INTRODUCTION
Concerns have been raised about the inappropriate use of polypharmacy, especially in older patients who may take a large number of medicines with varying levels of complexity (1). Overtreatment with unnecessary or inappropriate medicines does not represent the best possible medical care for a patient, and one approach to avoid this is through deprescribing. The term “deprescribing” was first introduced in 2003 by Woodward that suggested planning and undertaking deprescribing activities to improve health outcomes in older people (2). The article by Reeve et al. from 2015 exposed that most papers include deprescribing definitions with terms related to discontinuing medicines ( e.g., stop, cease, withdraw), while a limited number included the terms dose reduction, substitution, and tapering (3). Thus, they proposed one definition of deprescribing, which is described as the process of withdrawal of an inappropriate medication, supervised by a healthcare professional, with the goal of managing polypharmacy and improving outcomes. The definition of deprescribing is indeed still ambiguous and raises, for example, the question of whether dose reduction is deprescribing or not.
Moreover, studies evaluating the potential benefits and harms of deprescribing can vary widely in the provided intervention. In general, deprescribing studies can be divided into medication cessation vs. deprescribing intervention trials (4). In medication cessation trials, a target medicine is discontinued in all participants and it provides direct information on the effectiveness and safety of deprescribing (5, 6). For example, such trials may explore abrupt deprescribing of proton pump inhibitors in all patients, regardless of the indication. On the other hand, deprescribing intervention trials examine the implementation of an intervention designed to encourage deprescribing, but not required to do so (7). In these trials, the appropriateness of a medicine is first assessed for each individual patient. Later, deprescribing is proposed only for those patients for whom it is deemed necessary. These studies may involve interventions that focus only on discontinuation of medicine, such as patient education on the cessation of proton pump inhibitors, or a broader medication optimisation intervention that includes adjustment, discontinuation, or even initiation of a more appropriate medicine ( e.g., medication review, medicines reconciliation). Deprescribing intervention trials typically evaluate the success of the intervention implementation, such as the proportion of participants for whom deprescribing was deemed necessary and who successfully discontinued their proton pump inhibitor. Effectiveness and safety outcomes are also commonly reported, but are likely to depend on the success of the intervention. Additionally, these trials may provide qualitative insights that are important, for example, to understand the potential ineffectiveness, to plan further improvement, or possibly implement deprescribing interventions. Although both types of studies are undoubtedly important, the results may not be directly comparable and require cautious interpretation (1).
Not only can the definition of deprescribing and interventions themselves vary, but deprescribing approaches can also target populations with different characteristics, and be specific to certain medicines, settings, or deprescribing tools. In order to provide insights into the current state of research and potential avenues for future exploration, this umbrella review examined and summarised systematic reviews and meta-analyses of deprescribing studies by characteristics of intervention, population, medicine, and setting. Moreover, clinical and humanistic outcomes, barriers and facilitators to deprescribing, and tools for deprescribing are presented.
EXPERIMENTAL
The PRIOR guidelines 2022 for the overview of reviews were used as a guide in the preparation of this umbrella review.
Data sources
The Medline bibliographic database was used. The search was limited to systematic reviews and meta-analyses published in English up to April 2022. A set of terms was selected prior to beginning the search to cover deprescribing and all possible related terms. Therefore, we used synonyms for discontinuation ( e.g., withdraw) in conjunction with terms related to healthcare services ( e.g., medication review), treatment with multiple medicines ( e.g., polypharmacy), or appropriateness of prescribing ( e.g., inappropriate prescription). Additional references were sourced through reviewing bibliographies of identified reviews. A full search strategy is provided in Appendix 1.
Selection of reviews and meta‑analyses
Reviews reporting any type of deprescribing approach regardless of the definition of deprescribing were included. Reviews were excluded if deprescribing was not planned and supervised by a healthcare professional, as delineated in the definition by Reeve et al. (3). Reviews examining only temporary discontinuation of medicines were also excluded.
Data extraction
A reviewer (NJ) extracted the relevant data using a standard data extraction form designed for this review. The data extracted included general characteristics of the review (population included, target medicines, healthcare settings), methodology (objective, number and type of studies included, review type), deprescribing approach ( medication cessation trials or deprescribing intervention trials), and outcomes. Key findings regarding both clinical and humanistic outcomes were extracted from the reviews analysed. Clinical outcomes included mortality, hospitalisation, medication use, adverse drug withdrawal events, and falls, while the humanistic outcome was quality of life. For reviews describing attitudes, barriers, or facilitators to deprescribing approaches, these qualitative outcomes were retrieved. For reviews with descriptions of the deprescribing tools, the most important results about the reviewed tools were retrieved. For systematic reviews, the narrative conclusions of the authors were extracted. For meta-analyses, the pooled relative risks (RR), odds ratios (OR), or mean differences were extracted along with the 95 % confidence intervals (CI).
Data synthesis and analysis
Regarding the deprescribing approach, reviews were divided into two main categories: reviews that mainly included medication cessation trials and reviews that mainly included deprescribing intervention trials. The latter were further subdivided into reviews that mainly included specific deprescribing interventions with only deprescribing and no probability of a prescribing component ( e.g., medication review conducted solely to identify deprescribing targets, excluding the initiation of new medicines) or broad medication optimisation interventions with deprescribing and also a high probability of a prescribing component ( e.g., starting a new medicine). The reviews describing attitudes, barriers, or facilitators to deprescribing approaches and the reviews describing tools for deprescribing were summarised separately. Findings are presented in a narrative form. Given the variety of deprescribing approaches presented across all reviews, statistical pooling in meta-analyses was not appropriate.
Quality and overlap assessment
The PRISMA 2020 checklist (9) was used to assess the quality of reviews focused on clinical and humanistic outcomes of deprescribing approaches, as well as those concentrating on tools for deprescribing. Meanwhile, the ENTREQ 2012 checklist (10) was used to assess the quality of reviews that reported on attitudes, facilitators, or barriers to deprescribing approaches. The overlap of primary studies within the included reviews was also assessed. Primary studies were extracted, and their overlaps were separately evaluated for reviews focused on the clinical and humanistic outcomes of deprescribing approaches, reviews exploring attitudes, barriers, or facilitators to deprescribing approaches, and reviews examining tools for deprescribing. Additionally, the overlap based on the types of medicines was separately addressed in reviews focused on the clinical and humanistic outcomes and those exploring attitudes, barriers, or facilitators to deprescribing approaches.
RESULTS AND DISCUSSION
A total of 94 systematic reviews, including 23 meta-analyses, were included in the umbrella review. Fig. 1 provides an overview of the selection process.
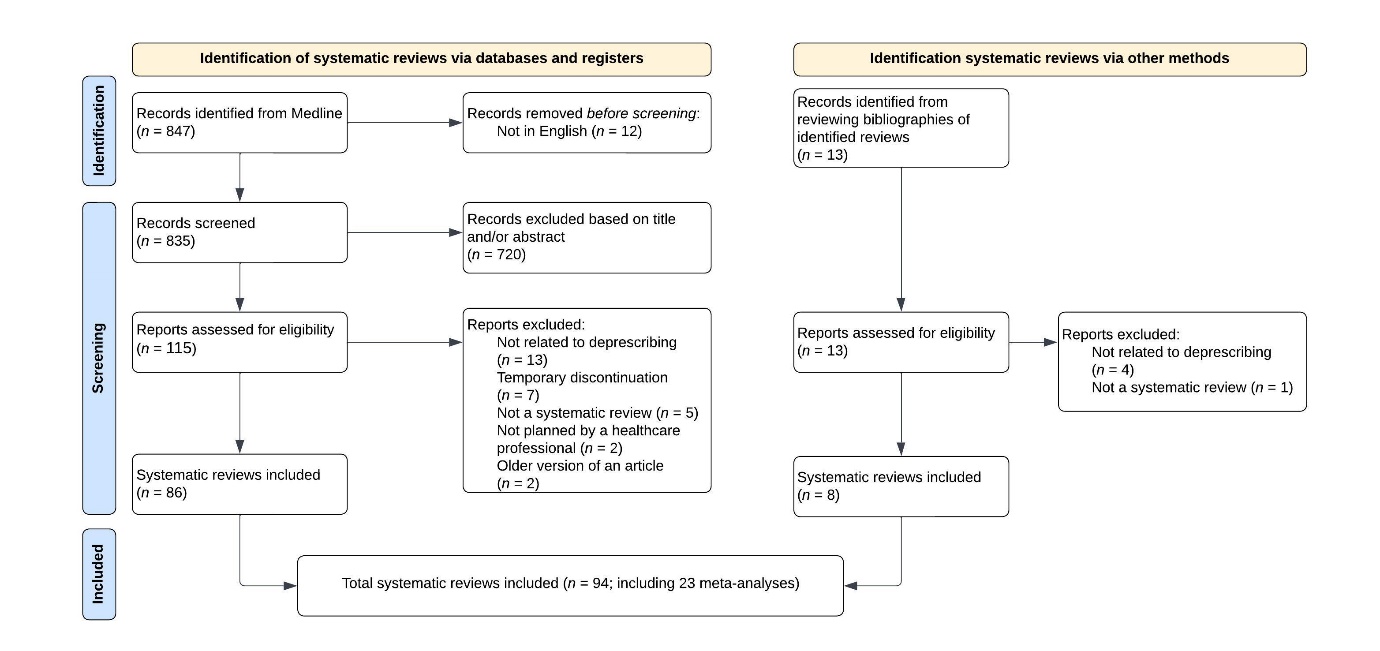
Fig. 1. Flowchart of systematic reviews inclusion.
General characteristics of included reviews and meta‑analyses
All 94 reviews were published between 2008 and 2022. Most reviews reported clinical and humanistic outcomes, namely mortality, quality of life, hospitalisation, medication use, adverse drug withdrawal events, or falls (70/94, 74 %). Fewer reviews reported attitudes, facilitators, or barriers to deprescribing approaches (17/94, 18 %). One of the reviews reported both clinical outcomes and qualitative findings related to facilitators and barriers to deprescribing. Few reviews focused specifically on identifying or examining tools for deprescribing approaches (8/94, 8.5 %).
Reviews describing clinical and humanistic outcomes of deprescribing approaches
Most reviews reported clinical and humanistic outcomes (70/94, 74 %), and 23 of the reviews were upgraded to meta-analysis. The number of randomised or non-randomised studies included in a single review ranged from 2 to as many as 116, on average 17 per review, and the number of participants ranged from 15 to more than 500,000 (Supplementary Table I).
The populations included were of different ages, usually older people over 65 years of age (36/70, 51 %; Table I), adult people over 18 years of age (30/70, 43 %), and only four (4/70, 6 %) reviews addressed younger people under the age of 18. The reviews focusing on an adult population over 18 years of age were not limited to those under 65 years but also included older individuals aged 65 and above, who may even represent the majority of patients in these studies. Slightly less than half of the reviews were limited to patients with specific conditions (33/70, 47 %), most commonly patients with mental disorders (17/70, 24 %), pain (4/70, 6 %), or specific chronic diseases such as diabetes, reflux disease, heart failure and others (Table I). In the older population, the reviews did not focus on a specific disease, with the exception of mental disorders (11–16), especially dementia and sleep disorders. In the adult population, most reviews focused on mental disorders (17–24) or chronic medical conditions (5, 6, 25–34). Of the four reviews that addressed populations younger than 18 years, three focused on children and adolescents with mental disorders such as attention-deficit hyperactivity disorder (35, 36) or epilepsy (37), and one review focused on paediatric patients with asthma (38).
Table I. Number of included systematic reviews describing clinical and humanistic outcomes according to age and characteristics of population
Most reviews focused on a group of medicines, most commonly medicines for mental disorders (15/70, 21 %), in particular benzodiazepines (13, 16, 21–23) and antipsychotics (15, 18, 35, 36). Two other commonly covered groups of medicines were cardiovascular medicines (7/70, 10 %) (39, 40), including antihypertensives (11, 41, 42) or heart failure medicines (29, 43), and analgesics (5/70, 7 %), especially opioids (14, 27, 31, 33, 34).
The reviews were generally not restricted to a particular setting (46/70, 66 %). Few of the reviews were specifically limited to an inpatient (12/70, 17 %) (31, 34, 44–53) or outpatient setting (11/70, 16 %) (5, 21, 22, 54–61). Long-term care ( e.g., nursing homes) was addressed in only one review (62). The main characteristics of reviews and included studies as well as outcomes, namely mortality, quality of life, hospitalisation, medication use, adverse drug withdrawal events, and falls, are summarised in Supplementary Table I.
To summarise, deprescribing approaches were most commonly employed in older populations, with mental disorders, polypharmacy, limited life expectancy, or specific chronic conditions such as diabetes, reflux disease, or heart failure. All the most frequently recognised characteristics in the reviews are expected in the older population and outline patients with high risk for medicine misadventures that should be prioritised for considering deprescribing where appropriate (63). Importantly, successful deprescribing requires more than just considering a patient's age, especially it must address an individual's health condition and medication complexity, including polypharmacy or inappropriate medication use (62, 64). Indeed, patient-specific interventions, such as medication reviews conducted by pharmacists or physicians aimed at identifying deprescribing opportunities in older patients, have been shown to be more effective at reducing mortality than broad deprescribing educational initiatives directed at healthcare providers or older patients (55, 64).
Type of deprescribing intervention and outcomes
The reviews were divided according to the main type of intervention performed in the studies. In many cases, the reviews did not contain only one type of trial but were classified according to the predominant type. More reviews included deprescribing intervention trials (39/70, 56 %; Fig. 2, Supplementary Table I) ; 16 with specific deprescribing interventions, and 23 with broad medication optimisation interventions. Fewer included medication cessation trials (31/70, 44 %).
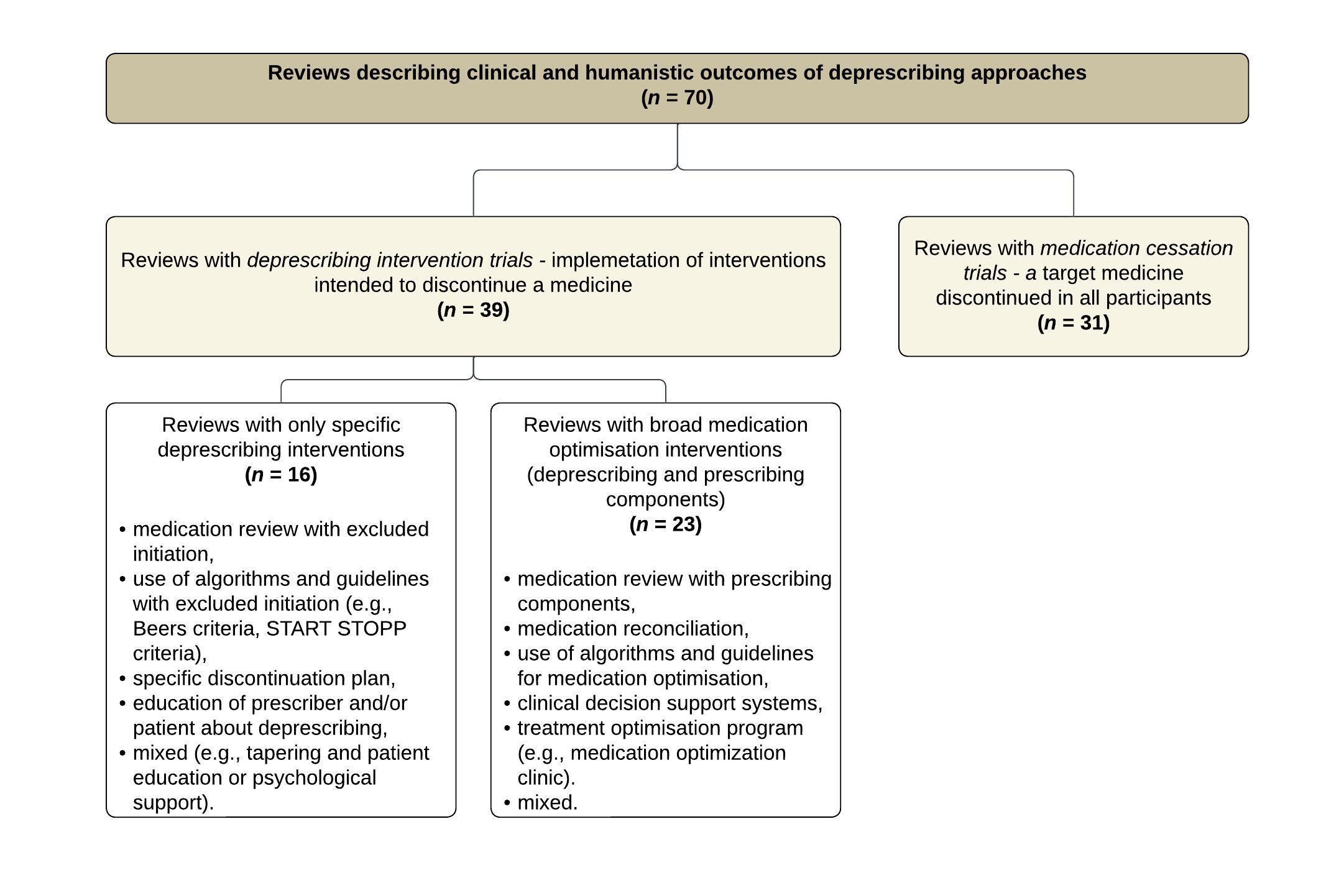
Fig. 2. Distinction between reviews describing clinical and humanistic outcomes of deprescribing approaches.
Reviews which mainly included deprescribing intervention trials primarily reported medication use outcomes (34/39, 87 %), as well as adverse drug withdrawal events (19/39, 49 %), hospitalisation (16/39, 41 %), quality of life (15/39, 38 %), mortality (14/39, 36 %), and falls (13/39, 33 %). In addition to clinical and humanistic outcomes, some also reported implementation outcomes such as the feasibility of the interventions in clinical practice (33, 64). These reviews were further divided into reviews that examine specific deprescribing interventions, and reviews that include broad medication optimisation interventions, which were mostly medication reviews.
Specific deprescribing interventions have been proven to reduce the total number of inappropriate medications (13, 19, 21, 22, 24, 27, 31, 33, 34, 64–66). In particular, educational deprescribing interventions have been shown to reduce opioid use. Additionally, a wide range of interventions, ranging from minimal deprescribing interventions like providing self-help information to patients, to more complex interventions such as cognitive behavioural therapy or mindfulness-based cognitive therapy, have been effective in reducing the use of benzodiazepines or antidepressants. Mostly no detrimental consequences of specific deprescribing interventions were reported, as no increase in adverse events (13, 21, 31, 64, 67), emergency department visits, or rehospitalisations (66, 68), also in the very fragile patients with limited life expectancy were noted. The benefit of deprescribing on other important clinical outcomes was not found, with the exception of two meta-analyses that were able to show a mortality risk-benefit (64, 66) when very targeted patient-specific geriatric interventions were applied. These interventions included medication reviews that excluded initiation but solely focused on identifying targeted medicines for deprescribing, using predefined algorithms such as the Screening Tool of Older Persons Prescriptions (STOPP) in older adults or older adults with limited life expectancy. When interpreting clinical outcomes of deprescribing interventions, it should be noted that it is not always necessary to demonstrate that discontinuation of a medicine leads to improved patient health. Rather, if it can be demonstrated that ceasing the medicine does not result in any deterioration of the patient's health, this can also be considered a favourable outcome (69).
Broad medication optimisation interventions included a variety of interventions, most notably multidisciplinary interventions, pharmacist-led medication reviews, physician-led interventions, prescriber or patient education programmes, and clinical decision support systems. These interventions have been proven to reduce the use of potentially inappropriate medications (45, 48, 53, 55, 57, 62, 70–72) and have also been found to be cost-effective (50, 59). Deprescribing was safe and feasible, and more complex interventions such as medication reconciliation, medication review, and patient education by pharmacists even reduced potential adverse drug events (45, 57) and hospitalisation rates (43, 46). Medication review-directed deprescribing interventions reduced falls by 24 % in older residents in nursing homes (62). In most cases, no difference in mortality was found after deprescribing, with the exception of two meta-analyses that reported deprescribing interventions within medication review reduced all-cause mortality in older adults in randomised controlled studies (55, 62). Therefore, complex interventions, including medication reconciliation and other services that frequently involved pharmacists, demonstrated improvements in important clinical outcomes. However, due to the wide range of interventions that consider a patient's overall medication regimen, it is challenging to ascertain whether the benefits can be solely attributed to deprescribing. In fact, one review (58) reported a higher medication burden following a comprehensive geriatric assessment, indicating that factors beyond deprescribing alone can influence medication usage and other outcomes.
Reviews which mainly included medication cessation trials primarily reported adverse drug withdrawal events (29/31, 93 %) and medication use outcomes (17/31, 54 %), as well as mortality (14/31, 45 %), quality of life (7/31, 23 %), hospitalisation (6/31, 19 %), but rarely falls (4/31, 13 %). Notably, one specific intervention, procalcitonin-guided discontinuation of antibiotics, demonstrated a lower mortality rate (47, 51). Furthermore, deprescribing psychotropic medicines resulted in a reduction in the number of falls (39), and discontinuation of statins showed potential improvement in quality of life (40). Reviews with medication cessation trials typically examined the best strategies for discontinuing medicine in all participants, who may differ in the appropriateness and importance of the indication for the medicine to be discontinued. Consequently, the occurrence of adverse events was frequently reported for these strategies. The outcomes varied, ranging from the absence or occurrence of transient adverse drug withdrawal events (6, 11, 14–16, 25, 41, 42, 73) to more severe reactions (5, 12, 20, 28, 29, 35, 36, 74, depending on the specific medicine and individual characteristics. It is important to distinguish between different reasons for deprescribing. Reviews may focus on the discontinuation of potentially inappropriate medications without an existing indication or on the discontinuation of medicines with an existing indication but with a desire to treat the condition less intensively. For example, when proton pump inhibitors were discontinued in patients with reflux disease or mild esophagitis, an increased risk of poor symptom control and lower patient satisfaction was observed (5), whereas, most patients without a clear indication can reduce or completely discontinue proton pump inhibitors without worsening symptom control (6). If the reason for deprescribing is to reduce the intensity of treatment for an existing disease, this must be done with special caution as deprescribing medicines in patients with pre-existing, yet stable, conditions carries the possibility of recurrence. For instance, discontinuing heart failure medicines in patients with stable chronic heart failure (29), ceasing attention-deficit hyperactivity disorder medicines in children diagnosed with the condition (36), or discontinuing biological therapies for rheumatic diseases (28) can potentially lead to relapse. Two reviews even reported higher mortality rates associated with discontinuing heart failure medicines (29) and warfarin in high-risk patients, such as frail individuals or those with limited life expectancy (40). Careful consideration should be given to the appropriateness of indication and the potential for recurrence when deprescribing medicines in cessation trials.
Reviews describing attitudes, barriers, or facilitators to deprescribing approaches
The reviews describing attitudes, barriers, or facilitators to deprescribing approaches (17/94, 18 %) included from 2 to 42 randomised or non-randomised studies in a single review, on average 19 per review (Supplementary Table II). The number of participants ranged from 48 to as many as 400.000. Participants were patients (13/17, 76 %) (75–87), healthcare professionals (12/17, 71 %) (45, 79–83, 85–90), such as prescribers, nursing home staff, or caregivers (11/17, 65 %) (75, 76, 78–80, 82–87). Most reviews did not address a specific medicine or were limited to any long-term medicine (10/17, 59 %), five reviews were focused on a specific medicine, such as cancer therapy (86, 87), benzodiazepines (83), psychotropic medicines (90), and anticholinergics (85), two reviews were tied to a group of potentially inappropriate medications (45, 89). The reviews were generally not restricted to a particular setting (12/17, 71 %), three were specifically limited to inpatient settings (45, 86, 87), one to outpatient settings (80), and one to long-term care settings ( e.g., nursing homes) (90).
The main findings related to attitudes, barriers, or facilitators to deprescribing approaches are summarised in Supplementary Table II. The reviews examining attitudes toward deprescribing with the patients' attitudes towards deprescribing questionnaire (PATD) or modifications, reported that 70–88 % of patients are willing to discontinue medicines if told to do so by a healthcare professional (75–78). Additionally, patients showed a willingness to specifically discontinue benzodiazepines (83). In contrast, it is worth noting that healthcare professionals consider the discontinuation of benzodiazepines particularly challenging (83). This highlights the importance of a collaborative approach that involves patients, carers, and multiple healthcare professionals to successfully navigate the deprescribing process. The niche area of cancer therapy discontinuation for end-of-life situations further emphasizes the critical need for patient care that is empathetic, well-informed, and consistent with the patient's values and desires (86, 87). Effective communication is essential when presenting deprescribing also to other patients in late palliative care, highlighting the need for these strategies to be embedded in deprescribing tools (91).
Facilitators and barriers to deprescribing are mostly organisational, professional, and patient-related. The establishment of other clinical pharmacy services, such as the involvement of multidisciplinary teams in the process, medication reconciliation, medication review, as well as the presence of a clinical pharmacist in the clinical setting, may serve as a framework for deprescribing interventions and was the most frequently cited facilitator of deprescribing (45, 82, 88, 89). Patient-related facilitators primarily included patient's or family's involvement, good communication with the patient, especially about possible adverse drug withdrawal events (78, 83, 90), and shared decision-making about stopping medicines (76, 79, 89). Professional-related facilitators included awareness of deprescribing, self-confidence and skills of healthcare professionals, good collaboration between healthcare professionals ( e.g., nurses and physicians), follow-up with monitoring (78, 80, 82, 88–90), and other factors listed in Supplementary Table II. Barriers included factors similar to those of facilitators, only in a negative direction. Additionally, unawareness of the benefits of deprescribing, fear of cessation, or fear of missing out on future benefits of treatment were highlighted as important barriers (78, 79, 84). Addressing fears and concerns about deprescribing is critical to overcoming barriers to deprescribing and should be done through effective patient education and open communication between patients and healthcare professionals. Shared decision-making with patients plays a vital role in this regard.
Reviews describing tools for deprescribing approaches
Eight reviews described tools for deprescribing approaches and are summarised in Supplementary Table III. Three of the reviews examined all of the tools developed for deprescribing and reported the following types of tools: general framework, detailed tools for medicine assessment, and comprehensive discontinuation guidelines (91–93). One of these reviews focused on frail older populations and one on palliative care patients, including cancer patients. Three other reviews focused on guidelines for discontinuation of specific medicines, e.g., cholinesterase inhibitors (94), statins (95), and dermatological therapy (96). One review described only educational materials on deprescribing one or more medicines (97), and another review determined the applicability of the N-of-1 trial method (98).
Tools for deprescribing approaches could be applied in different stages of deprescribing, e.g., preparation, which includes assessment of the current status of the patient and his medication history, medicine evaluation, decision making, and implementation (91). The identified weaknesses of the tools in the reviews include poor descriptions of development methodology and their limited application in clinical practice (92), no or few specific recommendations for the discontinuation of specific medicines (94–96), and in the case of educational material, a requirement of high levels of reading, thus making it inappropriate for populations with low health literacy (97). An N-of-1 or single-subject clinical trial is a randomized crossover study design in which a single patient acts as their own control (98). This design uses the random allocation of an experimental and a control intervention while keeping the patient unaware of the sequence (98). This method, considered safe, may be particularly useful for studying deprescribing in specific individuals, such as older adults who are cognitively impaired, addressing the inherent complexities and variabilities of this group (98). However, while the overall feasibility of N-of-1 trials remains to be evaluated, it is important to note that they are not suitable for investigating long-term outcomes, risks, or benefits (98). Practical and validated tools are needed to provide clinicians with guidance on the discontinuation process, communication aspects, and to encourage patient involvement in order to align with patients' evolving priorities and care goals (91). Patients with certain medical conditions such as cancer patients or near the end of life may have unique opportunities and challenges to deprescribing.
Quality and overlap assessment
Reviews focusing on clinical and humanistic outcomes had an average score of 0.77, while those concentrating on tools for deprescribing scored an average of 0.79 out of a maximum of 1.00, reflecting a robust reporting approach. Reviews exploring attitudes, barriers, or facilitators to deprescribing approaches had a notably lower average score of 0.54 out of 1.00, highlighting areas for improvement in reporting and methodology. Regarding ENTREQ guidelines, deficiencies were noted in approaches to searching and in the use of quotations. Reviews focusing on qualitative research could benefit from more structured search strategies and more effective integration of direct evidence, such as quotations. Detailed assessments of the quality are available in Supplementary Tables IV to VIII. The overlap of all primary studies was 28 % between reviews focused on the clinical and humanistic outcomes of deprescribing approaches, 24 % between reviews exploring attitudes, barriers, or facilitators to deprescribing approaches, and only 3 % between reviews examining tools for deprescribing. The low overlap in reviews examining tools for deprescribing indicates diverse research in this area, with potential for new studies. The overlap based on the types of medicines is reported in Supplementary Tables I and II.
The diversity of the terminology in the field of deprescribing represented a major challenge and has made an effective retrieval of all published reviews uncertain. Intentionally, the search was extensive and the search profile broad, hence ensuring the majority of applicable reviews were identified and included. During the writing of this paper, additional strategies for searching deprescribing literature were published (99), but the search profile of this umbrella review was not compared with these recommended strategies. Reviews were classified by the predominant intervention type and may include different types of studies, including both deprescribing intervention trials and medication cessation trials. The wide range of qualitative and quantitative reviews did not allow comparability or correlation between specific interventions and outcomes. The list of excluded reviews is not provided. The protocol of this umbrella review was not registered.
Practice, policy, and future research
In clinical practice, it's crucial to acknowledge that successful deprescribing requires individualized approaches that consider the patient's health conditions and medication complexity, including inappropriate medication use. Collaboration among patients, caregivers, and healthcare professionals is essential for successful deprescribing. This should be followed also on a policy level. The policy should support the implementation of deprescribing in clinical practice by producing practical and validated tools for deprescribing, considering factors like health literacy and patient populations with unique needs. Future systematic reviews on deprescribing should carefully differentiate between deprescribing intervention trials and medication cessation trials and then focus on the composition and clear description of the deprescribing approach examined in specific populations, medicines, and settings. The description of the study design of included studies in future reviews must be carefully considered, especially the selection of deprescribing intervention, to ensure sufficient quality of evidence and the implementation of evidence-based interventions into routine practice (69). Researchers should focus more on one type of medicine and, when appropriate, also on patients’ groups, to make a specific recommendation for discontinuation in the absence of compelling indications.
CONCLUSIONS
The current umbrella review provides a comprehensive and nuanced overview of the existing evidence and identifies specific reviews that can inform clinical practice and researchers in the field of deprescribing. A total of 94 systematic reviews were included. Most reviews examined outcomes related to mortality, quality of life, hospitalisation, medication use, adverse drug withdrawal events, or falls of deprescribing approaches (70/94, 74 %). Reviews with deprescribing intervention trials, qualitative findings, and tools provide insights into successful implementation in clinical practice, considering factors such as individual patient needs and overall treatment goals. Reviews with medication cessation trials aim to identify the safest deprescribing strategies in all participants, including those who may have an appropriate and established indication and therefore these reviews primarily examined adverse drug withdrawal events. Distinguishing between deprescribing intervention trials and medication cessation trials in a systematic review is crucial, as it ensures an accurate assessment of the evidence to inform decisions regarding deprescribing strategies.
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
In the supplementary file, the search profile, characteristics of the included reviews, and the quality assessment of the reviews are available.
Availability of data and materials. – The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding. – This work was supported by the Slovenian Research Agency (Programme Group No. P1-0189 and P3-0360). The Slovenian Research Agency had no role in the design and conduct of the study; analysis and interpretation of data; preparation, approval, and submission of the manuscript for publication.
Conflict of interest. – N. Japelj, N. Horvat, and M. Kos declare that they have no competing interests. L. Knez has received a speaker honorarium from MSD, Pfizer, and Roche.
Authors contributions. – Conceptualization, N.J., N.H., L.K., and M.K.; methodology, N.J. and M.K.; investigation, N.J.; analysis, N.J. and M.K.; writing, original draft preparation, N.J.; writing, review and editing, N.J., N.H., L.K. and M.K. All authors have read and agreed to the published version of the manuscript.
