Thus, it is evident that most of the isolates possessed multiple antibiotic resistance traits. Though the development of antibiotic-resistant determinants in S. aureus is associated with the uncontrolled usage of antibiotics in human and veterinary medicine, the incidence of drug-resistant S. aureus in raw milk samples warrants closer monitoring. Our results have shown that 12 of the total 100 investigated samples were positive for S. aureus. Five raw cow milk samples from Albania (41.66%), and seven raw milk samples from Serbia (58.33 %) were contaminated with S. aureus. Resistance to ampicillin of S. aureus isolates from both milk samples from Albania (36.7 %) and Serbia (34.1 %) was the most common, followed by resistance to tetracycline (16.9 %) of isolates from Albanian milk samples, and towards oxacillin (18 %) of isolates from Serbian milk samples. The recorded resistance towards erythromycin (13.2 %; 13.1 %), and sulfatrim (7.6 %; 6.9 %) was similar between isolates from both samples of milk. The obtained results have shown higher resistance towards cephalothin of S. aureus isolates from milk samples from Serbia (3.9 %), compared to milk samples from Albania (1.6 %), respectively. The obtained results regarding MICs of antibiotics indicated that isolates were resistant to tetracycline, oxacillin, erythromycin, and ampicillin, respectively. Milk contamination is a matter of a serious public health concern since they are widely consumed by infants, children, and the elderly population. This is crucial since young children may be highly exposed to the residues of antibiotics and could be at a higher risk of adverse health effects due to their physiology and metabolism.
Antimikrobna rezistencija sojeva Staphylococcus aureus izoliranih iz uzoraka sirovog kravljeg mlijeka s područja Albanije i Srbije
Sažetak
Cilj ovog istraživanja bio je utvrditi stopu prevalencije antimikrobne rezistencije (AMR) Staphylococcus aureus izoliranog iz uzoraka sirovog kravljeg mlijeka u Albaniji i Srbiji. Na nasumično odabranim farmama krava prikupljeno je ukupno 100 uzoraka sirovog mlijeka, 50 iz Albanije i 50 iz Srbije. Dvanaest uzoraka (12 %) je bilo pozitivno na S. aureus, od čega je pet bilo porijeklom iz Albanije (41,66 %) i sedam iz Srbije (58,33%). Rezistentnost izoliranih sojeva S. aureus na ampicilin u oba uzorka mlijeka iz Albanije (36,7 %) i Srbije (34,1 %) bio je najčešći. Neki sojevi S. aureus iz uzoraka mlijeka iz Albanije bili su rezistentni na tetraciklin (16,9 %), dok su izolati iz uzoraka mlijeka iz Srbije bili rezistentni na oksacilin (18 %). Zabilježena rezistencija na eritromicin (13,2 %; 13,1 %) i sulfatrim (7,6 %; 6,9 %) bila je slična između oba uzorka mlijeka. Dobiveni rezultati pokazali su veću rezistentnost sojeva S. aureus prema cefalotinu u uzorcima mlijeka iz Srbije (3,9 %), u odnosu na uzorke mlijeka iz Albanije (1,6 %). Svi izolati testirani na rezistentnost na antibiotike bili su osjetljivi na meticilin, vankomicin, kloramfenikol i ciprofloksacin. Rezultati dobiveni određivanjem minimalnih inhibitornih koncentracija (MIC) antibiotika pokazali su da su izolati rezistentni na tetraciklin, oksacilin, eritromicin i ampicilin. Rezultati našeg istraživanja pokazali su kako su pronađeni ostaci antibiotika povezani s upotrebom eritromicina (72,6 % i 73,9 %) i ampicilina (70,4% i 71,2%), dok ostaci ciprofloksacina u ispitivanim uzorcima mlijeka nisu registrirani.
Ključne riječi: mlijeko, sigurnost mlijeka, AMR; antibiotici, S. aureus.
Acknowledgments
This research was supported by the Ministry for Education, Science, and Technological Development of the Republic of Serbia.
References
https://doi.org/10.1186/s12917-016-0905-3
https://doi.org/10.1007/s13594-013-0128-3
https://doi.org/10.1016/j.ijfoodmicro.2008.08.011
https://doi.org/10.10520/EJC96992
https://doi.org/10.1016/j.tvjl.2008.08.024
https://doi.org/10.1080/10408398.2010.519837
https://doi.org/10.3168/jds.S0022-0302(00)74856-9
https://doi.org/10.1016/j.ijfoodmicro.2010.06.002
https://doi.org/10.14202/vetworld.2019.1240-1250
https://doi.org/10.1186/1476-511X-6-25
https://doi.org/10.1002/mbo3.1035
https://doi.org/10.1186/s12937-016-0147-z
https://doi.org/10.1155/2014/827965
https://doi.org/10.3390/antibiotics10020205
https://doi.org/10.1016/j.vetmic.2015.05.010
https://doi.org/10.1155/2018/3801479
https://doi.org/10.1371/journal.pone.0183899
https://doi.org/10.1016/j.ijfoodmicro.2007.04.006
https://doi.org/10.1016/j.ijfoodmicro.2006.10.049
https://doi.org/10.3390/ijerph15102223
https://doi.org/10.1016/j.ijfoodmicro.2007.07.010
https://doi.org/10.1016/j.fm.2008.12.008
https://doi.org/10.3390/antibiotics10010069
https://doi.org/10.15567/mljekarstvo.2020.0102
https://doi.org/10.3390/antibiotics10050546
https://doi.org/10.1111/tbed.12698
https://doi.org/10.1186/s40550-016-0027-5
https://doi.org/10.1007/0-387-28801-5_16
https://doi.org/10.1111/lam.12735
https://doi.org/10.1371/journal.pone.0124845
te Giffel, M.C., Wells-Bennik, M.H.J. (2010): 7-Good hygienic practice in milk production and processing. In M.W. Griffiths (Ed.), Improving the Safety and Quality of Milk (pp. 179-193). Woodhead Publishing.
https://doi.org/10.1533/9781845699420.2.179
https://doi.org/10.3168/jds.2018-16208
https://doi.org/10.1016/j.vetmic.2009.12.044
https://doi.org/10.1111/ceo.13364
https://doi.org/10.1089/fpd.2010.0707
