Introduction
Mastitis is a highly prevalent disease in dairy cows and one of the most costly diseases in the dairy industry. The most important factors in the formation of mastitis are bacterial agents. Considering the bacterial etiology of mastitis, mastitis is mostly caused by contagious and environmental pathogens. Staphylococcus aureus ( S. aureus) and Streptococcus agalactiae ( S. agalactiae) are among the contagious pathogens, while Escherichia coli ( E. coli) is among the environmental pathogens (Halasa et al., 2007; Kalińska et al., 2018).
It is well known that cytokines have an effect on the pathophysiology of mastitis and play an important role in the defense system of the mammary gland. The cytokines released from CD4+ (cluster of differentiation 4) T lymphocytes play an important role in the regulation of immunological reactions. Th0 lymphocytes differentiate into two subgroups: T helper (Th)1 and Th2. Among the cytokines released from the Th1 group CD4+ T lymphocytes, are interferon (IFN)-γ, interleukin (IL)-2, and tumor necrosis factor (TNF)-α, while IL-4, IL-5, and IL-10 are released from the Th2 group CD4+ T lymphocytes (Romagnani, 2000; Sordillo, 2018). Th1 cells are responsible for cellular immunity, and they suppress the Th2 cells. On the other hand, Th2 cells are mainly associated with humoral immunity and immunoglobulin class switching to IgG1 and IgE, and Th2 cells are responsible for humoral immunity (Zhu and Paul, 2008).
It has been reported that experimentally-induced or naturally occurring mastitis results in an increase in the somatic cell count (SCC) and cytokine changes (TNF-α, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and IL-12) concentrations in milk (Hisaeda et al., 2001; Bannerman, 2009). These changes in the concentrations of cytokines, which play a central role in immune regulation, differ according to the types of bacteria that cause subclinical mastitis (Alluwaimi, 2004; Sordillo, 2005). In mastitis induced experimentally by introducing E. coli, there was an increase in TNF-α concentration during the first 18 h, parallel to the increase of SCC after this hour, and although the SCC increased, TNF-α concentration decreased. Also, it has been reported that the increase in the concentration of IL-1β begins to form after the TNF-α starts to increase (Bannerman, 2009). It has also been reported that in mastitis caused by S. aureus, there were changes in the concentrations of IL-8, TNF-α, and IL-1β, but correlation could be established with the change of SCC (Riollet et al., 2000). An increase in IFN-γ concentration has also been reported in mastitis milk experimentally-induced by pathogens, such as Mycoplasma bovis (Kauf et al., 2007), Streptococcus uberis (Bannerman et al., 2004a), Klebsiella pneumoniae (Bannerman et al., 2004b), S. aureus, and E. coli (Bannerman et al., 2004c). Also, the milk IL-2 concentration increases in the case of S. aureus-induced mastitis (Alluwaimi et al., 2003). IFN-γ concentration was higher in E. coli-induced mastitis than S. aureus-induced mastitis. Moreover, in mastitis caused by E. coli and S. aureus, during the infection process in which the milk SCC level is high, IL-6, TNF-α, and IFN-γ concentrations decrease (Lee et al., 2006). In coagulase-negative staphylococci (CNS)-induced mastitis, the milk IL-4 and IL-10 concentrations decrease, while it is known that there is an increase in IL-6 (Bochniarz et al., 2017). It has also been reported that there is no difference in the concentration of IL-4 between mastitis and healthy milk (Fonseca et al., 2009).
The aim of the present study was to determine in which direction the Th1/Th2 cytokine-based balance changes by evaluating the cytokine groups produced from Th1 and Th2 lymphocytes in subclinical mastitis caused by S. aureus, S. agalactiae, and E. coli.
Material and methods
Establishing study groups and somatic cell count
In this study, clinically healthy, mid-lactation, multiparous (between parities two and three) Holstein and Simmental cows, weighing 450–500 kg, with body condition scores between 3.5 and 3.8, milked twice per day and with a daily milk yield of 10-25 liter (mean = 14.54) were selected among the cows in seven commercial dairy farms (four free-stall or loose and three tie-stall) in the Elazig province of Turkey. Statistical power analysis was performed to determine the total number of animals in the groups. In this study, the required minimum sample number was determined as 180 using effect size = 0.25, alpha = 0.05, and power = 0.80 (Cohen, 1988). The study was conducted between 01.11.2019 and 30.01.2020. For the study, the ethics committee approval was obtained from the Fırat University Animal Experiments Local Ethics Committee (FU-2018/98).
The California Mastitis Test (CMT) was applied to the cows, as described by Ruegg and Reinemann (2002). The first three squirts from each quarter were discarded before morning milking, and a milk sample was obtained from each quarter to the cup of the CMT container representing its quarter. Then, a volume of the commercial reagent (BOVI-VET, CMT-Test, Kruuse, Denmark) equal to the milk was added to each cup of the container. A gentle circular motion was applied in a horizontal plane for 10-15 s to mix milk with the reagent. Then, according to the consistency of the gel formed in each cup, CMT scoring was done (always by the same person). According to the score, the results were divided into CMT negative (-) and positive (+, ++, and +++ degrees). If at least one quarter received one of the +, ++, and +++ values, the cow was considered to have subclinical mastitis. In the CMT, when all mammary quarters were negative, they were evaluated as CMT (-), namely healthy cows (n = 45) (Tolosa et al., 2013).
To establish the other groups, milk samples (approximately 2 mL) were collected aseptically after disinfection of the teat with 70 % alcohol on a cotton ball. Milk samples were bacteriologically tested in the Microbiology Laboratory of the Faculty of Medicine, Firat University, according to the National Mastitis Council (NMC) guidelines (Hogan et al., 1999). After isolation and identification of microorganisms, E. coli group was designated as cows with only E. coli growing in milk samples of CMT-positive (n = 45), S. agalactiae group as cows with only S. agalactiae growing in milk samples of CMT-positive (n = 45), and S. aureus group as cows with only S. aureus growing in milk samples of CMT-positive (n = 45). Study groups were designed as reported by Safak et al. (2021). In the bacteriological examination of milk from healthy cows, no growth of any microorganisms was observed. The cows that grew two or more bacteria in their milk samples were excluded. Two-thousand quarters belonging to 500 cows were examined until the number of cows in the groups was completed. Also, 2 mL of milk samples for SCC measurement and cytokine analysis were obtained from cows of the four groups. The SCC was measured in fresh milk. Somatic cell count was measured using a DeLaval Cell Counter® (Cell counter DCC; DeLaval, Tumba, Sweden) according to the manufacturer’s instructions in all cows in four groups (Jaeger et al., 2017). After SCC analysis, milk samples were frozen at -20 °C and were stored at -70 °C until analysis of TNF-α, IFN-γ, IL-2, IL-4, IL-5, and IL-10.
Cytokine analysis in milk samples
To determine the cytokine levels of Th1 (TNF-α, IFN-γ, IL-2) and Th2 (IL-4, IL-5, IL-10), species-specific commercial IFN-γ (catalog number: SEA049Bo, all USCN Life Science Inc., Wuhan, China), IL-2 (catalog # SEA073Bo, all USCN Life Science Inc., Wuhan, China), TNF-α (catalog #: SEA133Bo, all USCN Life Science Inc., Wuhan, China), IL-4 (catalog # SEA077Bo, all USCN Life Science Inc., Wuhan, China), IL-5 (catalog # SEA078Bo, all USCN Life Science Inc., Wuhan, China), and IL-10 (catalog # SEA056Bo, all USCN Life Science Inc., Wuhan, China) ELISA kits were used. The application stages of the ELISA test were carried out according to the manufacturer’s instructions and methods in the literature (Can-Sahna and Risvanli, 2015). After all, stages were completed, the results were read by an automatic microtiter plate reader (Bio Tek Instruments, USA) at 450 nm. The detection ranges of TNF-α, IFN-γ, IL-2, IL-4, IL-5, and IL-10 for cattle were 7.8 to 500, 15.6 to 1,000, 15.6 to 1,000, 15.6 to 1,000, 15.6 to 1,000, and 15.6 to 1,000 pg/mL, respectively, as reported by the manufacturer.
Statistical analysis
The SPSS 22.0 package program (Statistical Package for the Social Sciences for Windows SPSS 22.0 Edition for Windows, Chicago, Illinois, USA) was used for statistical analysis of the data.
The normality distribution of the cytokines (TNF-α, IFN-γ, IL-2, IL-4, IL-5, and IL-10) and SCC were tested using the visual (histogram and probability graphs) and Kolmogorov-Smirnov test. It was determined whether the data showed a normal distribution or not. As a result of the evaluation, it was determined that there was no normal distribution of characteristic values in each group. Therefore, the Kruskal-Wallis test, one of the non-parametric tests used in the comparison of multiple groups, was used in inter-group comparisons. The Bonferroni corrected Mann-Whitney-U test was used for the post-hoc pairwise group comparisons after the Kruskal-Wallis. As a result of the Bonferroni correction, the value obtained as a result of dividing the statistical significance limit value of 0.05 by the number of comparisons to be made (four groups are compared in six different ways) was used as the significant value in the pairwise group comparisons. The Bonferroni corrected Mann-Whitney-U significance value was calculated as P = 0.05/6 = 0.0083; however, when the significance value of 0.01 or less is used for this test, the power of the Mann-Whitney-U test decreases. Therefore, a value of P˂0.01 was accepted in the pairwise group comparisons.
Results and discussion
The SCC value in the milk samples of the cows in the CMT (-) (48,000.87 ± 7,000.09 cells/mL) group was significantly lower than that of the milk samples of the cows in the other three groups ( E. coli, S. aureus, and S. agalactiae) ( P˂0.01). However, no statistically significant difference was found between E. coli (580,000.00 ± 88,000.36 cells/mL), S. aureus (1,162,000.76 ± 214,000.29 cells/mL), and S. agalactiae (1,701,000.58 ± 312,000.39 cells/mL) ( P˃0.01) (Table 1).
Table 1. Comparison of milk SCC among groups
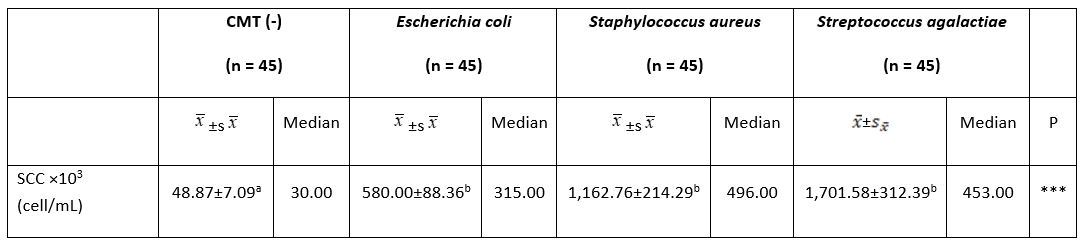
***: P˂0.001. a, b: The difference between groups with different letters on the same row is statistically significant, (P˂0.01)
Table 2. Comparison of milk cytokine concentrations among groups
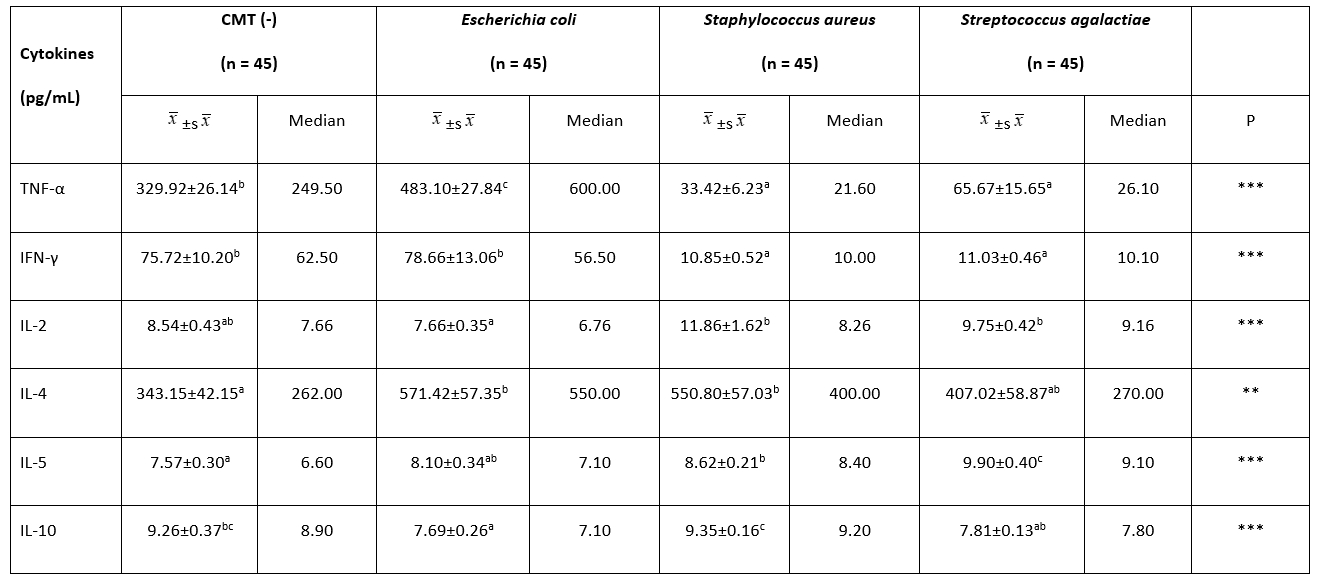
**: P˂0.01, ***: P˂0.001.
a, b, c: The difference between the groups with different letters on the same row is statistically significant, (P˂0.01)
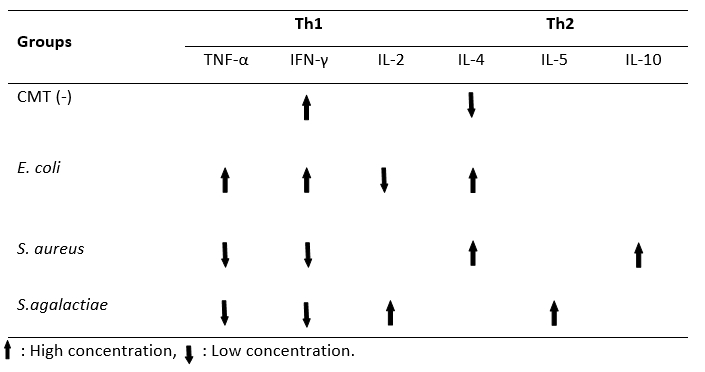
Table 2 gives the findings of the milk cytokine concentration between groups. The TNF-α concentration of the E. coli group (483.10 ± 27.84 pg/mL) was significantly higher than the other groups ( P˂0.01). There was no statistical difference between the S. aureus and S. agalactiae groups in terms of milk TNF-α concentration ( P˃0.01). The highest milk IFN-γ concentration was found in the E. coli group (78.66 ± 13.06 pg/mL) (P˂0.01). However, no significant difference was observed between the CMT (-) (75.72 ± 10.20 pg/mL) and E. coli groups (75.72 ± 10.20 pg/mL) with regard to IFN-γ ( P˃0.01). There was no statistical difference between the S. aureus and S. agalactiae groups in terms of milk IFN-γ concentration ( P˃0.01). The lowest concentration of milk IL-2 was detected in the E. coli (7.66 ± 0.35 pg/mL) group. No statistical difference was found between the other three groups ( P˃0.01). While the milk IL-4 concentration was the lowest in the CMT (-) group (343.15 ± 42.15 pg/mL), there was no significant difference between the E. coli, S. aureus, and S. agalactiae groups in terms of the milk IL-4 concentration ( P˃0.01). The level of IL-5 was high in the S. agalactiae group (9.90 ± 0.40 pg/mL), while the lowest was in the CMT (-) group (7.57 ± 0.30 pg/mL) ( P˂0.01). The concentration of IL-10 in milk was higher in the S. aureus group (9.35 ± 0.16 pg/mL) than in the S. agalactiae (7.81 ± 0.13 pg/mL) and E. coli (7.69 ± 0.26 pg/mL) groups ( P˂0.01). No statistically significant difference was found between the S. aureus (9.35 ± 0.16 pg/mL) and CMT (-) (9.26 ± 0.37 pg/mL) groups ( P˃0.01). Moreover, Th1/Th2 polarization shifted towards Th1 in milk with mastitis caused by E. coli. Th1/Th2 polarization was determined to be Th2 in milk with mastitis caused by S. aureus and S. agalactiae (Table 3).
Table 3. Th1/Th2 polarization in different samples of mastitic milk (E.coli, S.aureus, S.agalactiae) in comparison to he negative control (CMT -)
A great number of cytokines have been identified so far, which are secreted from various cell types to ensure communication between cells. In particular, the Th1 (IFN-γ, IL-1β, IL-2, IL-8, IL-12, and TNF-α) and Th2 (IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, IL-25, IL-31, and GM-CSF) cytokine groups play an important role during inflammation. These cytokine groups increase or decrease according to the course of the infection. However, in these changes, an increase or decrease in all cytokines belonging to the same group is not expected at the same time. It is interpreted according to the group with the majority of increasing or decreasing cytokines (Sordillo, 2018; Dembic, 2015).
The level of TNF-α increases in bovine mastitis as well as in other bacterial diseases. It was reported in studies conducted in both, experimentally and naturally occurring mastitis, that the concentration of TNF-α increased (Hisaeda et al., 2001; Slebodzinski et al., 2002; Bannerman et al., 2005). It has been reported that there is an increase in TNF-α concentration in cases where mastitis occurs and SCC increases (Safak and Risvanli, 2021). In the present study, the TNF-α concentration was higher in the E. coli group than in the S. aureus and the S. agalactiae groups.
The concentration of IFN-γ was relatively high in milk with mastitis caused by S. aureus and E. coli (Lee et al., 2006; Riollet et al., 2001). The level of IFN-γ increases in experimentally-induced mastitis cases (Bannerman et al., 2004a, b, c) in addition to naturally occurring mastitis (Hisaeda et al., 2001). In the present study, the IFN-γ concentration of the E. coli group was higher compared to other groups.
The concentration of IL-2 in milk was high in mastitis caused by S. aureus and coliform mastitis (Alluwaimi, 2000; Alluwaimi and Cullor, 2002; Alluwaimi et al., 2003). It has been noted that the transcriptional activity of IL-2 decreased 24 h after infection in the bovine mammary gland infected with S. aureus. This significant reduction in IL-2 demonstrates its importance in the regulation of the immune responses in the bovine mammary gland. Since it has a pro-inflammatory character, it decreases in the later stages of the infection, while it increases in the early stages of the infection, in contrast to the anti-inflammatory cytokines (Alluwaimi et al., 2003).
In the present study, the level of IL-4 was lower in the CMT (-) group compared to the S. aureus and E. coli groups, while no statistical difference was found compared to the S. agalactiae group. Fonseca et al. (2009) also found no difference in the IL-4 concentration between healthy and mastitis milk. On the other hand, Bochniarz et al. (2017) found low concentrations of IL-4 in milk with CNS mastitis. Based on these findings, it can be stated that the bacterial species affected the secreted IL-4 concentration at different levels.
IL-10 plays a central role in limiting inflammation and forming the humoral immune response to infection (Asadullah et al., 2003). Moreover, this cytokine plays a role in changing the Th1/Th2 balance by suppressing the production of IFN-γ and IL-12, which play a role in promoting Th1 polarization, and shifts the balance (Moore et al., 2001; Mocellin et al., 2004). An increase in the level of IL-10 has been detected in mastitis caused by various pathogenic species such as E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Streptococcus spp. (Bannerman, 2009). In the present study, changes in the milk IL-10 concentration were found in mastitis groups caused by E. coli, S. aureus, and S. agalactiae. Contrary to the data in this study, in a study by Bochniarz et al. (2017) conducted on CNS mastitis, the concentration of IL-10 in milk was lower than in the healthy group. It was suggested that these changes in the concentration of IL-10 have resulted from an increase in the later stages of the infection due to its anti-inflammatory properties.
Despite all the screenings, no studies cold be found in the literature regarding the direction in which the Th1/Th2 cytokine based balance changes, by evaluating the cytokine groups produced from Th1 and Th2 lymphocytes separately according to the bacterial species in milk with subclinical mastitis. Previous studies were mostly limited to partial cytokine changes caused by bacteria causing mastitis, but information on the polarization of Th1/Th2 cytokine based balance was not provided. In the present study, there was an increase in TNF-α and IFN-γ in the E. coli group, and consequently, the Th1/Th2 polarization balance shifted towards Th1, while in the S. aureus and S. agalactiae groups, TNF-α and IFN-γ decreased and therefore, the Th1/Th2 polarization changed. It could be observed that the balance was in the Th2 direction, which led to a conclusion that cellular immunity should be strengthened in E. coli mastitis through applications related to the Th1 direction. To strengthen the cellular immunity, it is recommended to take vitamin D supplements in D2 (Ergocalciferol) and D3 (Cholecalciferol) forms together with minerals, such as selenium, copper, and zinc, and also vitamin E and vitamin A (Hogan et al., 1990; Heinrichs et al., 2009; Lippolis et al., 2011). Also, it is among the recommended practices to keep blood ketone levels low by preventing the cow from entering a negative energy balance with the regulations in nutrition programs (Waller, 2000). Moreover, in the light of the data obtained from the present study, it was understood that it was necessary to support the Th2 direction, that is, humoral immunity, in mastitis caused by S. aureus and S. agalactiae. To achieve this, Corynebacterium cutis lysate applications (Saat et al., 2016), homeopathic drugs (Healwell VT-6, Dolisovet) (Varshney and Naresh, 2005; Aubry et al., 2013) various peptides (Jeong et al., 2017), and vaccine practices that keep immunoglobulin levels high are recommended (Middleton et al., 2009).
Conclusion
The results of the present study revealed that the cytokine concentration in milk with subclinical mastitis caused by E. coli, S. aureus, and S. agalactiae changed according to the bacterial species. In the case of E. coli-induced subclinical mastitis, it was observed that the Th1/Th2 cytokine based balance of milk shifted towards the Th1. If there were subclinical mastitis caused by S. aureus and S. agalactiae, the Th1/Th2 cytokine based balance was determined to be in Th2. Mastitis remains a major issue in dairy production, even though prevention and control programs have been available since the 1960s and have been successfully implemented on many herds. It is also well known that prevention and control programs are in the foreground rather than the treatment of mastitis. Based on our findings, the immune system should be supported in the Th1 direction to protect against E. coli-induced mastitis, and in the Th2 direction to protect it from the mastitis caused by bacteria such as S. aureus and S. agalactiae. In other words, it was concluded that keeping the cellular immunity strong in cases of E. coli-induced mastitis and keeping humoral immunity strong in cases of S. aureus and S. agalactiae-induced mastitis would provide advantages to the dairy businesses to prevent E. coli, S. aureus, and S. agalactiae-induced mastitis, which cause huge economic losses.
Acknowledgements
All data of this article is part of the Ph.D. thesis of Tarik SAFAK. The manuscript was previously published on the preprint server Research Square.
Funding
This study was supported by the Scientific and Technological Research Council of Turkey [TUBITAK-119O787].
Polarizacija citokina Th1 / Th2 u mlijeku u odnosu na patogene uzročnike subkliničkog mastitisa kod krava
Sažetak
Cilj ovog rada bio je odrediti ravnotežu između citokina Th1 i Th2 u odnosu na bakterijske uzročnike subkliničkog mastititsa kod krava. Pri tom su krave bile podjeljene u sljedeće testne skupine: krave s negativnim testom na mastitits CMT- (n = 45); grupa oznake Escherichia coli ( E. coli) uključivala je jedinke kojima je određen samo porast soja E. coli u uzorcima s pozitivnim CMT testom (n = 45); Streptococcus agalactiae ( S. agalactiae) grupa uključivala je jedinke kojima je određen samo porast soja S. agalactiae u uzorcima s pozitivnim CMT testom (n = 45); Staphylococcus aureus ( S. aureus) grupa uključivala je jedinke kojima je određen samo porast soja S. aureus u uzorcima s pozitivnim CMT testom (n = 45). Broj somatskih stanica (SCC) u uzorcima svježeg mlijeka određivan je pomoću brojača DeLaval Cell Counter. Analize koncentracije citokina provedene su ELISA metodom koristeći gotove selektivne kitove za pojedinu bakterijsku vrstu koja je određivana. Koncentracije alfa tumorskog faktora nekroze (TNF-α) i gama-interferona (IFN-γ) bile su relativno visoke u grupi E. coli, no koncentracija interleukina (IL)-2 je bila niska. Najniža koncentracija IL-4 određena je u grupi CMT- odnosno u jedinki koje nisu imale mastitis. Najviša koncentracija IL-5 određena je u grupi S. agalactiae, dok je najviša koncentracija IL-10 određena u grupi S. aureus. Također, ravnoteža pomoćničkih T-limfocita (Th1/Th2) polarizirala se u smjeru koncentracije Th1 u uzorcima mastitičnog mlijeka grupe E. coli. S druge strane u grupama S. aureus i S. agalactiae ravnoteža Th1/Th2 se polarizirala u smjeru Th2. Uzimajući u obzir rezultate ovog istraživanja, nalaže se zaključak da je kod mastitisa uzrokovanog bakterijom E. coli potrebno održavati stanični imunitet, dok je kod mastitisa uzrokovanog bakterijama S. aureus i S. agalactiae potrebno održavati humoralni imunitet.
Ključne riječi: Th1/Th2 citokini; subklinički mastitis; krave; California Mastitis Test
