UVOD
Most changes made by humans disrupt the natural balance of many ecosystems that have evolved over a long period of time (Stafilov et al. 2010). The industrial activities that contribute to the pollution of the environment with heavy metals in the Republic of North Macedonia are mainly mining and metallurgical activities (Stafilov 2014). Most of these industries use raw materials or auxiliary chemical substances in their operations. The waste from these industries is dangerous for people and the environment, often even in very small quantities. The main industrial facilities in the Republic of North Macedonia are located in Radovish (copper mine and flotation), Veles (abandoned lead-zinc and cadmium smelter), Kavadarci (ferronickel smelter), Skopje (steel and ferroalloy production and steel processing), Tetovo (abandoned ferrochrome smelter and current ferrosilicon smelter), and Kichevo and Bitola (thermal power plants, where lignite fuel is used). There are also three Pb-Zn mines and flotation plants (Sasa, Toranica, Zletovo), which are located in the eastern part of the country and the previously active As-Sb-Ti mine Allchar, which is located in the southern part of North Macedonia (Barandovski et al. 2020). The industrial and the agricultural activities (mining, smelting, etc.) are often associated with local contamination of water, soil, air and plants grown near these areas (Lénárt et al. 2023). The accumulation of toxic metals in plants, water and soil may increase the risk of its transfer to wild mammals and game animals as the results of the ingestion through diet (Bilandzić et al. 2010, Falandysz et al. 2005, Reglero et al. 2008). Especially dangerous are compounds that are easily accumulated in the living organisms, such as in the case of mercury and arsenic.
Mercury (Hg) is a global toxic pollutant, which tends to accumulate within the food chain. Its presence in humans and animals is an indicator of environmental pollution with mercury from both natural and anthropogenic sources, so the presence of this heavy metal in the natural environment is considered undesirable and potentially dangerous (Dobrowolska and Melosik 2002). The inorganic mercury with high doses can affect the nervous, renal, cardiovascular and gastrointestinal system (Grupta 2012). The reproductive effects induced by the inorganic mercury include decreased fertility and reductions in both live births and litter sizes (Anonymous 2011). Arsenic is a metalloid from group V of the Periodic Table of Elements (Stafilov and Šajn 2016), so it is not classified as a heavy metal, but it is toxic and dangerous for human health and is often studied together with heavy metals. The inorganic arsenic (As) is an environmental toxicant that occurs naturally in soil, water and air and reaches consumers mostly through the food supply contaminated by anthropogenic activities and geological releases. Arsenic is an element, which tends to accumulate in the living tissue, i.e. once ingested by any organism it is passed out of the organism very slowly (Mandal and Suzuki 2002). The ingestion of large doses of As leads to gastrointestinal symptoms, disturbances of cardiovascular and nervous system functions, and eventually death (Gomez-Caminero et al. 2001).
The bioindicators have recently been defined as the organisms in which changes in known characteristics can be measured in order to assess the extent of the environmental contamination. In this way the conclusions can be drawn about health implications for the other species in the environment as a whole (Baloš et al. 2015). The identification of heavy metal concentrations in the organs of free-living animals provides an indirect measure of environmental pollution, allowing the determination of the degree of exposure of animals to these elements in a given area (Wieczorek-Dąbrowska et al. 2013). Due to its biological characteristics (life habits, diet, relatively long lifespan) and relatively simple sampling procedure during the regular hunting season in the last decade, a significant number of studies have been done, in which game animals were used as a bioindicators of environmental pollution, especially wild boar ( Sus Scrofa L.), red deer ( Cervus Elaphus) and roe deer ( Capreolus capreolus) (Bilandzić et al. 2009, Gašparík et al. 2017, Markov and Ahmed 2019, Santiago et al. 1999, Srebočan et al. 2012). The long period of accumulation of the chemicals during their lifetime can provide an early warning of negative toxic effects in the ecosystem as a whole. In the Republic of North Macedonia the wild boar, one of the most numerous game species, is represented in the entire territory of the country, and in the last decade its number has increased drastically. This has been confirmed by the data of the State Statistics Office, where the population size increased 2 to 3 times from 2010 to 2020 (Anonymous 2021a). The largest number of hunters in North Macedonia are focused on wild boar hunting. Therefore, in the last few years, the number of harvested wild boars has increased 3 to 4 times compared to the previous years (Anonymous 2021b). For these reasons, it is very easy to obtain wild boar samples during the regular hunting seasons and to use this species for biomonitoring purposes.
The deposition of the heavy metals and their movement through the environment depends on many physical and biological processes and factors. In order to obtain a good assessment of the transfer and the potential of a particular pollutant, it is necessary to constantly monitor and measure various physical, chemical and biological properties of the ecosystem by means of appropriate bioindicators. The content of heavy metals in game tissues can serve as a good basis for providing the important data on their presence in ecosystems. In this study we used free-living wild boars to provide new data for the concentration of environmental contaminants Hg and As in the Republic of North Macedonia.
MATERIJALI METODE
Study area – Studijsko područje
The Republic of North Macedonia is a country situated in the central part of the Balkan Peninsula, borderingSerbia to the north, Bulgaria to the east, Greece to the south and Albania to the west, and it has a total area of 25,713 km2. The country is mountainous and has deep basins and valleys with three large lakes, and it is bisected by the Vardar River. It has a water area of 857 km2, while its land area is 24,856 km2 (Barandovski et al. 2020). This study was carried out on 11 hunting areas that cover the territory of the entire country (Figure 1), namely: Pelagonisko hunting area (1), a total area of 408,279 ha; Ohridsko-Prespansko hunting area (2), a total area of 183,743 ha; Kičevsko-Brodsko hunting area (3), a total area of 173,624 ha; Pološko hunting area (4), a total area of 168,717 ha; Skopsko-Kumanovsko hunting area (5), a total area of 276,105 ha; Sredno-Vardarsko hunting area (6), a total area of 220,525 ha; Krivorečansko hunting area (7), a total area of 109,524 ha; Bregalnčko hunting area (8), a total area of 214,435 ha; Vlainsko-Maleševsko hunting area (9), a total area of 139,233 ha; Strumičko hunting area (10), a total area of 168,695 ha; and Dolno-Vardarsko hunting area (11), a total area of 282,772 ha (Trpkov and Maletic 1997).
Sampling – Uzorkovanje
From the mentioned 11 hunting areas (number 1 to 11), a total number of 608 liver samples of wild boar (aged between 2 and 4 years), shot by the hunters, were collected during the regular hunting seasons (between 2016 and 2022). The collected samples did not have a normal distribution according to the hunting areas. Samples were collected, individually packed in polyethylene bags, and transferred to the laboratory in refrigerated bags. The tissue samples were frozen and stored at -20 °C until analysis. During sampling operations, special care was taken to avoid areas near the bullet pathway; all tissue samples were taken at least >40 cm away from areas of bullet damage (Danieli et al. 2012, Dobrowolska and Melosik 2008). It is important to emphasize that all samples were collected by active hunters during the regular hunting seasons and no wild boar was shot for the purpose of this research.
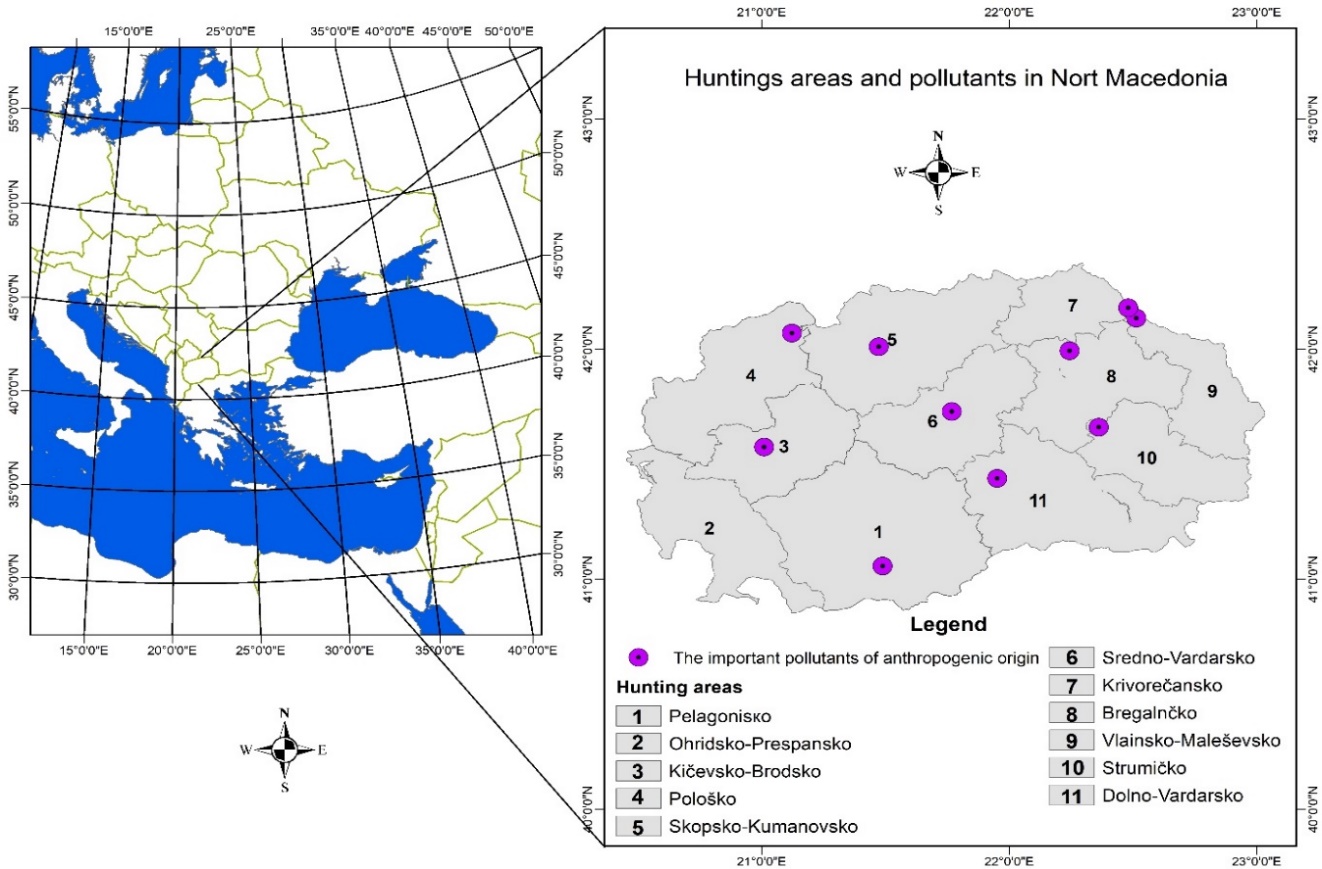
Figure 1. Eleven hunting areas (1-11) in the Republic of North Macedonia and the locations of pollutants.
Slika 1. Jedanaest lovnih područja (1-11) u Republici Sjevernoj Makedoniji i lokacije zagađivača.
Analysis – Analiza
Sample preparation: Around 1 g of defrosted homogenized sample was weighted with an accuracy of ±0.01 g into a digestion vessel; 5 mL of concentrated HNO3 with purity for atomic absorption and 1 mL of 30% hydrogen peroxide was added to the sample (both reagents supplied by Merck, Darmstadt). The digestion was performed with a high-performance microwave oven (model Ethos Up, Milestone Srl, Sorisole, Italy) according to the procedure given in EN 13805:2014 (Anonymous 2014). Briefly, the microwave temperature was ramped for 20 min up to 220 °C, followed by 15 min digestion step at the same temperature. The microwave potency was automatically adjusted by temperature/pressure control sensors in each digestion vessel. The cooled samples were diluted to a volume of 25 mL with Milli-Q quality deionized water. Analysis of As was performed by electrothermal atomic absorption spectrometer (ETAAS) model AAnalyst 600 (Perkin Elmer, Waltham, Massachusetts) with Zeeman background correction. As a matrix modifier for arsenic atomization at wavelength of 193.7 nm (slit width 0.7 nm), 0.005 mg Pd ((NO3)2) solution was used according to EN 14332:2004, (Anonymous 2004). Furnace program for As determination in the liver matrix was optimized for maximal method sensitivity (Table 1). Mercury was analyzed by cold vapour atomic absorption (CVAAS) technology (EN 13806:2002) (Anonymous 2002), using flow-injection analysis system for mercury, model FIMS 100 (Perkin Elmer, Waltham, Massachusetts). For Hg analysis, electrodeless discharge lamp at 253.7 nm was used, and slit width was 0.7 nm. The reagents used were 3% ( V/ V) HCl as carrier solution and 0.2% NaBH4 in 0.05% NaOH as reducing reagent. The optimized FIMS 100 program for mercury determination is presented in Table 1. For instrument calibration, suitably diluted solutions of certified reference materials (CRM) of Hg and As (all purchased from Carl Roth GmbH, Karlsruhe, Germany) were used. Data acquisition and quantification of analyses was performed by WINLAB 32 software for AA, version 6.2.0.0079 (Perkin Elmer, Waltham, Massachusetts).
Table 1. Furnace program for arsenic determination and FIMS program for mercury determination
Tablica 1. Furnace program za određivanje arsena i FIMS program za određivanje žive
Method quality assurance: The methods for As and Hg determination were validated according the requirements proscribed by the Commission Regulation (Anonymous 2007). Method validation was performed by analysis of certified reference material (CRM) - offal liver FAPAS test material 07199. Thus, the method performance characteristics were the following: linearity from 5 calibration points was in the range of 0.018-20.0 µg/L for As and 0.014-5.0 µg/L for Hg, respectively, with R2> 0.99; limits of quantification were 0.66 µg/L (As) and 0.048 µg/L (Hg); the method precision (RSD) and recovery was 4.42% and 80.58% for As, and 5.06% and 91.1% for Hg, obtained from six replicate analyses of CRM. The obtained method performances fulfilled the requirements laid down in the respective regulative (Anonymous 2007). Each batch of samples was followed by one reagent blank sample and one CRM sample, thus providing internal quality control of the analysis. External quality control was assured by participation in proficiency test organized by FAPAS-FERA (UKAS accreditation) and the European Union Reference Laboratory for metals, EURL-MN, DTU (DANAK accreditation). The method was accredited according to ISO/IEC 17025:2017 (Anonymous 2017).
Statistical analysis – Statistička analiza
Statistical analysis was performed using the Statistica software version 14 (StatSoFT STATISTICA Software), while Microsoft Excel 2016 MSO (16.0.4312.1000) was used for descriptive analysis. To examine differences between sampling areas we used the two-way analysis of variance (ANOVA) test. Statistical significance was set at p ≤ 0.05.
REZULTATI I RASPRAVA
Тhe results of the analysis of Hg and As concentrations in wild boar liver tissues from 11 different hunting areas in the Republic of North Macedonia collected in the period of 7 years are presented in Tables 2 and 3, in the forms of the mean, median, minimum and maximum values. In our study, mercury was detected in 82.6% (502) of the total number of samples (N=608) and no statistically significant difference was found among all 11 hunting areas for this heavy metal. The mean concentration of Hg in livers of wild boar from 11 hunting areas was in the range from 12.7 μg/kg to 68.8 μg/kg and mean concentration for all was 45.67 μg/kg. The maximum mean value of 68.8 μg/kg was measured in hunting area number 5 (Skopsko-Kumanovsko).
The detected mercury values of 0.04 ±0.03 mg/kg in the liver of wild boars on hunting ground of state forests of Slovakia (Gasparik et al. 2012) shows similar concentrations to our study. On the other hand, when determining the concentration of heavy metals in meat and organs of wild boar, lower values of mercury in the liver were determined by Florijancic et al. (2015) in a study conducted on hunting grounds in eastern Croatia with the mean range of 0.012±0.002 to 0.026±0.003 mg/kg, and by Durkalec et al. (2015) in a study conducted on three locations in Poland, with the mean range of 0.019±0.013 to 0.027± 0.033. mg/kg. In another study, also done in Poland, on the basis of 14 samples originating from wild boars, it was determined that mercury values in the liver ranged (minimum-maximum) from 0.015 to 0.061, which is somewhat lower than our results (Dobrowolska and Melosik 2002). However, drastically higher values, not only in relation to our results, but also in relation to the other mentioned studies, were obtained in the Russky Sever National Park (North-West of Russia). At this location, the mean value of mercury in the liver was 0.419 mg/kg, which is almost 10 times higher than the mean value in our results (Eltsova and Ivanova 2021).
Table 2. Mercury concentrations (μg/kg) in the livers of wild boars from 11 hunting areas in The Republic of North Macedonia, in a period of 7 years (2016-2022).
Tablica 2. Koncentracije žive (μg/kg) u jetrima divljih svinja iz 11 lovnih područja u Republici Sjevernoj Makedoniji, u razdoblju od 7 godina (2016.-2022.)
1- 11 Hunting areas; N – number of samples; N < LODs % - number of samples bellow the limit of detection (%); N > P.v % - number of samples exceeding the permitted value (%)
1- 11 Lovna područja; N – broj uzoraka; N < LODs % - broj uzoraka ispod granice detekcije (%);
N > P.v % - broj uzoraka koji premašuje dopuštenu vrijednost (%)
The maximum level of Hg is not specified in the Commission Regulation (EC) no 1881/2006, setting the maximum level for certain contaminants in foodstuffs (Anonymous 2006). The situation is the same in the Republic of North Macedonia, because the regulation is fully harmonized with the European one. For that reason the suggestions of The European Union Reference Laboratory for Chemical Elements in Food of Animal Origin in Rome (EU-RL CEFAO) about the maximum level of Hg in the Commission Regulation (EC) no 149/2008 (Anonymous, 2008) should be respected. In this regulation specified for residues of pesticides in or on food and feed, of plant and animal origin, the maximum allowed level of Hg is 0.1 mg/kg of wet weight (Durkalec et al. 2015, Lénárt et al. 2023). In this study, looking at all locations, 6.56% (40/608) samples exceed the maximum allowed limits (N>0.1 mg/kg). The maximum value of 961 μg/kg, which is 9.5 times higher than the legal limit, was measured in hunting area number 1 (Pelagonisko). Accumulation and toxicity of Hg in aquatic biota, domestic animals, and humans have been well investigated and documented, but relatively little is known about these processes in wild terrestrial mammals. The concentration of heavy metals in animal tissue usually depends on the duration and speed of intake by the animal. Such bouncing values are probably the result of annual accumulation of mercury in the body through diet. As an omnivore, the wild boar eats both plant and animal food (Bilandzić et al. 2010, Lénárt et al. 2023), and searches for it on and below the surface of the soil. The strongly bound inorganic mercury in the soil may be the reason for this due to the wild boar's diet. Regarding all parts of plants (Florijancic et al. 2015), roots are the best known place of deposition of Hg, and are favourable food for wild boars. However, in the studies by Stafilov (2014), the occurrence of heavy metals such as mercury in the soil were relatively unimportant in the region of this hunting area where the maximum sample was determined. It is perhaps due to the nature of the wild boars’ behavior and long-distance movement (maximum daily movement varies between 4 and 27 km, on average 5.4 km according to Мiettinen et al. 2023), so this concentration integrates the contamination of much larger areas.
The statistically significant difference was found in the mercury concentration of liver samples between the years, i.e. the mean value for this heavy metal in 2022 was significantly higher than those measured in 2018, 2019 and 2021. In the same year (2022), the largest number of mercury values exceeding the maximum permitted limits by the regulation were measured, i.e. 2.3% out of 6.56% or 14 out of a total of 40 samples exceeding the permitted limits (N>0.1 μg/kg). Also, in the same year, the maximum value of 968.1 μg/kg and the maximum mean value of 74.89 μg/kg was measured.
Although there are some values that exceed the maximum allowable limits, it is important to note that the mean values for mercury are almost two times lower than the allowable limits, and even the maximum mean value does not exceed the limit. It is much easier to determine the reason and make a conclusion when the concentration of some heavy metal has an excessive increase or decrease over the years. However, regarding the occurrence of mercury in our results, many factors have influence on the final result. These factors are the place of sampling (since the samples are collected during the regular hunting season, most of the time the number of samples is not evenly distributed between the compared hunting areas and over the years), the geological origin of the heavy metals (whether the investigated heavy metal is naturally present in that area), anthropogenic impact on that area (whether there is a potential pollutant for the given location/hunting ground in the researched area), the age of individuals (heavy metals accumulate in animals in the course of their lives, so older individuals often have higher concentrations) and many other factors that require separate additional research in order to obtain a correct conclusion about such oscillations.
Table 3. Mercury concentrations (μg/kg) in the livers of wild boars from 11 hunting areas in the Republic of North Macedonia, in a period of 7 years (2016-2022).
Tablica 3. Koncentracije arsena (μg/kg) u jetrima divljih svinja iz 11 lovnih područja u Republici Sjevernoj Makedoniji, u razdoblju od 7 godina (2016.-2022.)
1- 11 Hunting areas; N – number of samples; N < LODs % - number of samples bellow the limit of detection (%); N > P.v % - number of samples exceeding the permitted value (%)
1- 11 Lovna područja; N – broj uzoraka; N < LODs % - broj uzoraka ispod granice detekcije (%);
N > P.v % - broj uzoraka koji premašuje dopuštenu vrijednost (%)
Similarly to mercury, the maximum permissible level of arsenic is not regulated for mammal food-producing animals in the EU legal regulation for foodstuffs of animal origin, including muscle and fat tissue (Regulation (EC) No 1881/2006) (Anonymous 2006). Also, in the regulation of the Republic of North Macedonia, which is fully harmonized with the European one, the maximum allowable limits for arsenic in animal organs are not specified. However, the national action level of arsenic in wild boar liver is 0.5 mg/kg in accordance with the Annual Plan for monitoring residues and illegal substances in live animals, products and raw materials of animal origin for 2020 (Anonymous 2020). Also, in the regulations for maximum allowed levels of certain contaminants of the Republic of Serbia, the maximum allowed level of arsenic is 0.5 mg/kg. For that reason, we have compared our results with this maximum level of As (0.5 mg/kg of wet weight). It is important to emphasize that from all the samples in which arsenic was detected (458 samples), none of them had the values which exceeded the maximum allowed limit (N>0.5 mg/kg). It indicates a relatively good condition of the ecosystem based on this metalloid.
The arsenic was detected in 75.3% (458) of the total number of samples (N=608). The same as for mercury, no statistically significant difference was found between all 11 hunting locations for arsenic. The mean concentration of As in the livers of wild boar from 11 hunting locations was in the range from 22 μg/kg to 55.3 μg/kg and mean concentration for all was 33.1 μg/kg. The maximum mean value of 55.3 μg/kg was measured in the hunting area number 9 (Vlainsko-Maleševsko).
In the study conducted by Reglero et al. (2009), in the Pb mining area of the valley of Alcudia and the Sierra Madrona mountains (Southern Spain), the mean values obtained for arsenic in the liver of wild boars were two times higher than the results in this study, i.e. the mean values for arsenic were 0.062 mg/g (in the mining area) and 0.062 mg/g (in the control area). Almost seven times higher values for arsenic in wild boar liver (mean value of 0.21 mg/) compared to our results were obtained by Piskorová, et al. (2003), in a study conducted in the Central Zemplin region of the Slovak Republic. However, lower values of arsenic in the wild boar livers were determined by Florijancic et al. (2015), in a study done on hunting locations from eastern Croatia, with the mean range of 0.013±0.001 to 0.019±0.0005 mg/kg.
In our results, similarly to mercury, the statistically significant difference was found in the arsenic concentration of liver samples between the years. The mean values for arsenic differed statistically in the following years: 2016 and 2019, 2017 and 2019/2022, 2019 and 2021, 2020 and 2021/2022. Although there are significant differences in mean arsenic values over the years, all mean values are well below the maximum levels allowed by law regulation, and this is clearly confirmed even by the maximum arsenic concentration of 465.5 μg/kg determined in 2022, which is also below the permissible limit. According to Stafilov and Šajn (2016), the mean value of arsenic content in topsoil in the Republic of North Macedonia was 9.2 mg/kg, which was lower than the European topsoil content of 12 mg/kg and it corresponds to our results knowing that the wild boar usually looks for food on the surface and below the surface of the soil. Due to the fact that the occurrence of arsenic can be a result of geological representation or anthropogenic influence, and that in this study there was no statistically significant difference in terms of locations as we could take into account the possible sources of arsenic, it is very difficult to explain such multiple oscillations over the years, except for additional research on the factors that were mentioned in relation to mercury. Some European countries have a problem with the natural presence of arsenic, but from the results of this study it can be concluded that this is not the case for the Republic of North Macedonia.
In general, edible internal organs of game are rarely used for human consumption and therefore their potential negative impact on public health is minimal. The game meat, which is most often used in human consumption, in numerous studies had a much lower incidence of the occurrence and concentration of heavy metals compared to the internal organs of game (Bilandzić et al. 2009, Durkalec et al. 2015).
ZAKLJUČAK
The results of this study are encouraging and it can be concluded that the Republic of North Macedonia does not have a problem with excessive contamination with the examined elements (Hg, As). Although in some samples the mercury values exceeded legal limits (number and %), the mean values for both mercury and arsenic were below the maximum allowable levels. Regarding these two elements, a statistically significant difference was not determined in relation to the locations, while the mean values in relation to the years differed statistically significantly Therefore, additional research is needed, where a larger number of influential factors will be taken into account to determine the specific cause of such oscillations.
