Introduction
Milk products are vital components of the food articles as added-value products (Calcium-enriched) and dairy-based functional foods with added probiotics (Ozer and Kirmaci, 2010; Ibrahim et al., 2021). Around the world, yogurt consumption with different flavours increased due to its excellent acceptable taste, balanced nutrients, and therapeutic value. Drinking yogurt is an elevating interest's level due to its appropriateness, easily carried, and advantages to human health (Olugbuyiro, 2011; Bhattarai and Das, 2016).
Worldwide, cheese is a popular product because of its health and flavour advantages. Besides, cheeses are satisfactory sources for dietary calcium, phosphorus, proteins, and decreasing the possibility of diabetes type 2 occurred. Based on the microbial content, the safety of food is represented with great attention to people, health authorities, and food manufacturers all over the world (Martínez-Ruiz et al., 2013; CDIC, 2014). Recently, the consumption of plant-origin analogous dairy products (cheese substitutes) has increased in Egypt, especially flavoured cheese, due to many benefits such as; lower cost-effectiveness and its simplicity of processing using cheaper vegetable products to use in the catering cheese sectors (Guinee, 2007).
Plant-origin cheese analogues are manufactured by blending various edible oils/fats, proteins, other ingredients, and water into a smooth homogeneous blend with the aid of heat, mechanical shear, and emulsifying salts. The proteolytic power of plant-origin coagulants as peppers and tomatoes are of higher specificity on αs1- and β-casein than the chymosin (Dublin, 2020; Sulejmani et al., 2021).
The pathogens occurrence in milk products is a significant concern in people's health in various world countries since dairy items can aid the food microbe growth. Food pathogens can contaminate the milk products from different sources and during manufacturing steps. Salmonella spp., E. coli, and Coagulase Positive Staphylococci (CPS) are the main pathogenic bacteria associated with food diseases due to milk items eating, and these are ubiquitous. Examinations for pathogens contamination are pertinent for the dairy industry because microbes' existence in the products causes economic damages due to non-agreement with legal specifications and negatively affecting public health (EFSA and ECDC, 2014; Choi et al., 2016).
Aerobic spore-forming bacteria include Bacillus cereus are a significant concern to the dairy industry, less for their pathogenicity but more for their spoilage-causing capabilities. Food safety and product quality are affected by different mechanisms; production of toxins, spoilage enzymes, and negatively affecting the milk products processing especially, cheeses and yogurt. A particular concern for these spore form bacteria, they withstand harsh environmental conditions (Postollec et al., 2012).
Based on the ubiquitous distribution of Bacillus cereus, it is virtually impossible to obtain products that are free from Bacillus cereus spores (Griffiths and Schraft, 2017). However, high numbers in foods are responsible for disease occurring. Frequently this is associated with keeping foods under conditions permitting multiplication of Bacillus cereus, which can be through improper refrigeration, not enough cooling, or keeping foods warm at <60 °C. Bacillus cereus is responsible for 2 food poisoning models: the first, diarrheal syndrome and the second vomiting syndrome. The first syndrome occurs by heat-labile toxins in the small intestine like the enterotoxin ( nhe) without haemolysis and cytotoxin K. The second syndrome has been produced by the toxin cereulide, which is heat resistant (Ehling-Schulz et al ., 2015).
From 2010 to 2012, Bacillus outbreaks increased significantly (99 outbreaks in 2010; 220 outbreaks in 2011; 259 outbreaks in 2012) in Europe, also causing mastitis and meningitis (EFSA and ECDC, 2014; Nesma et al ., 2020). B. cereus has emerged as the second foodborne pathogen after S. aureus and identified as a causative agent in 19 % of foodborne outbreaks was reported from 1998 to 2008 in the United States (Bennet et al ., 2013; Glasset et al., 2016). WHO (2007) stated that B. cereus becomes a high spread in pasteurized dairy items.
Therefore, our study aimed to evaluate the microbial contamination levels of the selected milk products and investigate B. cereus virulence genes using PCR technique plus focusing on plant-origin flavored cheeses and flavored drinking yogurt, which have limited data around the world, especially in Egypt.
Materials and methods
Sample collection
A total of three hundred random samples of plant-origin flavoured (pepper and Tomato) cheese, flavoured yogurt (strawberry and banana), flavoured drinking yogurt (banana and strawberry), kareish, white soft, and Ras cheeses (representing 50 samples for each) obtained from retail markets at different districts in El Fayoum Governorate from November 2019 to January 2021. The quantity of each cheese type was 250 g, while yogurt and drinking yogurt samples, each package contained about 105 g and 440 mL, respectively. Samples were transported under completely aseptic conditions in a cool isolated icebox as soon as possible to the laboratory.
Microbiological examination
Preparation of dairy products homogenate and decimal dilutions
11 g of sample and sterile 99 mL of 2 % sodium citrate (Sigma-Aldrich) solution (cheese samples) or 99 mL of 0.1 % peptone (Himedia, RM001) water (yogurt samples) were mixed in a polyethylene bag and then placed into a Lab-Blender/400-Seward (Worthing UK) to obtain 1/10 dilution. 1 mL of the previously prepared homogenate and 9 mL of 0.1% peptone water have mixed to prepare tenth fold decimal dilutions (APHA, 2015).
Enumeration of microorganisms in the examined samples
Coliforms count using three tubes (MPN/g) method based on (ISO, 4831: 2006). Count for Total Staphylococci and Coagulase Positive Staphylococci was done according to (BAM, 2016). Total yeasts and molds count was done based on (ISO, 21527-1: 2008).
Aerobic Spore Formers (ASF) was count according to (APHA, 2015). The homogenized samples were exposed to the shock of heat in a water bath at 80±1 °C for 12 min. After heat treatment, the samples were cooled immediately in an ice bath. Then the samples were inoculated in a plate count (Himedia, M091A) agar supplemented with 0.1 % soluble starch, using the pour plate technique. The plates were incubated at 32±1 °C for 48 h. The count of aerobic spore formers/g was calculated. Mannitol Egg Yolk-Polymyxin (MYP) agar (Himedia, M636F) plates were surface plated by spreading 0.1 mL of appropriate previously prepared serial dilutions and then incubated inverted at 32 °C for 24 h. In low count samples, 1 mL of the first dilution was distributed and spread on four plates of (MYP): 0.3 mL (3 plates) and 0.1 mL. B. cereus count was calculated after complete confirmation based on Amore et al. (2018).
Isolation and identification of the selected microorganisms in the examined samples
Isolation of E. coli was performed according to (SO 7251: 2005, Salmonella spp. according to ISO 6579-1: 2017, and Pseudomonas aeruginosa by Pseudomonas agar base (Himedia, M085) plus CetriNex supplement (FD029, Himedia) according to ISO 11059:2017).
For all isolates of ASF: ancillary biochemical tests (using Gram stain, Voges-Proskauer, dextrose fermentation anaerobically, reduction using nitrate broth, hydrolysis of tyrosine, lysozyme resistance, rhizoid growth, stained Crystal Protein, staining motility, haemolytic activity, growth at fifty degrees C, proteolytic activity on milk agar (Oxoid CM0021) and polysaccharide starch hydrolysis) were applied according to Bennett et al. (2015). Matrix-assisted laser desorption ionization-time off light mass spectrometry (MALDI-TOF MS) based on Yan et al . (2020) was done to confirm the examined isolates identification. Suspected isolates of Salmonella spp., E. coli, and S. aureus were identified according to De Vos et al. (2009).
Molecular identification of B. cereus isolates and detection of its virulence genes (Deoxyribonucleic acid extraction and multiplex PCR for virulence genes detection)
Deoxyribonucleic acid from the identified B. cereus culture was extracted using a Gene JET purification kit (Thermo Fisher, K0721). DNA found in the supernatant is stored at -20 °C. GyrB gene detection was performed by multiplex PCR using the primer pair BC1F/BC2rR at 365bp, (ATT GGT GAC ACC GAT CAA ACA: TCA TAC GTA TGG ATG TTA TTC), recognized as B. cereus using B. cereus positive control (ATCC ® 10876 TM), and tested for enterotoxigenic genes ( bceT, nhe, hbl, cytK, and ces).
The sequences (F:R) of primers were (TTACATTACCAGGACGTGCTT: TGTTTGTGATTGTAATTCAGG;AAGCIGCTCTTCGIATTC:ITIGTTGAAATAA GCTGTGG;GTAAATTAIGATGAICAATTTC:AGAATAGGCATTCATAGATT; A CAGATATCGGICAAAATGC:CAAGTIACTTGACCIGTTGC; GGTGACACATT ATCATATAAGGTG:GTAAGCGAACCTGTCTGTAACAACA) for bceT, nhe, hbl, cytK and ces genes at 428, 766, 1091, 421, 1271 bp respectively. The amplification cycles were achieved by a PT -100 Thermo-cycler (MJ Research, USA). PCR were done 2 times/isolate and visualization of products was achieved in 1.5 % (Tris Borate EDTA) agarose gels through UV. The molecular identification steps and sequences of primers used were according to Choma and Granum (2002); Ehling-Schluz et al., (2006) and Park et al. (2020).
Results
Statistical analytical results of microbiological parameters of the examined dairy products
The highest mean count for aerobic spore formers, B. cereus, Total Staphylococci, Coagulase positive Staphylococci, Coliforms (MPN/g) and Total yeast and mold was 1.9×10 6 ± 1.4×10 6, 1×10 5 ± 9.9×10 4, 2.6×10 7 ± 1.3×10 7, 6.6×10 5 ± 1.3×10 4, 4.1×10 4 ± 2.3×10 4 and 1.7×10 6 ± 6.4×10 5 CFU/g respectively in the white soft and kareish cheeses samples. The highest prevalence of ASF (88.0 %) was in the flavoured drinking yogurt samples, Coagulase Positive Staphylococci (22.0 %) in the Romy cheese, while the highest Coliforms prevalence (88.0%) and fungi (78.0 %) were in the kareish samples (Tables 1 and 2). Based on statistical results of flavoured products, there was a significant difference (p<0.05) between all of them in aerobic spore formers count, while there was a significant difference (p<0.05) between plant-origin flavoured cheese and flavoured drinking yogurt samples for the B. cereus and total Staphylococci counts. In unflavoured products, there was a significant difference (p<0.05) between all of them for Coagulase +ve Staphylococci count, also there was a significant difference (p< 0.05) between kareish and soft cheeses and kareish and Ras cheeses samples for total yeast and mold count only.
Prevalence of Aerobic Spore Formers members in the examined samples
B. licheniformis was the most ASF in the investigated kareish and flavoured drinking yogurt samples in a percentage of 23.7 and 17.0 respectively, while B. subtilis was the most ASF in the surveyed Romy cheese (20.9 %) and plant-origin flavoured cheese (50.0 %). B. pumilus is the most species were detected in flavored yogurt samples with a percentage of 18.9 (Fig. 1).
Table 1. Microbiological analysis of the examined flavored dairy products (CFU/g) (n=150)
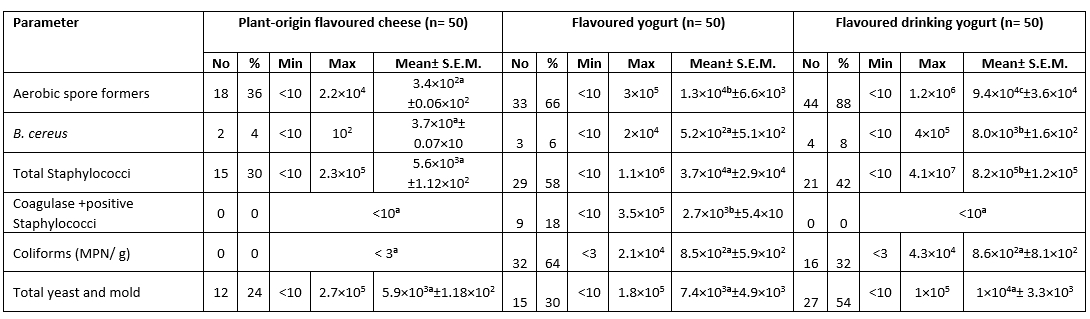
N = total number of the examined samples; Min: Minimum; Max: Maximum; No.: number of positive samples
Different superscript letters in the same row indicate significance difference (p<0.05)
Same superscript letters in the same row indicate non significance difference (p>0.05)
Table 2. Microbiological analysis of the examined unflavoured dairy samples (CFU/g) (n=150)
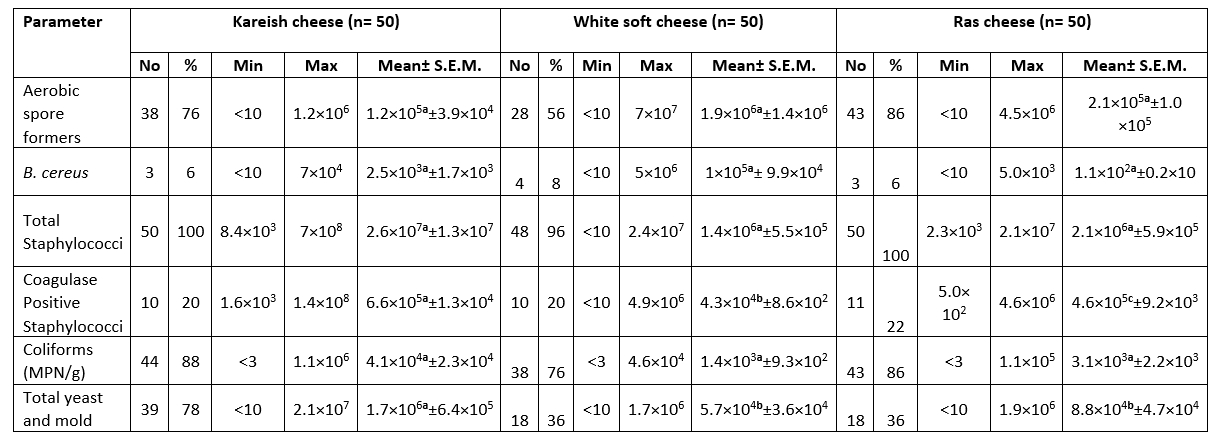
N = total number of the examined samples; Min: Minimum; Max: Maximum; No.: number of positive samples
Different superscript letters in the same row indicate significance difference (p<0.05)
Same superscript letters in the same row indicate non significance difference (p>0.05)
Prevalence of toxigenic genes of B. cereus and its haemolytic activity in the examined dairy products
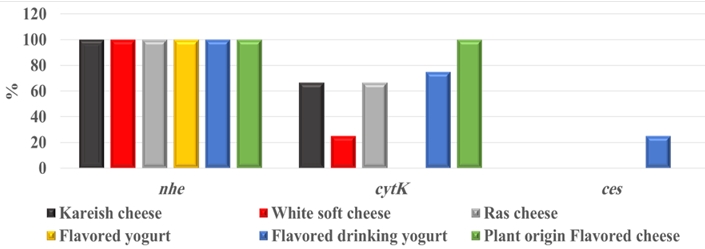
By testing suspected B. cereus isolates by PCR technique; the gyrB gene was found in 70.4 % that indicates B. cereus confirmation. The nhe was the most detected gene in all (100.0 %) the examined isolates of B. cereus, followed by cytK (52.6 %) and ces (5.3 %) genes. The hbl and bceT genes could not be found in all the examined isolates (Fig. 2, 3, 4, 5). Based on statistical results of nhe, cytK, and ces genes, there was a significant difference (p<0.05) between nhe and ces genes in all examined samples except in flavoured drinking yogurt samples.
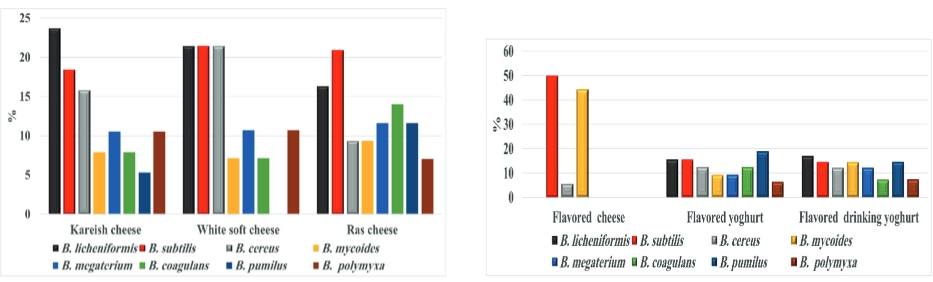
Figure 1. Percentages of identified ASF members obtained from the examined dairy products
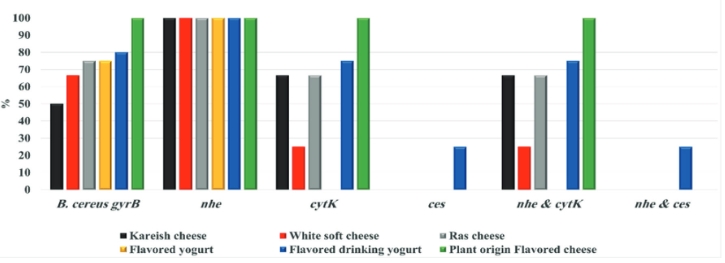
Figure 2. Prevalence of toxigenic B. cereus isolates from the examined dairy products samples hbl and bceT genes absence in all the examined isolates
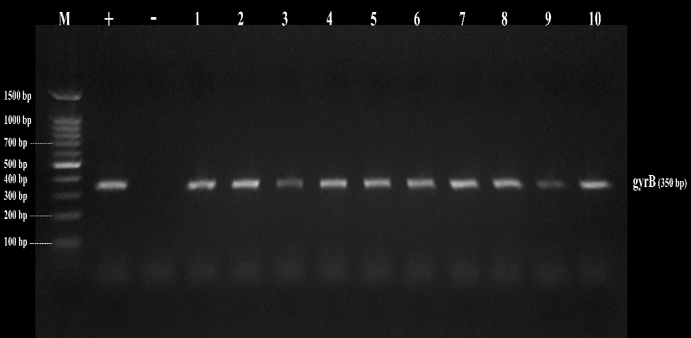
Figure 3. Amplicons of B. cereus isolates on agarose gel using gyrB (350bp). Lane (1-10) B. cereus isolates, and Lane (M) DNA ladder 100 bp molecular weight marker
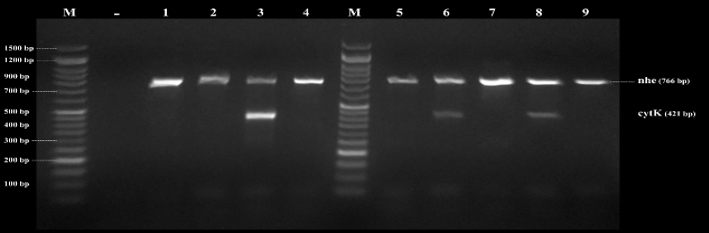
Figure 4. Agarose gel electrophoresis of PCR products of B. cereus genes found in examined samples by direct multiplex PCR. nhe (766bp) and cytK (421bp), Lane (1-9) B. cereus isolates, and Lane (M) DNA ladder 100 bp molecular weight marker
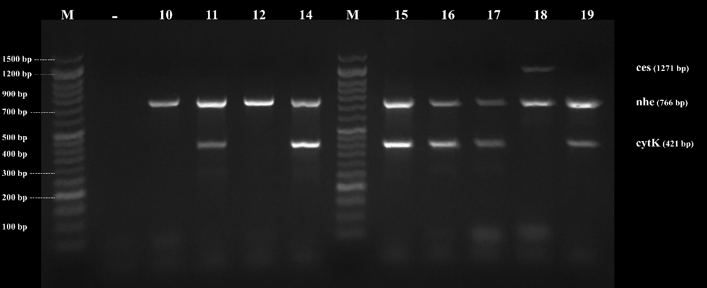
Figure 5. Agarose gel electrophoresis of PCR products of B. cereus genes found in examined samples by direct multiplex PCR. ces (1271) nhe (766bp) and cytK (421bp), Lane (10-19) B. cereus isolates, and Lane (M) DNA ladder 100 bp molecular weight marker.
All examined isolates were non weak haemolytic activity
No.: Number of the isolates harbouring gyrB gene
Figure 6. Relationship between haemolytic activities of B. cereus isolates obtained from different dairy products and the obtained toxigenic genes (No. =19)
By examination of B. cereus isolates for haemolytic activity, all isolates (100.0 %) were strong haemolytic (Fig. 6). More precisely, all strains (100.0 %) of B. cereus that carried nhe were able to show strong hemolysis.
Figure (7) is focused on the degree of acceptance of the examined milk products with the Egyptian Standards (ES). All the samples were complying with ES based on E. coli and Salmonella spp. results. According to other parameters; the degree of complying varied for each limit established by ES.
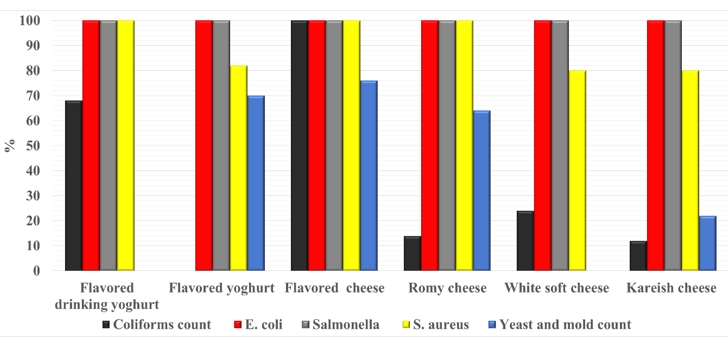
Drinking yogurt: (ES: 8042/2016): free from Coliforms, E. coli S. aureus, B. cereus, Salmonella spp.
Flavored yogurt (ES: 8042/2016): free from E. coli, S. aureus, B. cereus, Salmonella spp., molds < 10 cells/g.
Flavored cheese of plant-origin: (ES: 1867/2005): Coliforms < 10 cells/g, E. coli, S. aureus, B. cereus, Salmonella spp., molds < 10 cells/g, Yeast <400 cells/g.
Romy cheese (ES: 1007/4/2020): Coliforms < 10 cells/g, E. coli, S. aureus, B. cereus, Salmonella spp., molds < 10 cells/g, Yeast < 100 cells/g.
Soft cheese (ES: 8390/2020): Coliforms < 10 cells/g, E. coli, S. aureus, B. cereus, Salmonella spp., molds <10 cells/g, Yeast < 400 cells/g.
Kareish cheese (ES: 1008/4/2005): Coliforms < 10 cells/g, E. coli, S. aureus, B. cereus, Salmonella spp., molds < 10 cells/g, Yeast < 400 cells/g.
The missing columns indicate: not mentioned in ES.
Figure 7. Degree of acceptance of each the examined product type according to the Egyptian standards (ES)
Discussion
The hygienic quality of cheeses is critical to prevent their microbial contamination due to their high protein content, and children with weak immunity eat these types of cheeses (Youssif et al ., 2020). High counts of coliform bacteria in the investigated cheeses indicate contamination (direct and/or indirect), and reflect a bad hygiene that leads to an unsafe final product. The lower count of coliforms in surveyed plant-origin flavoured cheese may be due to bacteria of 2-Hydroxypropanoic Acid (LAB) which grow in unheated milk or cheese that makes the antagonism effect to undesirable microorganisms (Martínez-Ruiz et al ., 2013). Coliforms were in 42.0 % (lower than our results) of the examined yogurt samples (Adebisi et al., 2017).
Heikal et al . (2014) found those coliforms and staphylococci 2.5×10 4 (higher than our results) and 2.3×10 4 (lower than our results) CFU/g respectively in the white soft cheeses, while Staphylococcus aureus was detected in 6.7 % of the examined cheese samples (lower than our results). A lower count for S. aureus was reported by Worku et al. (2015) in the surveyed flavoured yogurt samples. Ibrahim et al. (2015) found that S. aureus in a higher percentage (66.0 %) than our results in the kareish cheese samples with a mean of 1.9 ×10 5 CFU/g, also the mean of coliforms was 3.9×10 3 cells/ g (similar to our obtained results).
The reduced power of hydrogen ion value of yogurt and flavoured drinking yogurt, participate in the multiplication reduction of organisms in samples with a low count of staphylococci (<10 CFU/g) and coliforms (<3 MPN/g) (Kailasapathy, 2006). Metwalli (2011) found mean counts of coliforms 1.5×10 5 CFU/g, which was lower than our results. S. aureus was detected in all kareish cheese samples with a mean of 0.2×10 6 CFU/g, which was similar to our results. The attributing reasons for the low enumeration of CPS (<10 CFU/g) was a decreasing of power of hydrogen ion of the examined drinking yogurt samples and the bacteria of 2-Hydroxypropanoic acid (Le Marc et al., 2009). Rahimzadeh et al . (2020) could not detect the S. aureus in the yogurt samples, and this result was similar to our results.
The high enumeration of molds and yeasts in the surveyed cheeses and flavoured products may be attributed to improper hygienic measures in processing and/or adding poor quality flavoured substances as an origin of fungal pollution (Graciela et al ., 2001). So, the manufacturers shall be careful when purchasing these substances. Metwalli (2011) found that fungi in all surveyed kareish cheese samples with a mean of 4×10 3 CFU/g, which was lower than our results. Heikal et al . (2014) found that fungi were present in 100.0 % of white soft cheese samples, with a mean of 1.9×10 3 CFU/g, which was lower than our results. Ibrahim et al. (2015) could detect fungi in all investigated kareish cheese samples with a mean count of 3.1×10 5 CFU/g. A nearly similar count of fungi (≥10 2 CFU/g) was reported by Worku et al. (2015) in the surveyed flavoured yogurt samples. Adebisi et al. (2017) found that fungi in 75.0% of the examined yogurt samples, which was higher than our results.
B. licheniformis spores cause milk products spoilage and have a bad effect on their sensory and technological characteristics (Dhakal et al ., 2014). B. pumilus, and B. subtilis are often isolated from animal surroundings and have been recorded to produce proteases and enterotoxin substances which damaging public health (Yoo et al., 2014). In yogurt samples, a decreasing value for the power of hydrogen ion participates in the multiplication reduction of ASF in low count samples (<10 CFU/g) (Kailasapathy, 2006).
Spores of ASF found in raw milk can resist pasteurization temperature and ultimately become incorporated into final products (Coorevits et al., 2008). Our results of ASF in flavoured drinking yogurt were mainly lower than the results obtained by Aly and Galal (2016). While the obtained results from Ras cheese samples were higher than those obtained by Khater and Abdella, (2017) and Nassib et al. (2018), white soft cheese results were higher than those obtained by Khater and Abdella (2017) and Nazem et al. (2020). Bacillus cereus was not detected in any cheese sample examined by Heikal et al. (2014) as well as in the study by Ibrahim et al. (2015).
The bceT gene of B. cereus does not cause diarrhoea and was not confirmed as a food-borne agent (Choma and Granum, 2002). Bacillus cereus strains have harboured more than one toxin. A total of 52.6 % of isolates have expressed 2 genes ( nhe and cytK), while 5.3 % of isolates harboured 2 genes ( nhe and ces). Our findings were almost like those of Zhao et al. (2020), who concluded that 94.4 % of B. cereus harboured nhe gene, while cytK gene was present in a higher percent (75.9 %) than our findings. Yibar et al. (2017) recorded that 42.3 % of B. cereus isolates from the examined different cheeses types harboured nhe gene. There was a significant difference (p<0.05) between nhe and ces genes in all investigated samples except in flavoured drinking yogurt, which indicates a high association of these genes with these products.
The B. cereus prevalence in yogurt was detected in 3 (6.0 %) out of 50 examined samples. This result was lower than those recorded by Ayoub et al. (2003), who found that 20.0 % of examined yogurts was polluted with B. cereus, while Abdel-Khalek (2002) reported no contamination for yogurt. The lower prevalence of B. cereus in yogurt may be attributed to the restrained action of LAB and bacteriocins, organic acids, and hydrogen peroxide production. Badly, a lower count (10 3/g) may cause health problems in immunosuppressive people (Granum, 2005). EFSA (2016) reported that more than 10 4 B. cereus colony-forming unit/g leads to diarrheal toxins secretion in the gastrointestinal tract of people.
B. cereus multiplication is prevented under the power of hydrogen ion 4.5, as yogurt is not related to B. cereus food poisoning on condition storage below 4 ⁰C. The investigated dairy products contain B. cereus with different enumeration, and most of the strains presented toxigenic power, so B. cereus may be considered as a hazard to people. However, the financial damages of B. cereus toxins shall not be ignored in the dairy industry. A famous technique of preservation to control B. cereus growth is acidification (Rossland et al., 2003). The suggested reason for the presence of B. cereus in the investigated dairy products especially in the surveyed white soft cheese and flavoured drinking yogurt is the use of raw milk contaminated with spores which are resistant to pasteurization. The origin of these spore is most probably the soil present in due to bad environmental milking practice (Gopal et al., 2015).
The use of raw materials with reduced spore enumeration is a highly preventive way for poisoning by B. cereus. Spores may transmit to multiple milk products by un-heat-treated milk and spores attached by biofilms to dairy utensils (Caro-Astorga et al., 2020). The lethal poisoning, cellular haemolytic, necrotic effects, hepatic damage, and sugar disorders cause by cytotoxic gene (Vangoitsenhoven et al., 2014). The nhe toxin has cytotoxic characteristics, while cytK toxin is the reason for bloody diarrhea (Jessberger et al., 2020).
Based on the data in (Fig.6), this proved the complete association between harboring toxic gene ( nhe) and exhibition of virulence feature (strong hemolysis). The beta-hemolytic B. cereus is the cause of food poisoning. More than 25.0 % of beta-hemolytic B. cereus isolates were not produced emetic toxin (Jessberger et al., 2020). Counting of B. cereus and examination for its toxic genes are essential for the microbiological quality and safety of these dairy products. It is the main threat in the food industry both by deteriorating the products and by endangering people’s life upon consumption. Applying excellent manufacturing principles and the HACCP plan are essential for elevating safe products (Hafiz et al., 2016; Ibrahim et al., 2020).
E. coli, Salmonella spp., and P. aeruginosa could not be found in any of the investigated milk products. The E. coli absence in the investigated flavoured soft cheeses was attributed to inhibitive effect of the starter cultures, competition for nutrients actions and areas of colonization, as well as vacuum packaging that reduces the oxygen concentration (Ganesan et al., 2012).
None detected Salmonella and E. coli was reported by Worku et al. (2015) in the surveyed flavoured yogurt samples. Rahimzadeh et al. (2020) could not detect E. coli in the examined yogurt samples, similar to our results. El-Ziney (2018) failed to detect Salmonella in the tested hard cheeses due to the inability of Salmonella to survive at low water activity. Metwalli (2011) reported that Salmonella spp. was not present in all samples. The most suggested causes of Pseudomonas aeruginosa absence is the non-formation of biofilms in milk tanks as well as their removal through heat treatment (pasteurization or UHT) (Simoes et al., 2009).
The reasons for unacceptable samples (Fig. 7) may be due to microbe species in un-heat-treated milk, degree of proper manufacturing, the degree of hygiene applied in dairy processing units, and milk handling practice during milk processing (Heikal et al., 2014). Finally, the ES needs to be more restricted and established the limit of parameters, which are not mentioned to ensure safe dairy product consumption.
Conclusions
Significant attention has been drawn to the use of flavoured yogurt, kareish, soft, and Romy cheeses with different pathogens types. Since it is of vital concern, it is recommended to adopt strict control measures during various stages of production and processing of such products. Finally, proper consumer handling and storage practices have affecting tools to control pathogens and protect milk products from deterioration.
Ispitivanje mikrobiološke kvalitete i sigurnosti odabranih mliječnih proizvoda s posebnim naglaskom na toksigene gene Bacillus cereus
Sažetak
Iako mliječni proizvodi igraju značajnu ulogu u prehrani ljudi, mogu predstavljati rizik za potrošače. Cilj ovog istraživanja bio je utvrditi razinu mikrobiološke kontaminacije u odabranim mliječnim proizvodima, prije svega prisutnost enterotoksigenih gena Bacillus cereus. Tristo sireva obogaćenih dodacima biljnog podrijetla, aromatiziranih jogurta, aromatiziranih jogurta za piće, kareish (sir od obranog mlijeka), mekani sir i sir Ras (Romy) (od svake vrste 50 uzoraka) nasumce je sakupljeno s maloprodajnih tržnica u različitim okruzima u guvernorati El Fayoum, Egipat. Primijenjena je mikrobiološka analiza i molekularna identifikacija Bacillus cereus multiplex PCR-om koja je otkrila potencijalni rizik povezan s konzumacijom ovih mliječnih proizvoda (bijeli mekani, ras i kareish sirevi). U svim analiziranim uzorcima sira (meki sir, Ras i kareish) utvrđena je visoka razina kontaminacije koagulaza pozitivnim stafilokokima (20,0, 22,0 i 20,0 %) i koliformnim bakterijama (76,0, 86,0 i 88,0 %). Najveći postotak (78,0 %) plijesni utvrđen je u uzorcima sira kareish. Bacillus licheniformis najaerobniji je tvorilac spora iz kareish sira (23.7 %) i aromatiziranog jogurta (17.0 %). Najveća prevalencija (8.0 %) Bacillus cereus zabilježena je u uzorcima aromatiziranog jogurta i mekog sira. Gen ( nhe) je bio najzastupljeniji gen (100,0 %) u svim ispitivanim uzorcima. Postojala je značajna razlika (p<0,05) između gena nhe i ces u svim ispitivanim uzorcima, osim u uzorcima aromatiziranog jogurta. Salmonella sp., Escherichia coli i Pseudomonas aeruginosa nisu detektirani u nijednom uzorku. Ovo je jedno od rijetkih istraživanja koje opisuje sigurnost i kvalitetu sireva obogaćenih dodacima biljnog podrijetla i aromatiziranih jogurta, posebno u Egipat.
Ključne riječi: Bacillus cereus; E. coli; CPS; mliječni proizvodi; multipleks PCR; enterotoksigeni geni
