Introduction
With advances in healthcare and improvements in living conditions worldwide, life expectancy has increased remarkably, leading to many societies having a greater proportion of older adults (1). In Slovenia, for example, life expectancy rose from 72.1 to 77.6 years for men and from 79.6 to 83.7 years for women between 2001 and 2021 (2). However, as individuals age, they become more likely to develop chronic conditions and experience age-related physiological changes that may necessitate pharmacotherapy interventions (3). Consequently, older adults often face a higher medication burden than younger individuals (4). Ensuring the appropriateness of medications and optimizing pharmacotherapy for older adults are paramount in maintaining their health and quality of life (3–5).
Sedative medications and medications with anticholinergic properties are commonly prescribed to older adults for various conditions, such as insomnia, anxiety, depression, urgent incontinence, and chronic pain (6). However, the use of these medications in older adults has been associated with increased risks of adverse events, including falls and fractures, cognitive impairment, delirium, dementia, and other geriatric syndromes (7, 8). These risks are further increased by the concomitant use of multiple medications with sedative or anticholinergic properties (9). In addition, older adults are more susceptible to drug-drug interactions and age-related pharmacokinetic changes that can complicate their medication regimens (3). Given the potential risks associated with sedative and anticholinergic medications, monitoring their utilization patterns among older adults over time is essential (10).
Several measurement tools are available to evaluate sedative load and anticholinergic burden, and they can offer valuable insights into the cumulative effects of these medications (11). Such tools include the Drug Burden Index (6), which quantifies the overall burden of sedative and anticholinergic medications, and criteria-based approaches such as the sedative load (SL) model (7, 8, 12) and the anticholinergic cognitive burden (ACB) scale (13, 14) that identify specific medications with these properties.
The SL model (8, 12) provides a comprehensive measure of sedative medication exposure among older adults. It assigns an SL score to each medication based on its sedative potential, enabling the calculation of an overall SL score for an individual (8). Higher scores have been shown to be associated with an increased risk of adverse outcomes, such as falls and fractures, cognitive impairment, and mortality in older adults (7, 15–17).
The ACB scale (13, 14) offers a standardized approach for quantifying the anticholinergic burden of medications. The ACB scale was initially published in 2008 and then updated in 2012. The cumulative score reflects the overall anticholinergic burden for an individual, and higher scores have been linked to cognitive impairment and functional decline in older adults (14, 18–20).
These tools assist healthcare professionals in quantifying medication exposure, guiding optimization strategies, and mitigating the risk of adverse health outcomes associated with sedative and anticholinergic medications in older adults (21–23). Additionally, they enable the evaluation of medication use patterns in database research, facilitating population-level assessments of prescribing practices and their impact on older adults' health (24).
Research examining sedative load and anticholinergic burden trends over the past three decades is limited. Direct prevalence comparisons are challenging because of methodological variations and demographic differences. In addition, higher prevalence is noted among institutionalized residents versus community-dwelling older adults (25–27). Despite such challenges, several studies (24, 25, 28, 29) found increasing trends up until 2005 or 2015, with more recent investigations showing declining patterns of use over the past decade for benzodiazepines (26, 30–32), psychotropics (27, 33), and atropinic medications (34).
Our study aims to investigate trends in the prevalence of sedative load and anticholinergic burden from 2009 to 2019 among older adults in Slovenia. Slovenia serves as a suitable setting for this research because of its aging population and a nationwide health claims database with accessible information. By analyzing data from national health databases and longitudinal studies, we seek to assess changes in prescribing practices in Slovenia and quantify the extent of sedative and anticholinergic use among older adults.
Experimental
Study design
This retrospective drug utilization study encompassed all dispensed outpatient prescriptions in Slovenia. Specifically, the study focused on medications with documented sedative or anticholinergic activity. The study population included all older adults (aged 65 years and older), for whom at least one prescription medication was dispensed from 2009 to 2019.
Data source
The study utilized national health claims data from the Health Insurance Institute of Slovenia. The database captures publicly funded outpatient prescriptions, while excluding over-the-counter medications, hospital prescriptions, and private (out-of-pocket) prescriptions. Private prescriptions constitute less than 5 % of all outpatient prescriptions in Slovenia (35). The Slovenian healthcare system, which provides obligatory health insurance for the entire population, is managed by the Health Insurance Institute of Slovenia. Detailed descriptions of both the database and the Slovenian healthcare system can be found in other published sources (36–38). The data used in the study were anonymized but contained unique patient identifiers, allowing analysis at the individual patient level. Patient-specific variables included sex and year of birth, while prescription variables comprised dispensed medications categorized by the Anatomical Therapeutic Chemical classification and prescription dates.
Sedative load and anticholinergic burden
The SL model was used in this study to evaluate the sedative burden of older adults (12, 15). The SL model is a method for assessing the sedative effects of medications that has been previously validated (7, 8, 15). The method is criteria-based and does not require dosage information to assess the sedative load for an individual. The SL model was developed by first identifying potential sedative medications through a comprehensive review of scientific literature. A panel of expert clinicians specializing in geriatric pharmacotherapy then categorized these medications based on their sedative properties. Four groups were defined and scored based on the sedative effects of the medications: (1) primary sedatives, or substances with a significant sedative effect (2 sedative points); (2) substances for which sedation is an important adverse effect (1 sedative point); (3) substances for which sedation is a possible adverse effect (0 sedative points); and (4) substances with no available data on sedation (0 sedative points). To calculate the sedative load for each patient, the scores of all concurrently prescribed medications are summed. A summed score ≥ 3 indicates a high sedative load (39).
The evaluation of the anticholinergic burden in this study utilized the ACB scale (13, 14), a criteria-based approach that does not require dosage information. During the development of this scale, a systematic review of published literature on the anticholinergic effects of medications was conducted to compile a list of possible anticholinergic medications. A panel of expert clinicians from various disciplines then further evaluated and categorized the medications. The final ACB scale assigns medications a score from 0 to 3. A score of 1 (possible anticholinergic effect) requires evidence of antagonist activity at muscarinic receptors from in vitro data. A score of 2 (definite anticholinergic effect) requires evidence of clinical anticholinergic effect from literature, prescriber’s information, or expert opinion. A score of 3 (definite anticholinergic effect) indicates evidence of the medication causing delirium based on literature, prescriber’s information, or expert opinion. Medications not meeting these criteria are assigned a score of 0. The ACB scores of all medications concurrently prescribed to a patient are summed to calculate the total anticholinergic burden for that patient. A summed ACB score ≥ 3 indicates a clinically significant anticholinergic burden (9).
Each medication in the health claims database was coded according to the SL model and the ACB scale, enabling the calculation of the sedative load and anticholinergic burden for each study participant. This information was then used for further analysis.
Statistical analysis
The participants served as the unit of analysis, and total sedative load and anticholinergic burden were calculated for each study participant. The presented results include the number of participants in the different categories: those with no sedative load (SL score = 0), those with a sedative load (SL score ≥ 1), and a subgroup of those with a high sedative load (SL score ≥ 3), as well as those with no anticholinergic burden (ACB score = 0), those with an anticholinergic burden (ACB score ≥ 1), and a subgroup of those with a clinically significant anticholinergic burden (ACB score ≥ 3). These categories were further broken down and grouped by sex (male and female) and age (65–69 years, 70–74 years, 75–79 years, 80–84 years, 85–89 years, and ≥ 90 years), and group percentages were calculated relative to the total study population.
The graphical presentation (figures) depicts the extent and trends of sedative load and anticholinergic burden from 2009 to 2019. Additionally, age-standardized trends are displayed, based on the standard European population of 2013. The presentation also includes chain and fixed-base indices to illustrate changes over the years. The trend analysis was conducted using a linear regression model. The yearly burden of sedative load and anticholinergic burden were also calculated for each person, and average values are presented.
Furthermore, the most commonly prescribed medications with sedative or anticholinergic activity were identified. The prescription frequency for each medication was determined by calculating the number of patients prescribed that particular medication relative to the total number of individuals receiving medications from a specific group. All statistical analyses were performed using IBM SPSS Statistics 26.0.
Results and discussion
Study population
A total of 6,734,045 prescriptions were prescribed to 321,259 older adult outpatients in Slovenia in 2009. The patients had an average age of 75.3 years (SD 7.7, median 74 years), and 57.6 % of them were female. In 2019, the number of prescriptions increased to 8,934,953, dispensed to 405,595 older adult outpatients. The average age of the patients in 2019 was 74.9 years (SD 7.0, median 74 years), with 61.4 % of them being female.
The total population of older adults in Slovenia in 2019 was 413,054. Notably, 98.2 % of the older adult population received at least one prescription (405,595 older adults included in our study). Our study included a substantial proportion of the older adult population, providing a comprehensive representation of this demographic.
Trends in sedative load
In 2009, the prevalence of sedative load among older adults was 50.7 % (age-standardized: 50.7 %), which decreased to 45.6 % (age-standardized: 45.1 %) by 2019. Similarly, the proportion of older adults with a high sedative load declined slightly over the years, with rates of 14.5 % (age-standardized: 14.5 %) in 2009 and 13.2 % (age-standardized: 13.0 %) in 2019. Fig. 1 illustrates these proportions across different age groups, using both crude and age-standardized data. Notably, the percentage of individuals receiving sedative medications increased with age, displaying a consistent trend across all age groups. Additionally, Fig. 2a,b demonstrates the prescribing trend of sedative medications by sex, with a higher proportion observed among women, a pattern also supported by age-standardized data. These findings suggest potential areas for improvement, particularly among the oldest population and women.
The linear regression analysis conducted on age-standardized data revealed a statistically significant decline in the proportion of individuals with a sedative load. The slope coefficient was –0.59 % per year (95 % CI: –0.68 to –0.49; R2 = 0.95; p < 0.001), indicating an average annual reduction of 0.59 % or an absolute decrease of 5.9 % over the 10-year period. Similarly, the proportion of individuals with a clinically significant sedative load decreased significantly, with a coefficient of –0.17 % per year (95 % CI: –0.21 to –0.12; R2 = 0.87; p < 0.001). This finding translates to an absolute decrease of 1.7 % or a relative decline of 11.7 % over 10 years. These findings were consistent with the chain and fixed-base indices presented in Fig. 1. The fixed-base index comparing the sedative load from 2019 to 2009 was 89.0, indicating a decrease of 89 % relative to the initial year. Similarly, the fixed-base index for high sedative load ≥3 in 2019 compared to 2009 was 89.3, demonstrating a similar level of reduction. Among older adults who were prescribed at least one medication with sedative activity, the average yearly burden of sedative load per patient remained relatively stable over the years, with a yearly burden of 8.11 in 2009 and 8.14 in 2019.
Few studies have examined the trends in overall sedative load, but several studies have focused on specific groups of sedative medications (26–31, 33). These studies generally reported increasing trends until around 2010, followed by decreasing trends in the subsequent decade. Our findings align with these results, confirming a decreasing trend in the use of sedative medications in recent years. A similar decreasing trend in overall sedative load was also observed among nursing home residents in Finland (27). In that study, the prevalence decreased from 84.6 % in 2003 to 69.1 % in 2017. Importantly, the higher absolute numbers in the Finnish study compared with our study may be attributed to differences in the study populations, as our study focused on older adult outpatients while the Finnish study was conducted in nursing homes (27). The decreasing trend in overall sedative load indicates progress in pharmacotherapy management in recent years. However, there is still room for improvement, particularly among the oldest adults and older women.
Trends in anticholinergic burden
The age-standardized proportion of older adults receiving at least one medication with anticholinergic activity decreased from 2009 to 2019 (50.4 % to 40.2 %, respectively), whereas the proportion of individuals with a clinically significant anticholinergic burden (ACB ≥ 3) remained relatively stable (10.9 % in 2009 and 10.9 % in 2019). Fig. 3 illustrates the trends by age groups and age-standardized data. The percentage of individuals prescribed anticholinergic medications increased with age, with varying trends observed across different age groups. Notably, in the age groups 85–89 years and ≥ 90 years, the proportion of individuals with clinically significant anticholinergic burden increased, unlike the other age groups. Fig. 2c,d presents the trend of prescribing medications with anticholinergic activity by sex, highlighting a higher proportion among women, as supported by the age-standardized data. Similar to the analysis of sedative load, the data on anticholinergic burden revealed areas for improvement, particularly in women and among the oldest individuals. The opportunity for optimizing pharmacotherapy is particularly evident in individuals aged over 85 years, among whom the proportion of individuals with clinically significant anticholinergic burden continues to rise.
The linear regression model demonstrated a statistically significant decrease in the overall proportion of individuals with an anticholinergic burden, with a coefficient of –1.0 % per year (95 % CI: –1.03 to –0.98; R2 = 0.99; p < 0.001). This finding indicates an average annual decrease of 1.0 % in the proportion of individuals with an anticholinergic burden in absolute terms. However, the proportion of individuals with a clinically significant anticholinergic burden remained unchanged over the years (coefficient 0.003; 95 % CI: –0.022 to 0.029; R2 = 0.009; p = 0.785). The chain and fixed-base indices corroborated these findings (Fig. 3). The fixed-base index for anticholinergic burden in 2019, relative to 2009, was 79.8, indicating a relative decrease to approximately 80 % from the initial year. Conversely, the fixed-base index for a clinically significant anticholinergic burden in 2019 was 100.3. Among older adults who received prescriptions for at least one medication with anticholinergic activity, the average annual anticholinergic burden per person displayed a consistent upward trend, increasing from 5.39 in 2009 to 6.01 in 2019.
Our findings align with other European studies (25, 32, 34), which have also observed a favorable decreasing trend in the proportion of older adults with an anticholinergic burden over the past decade. However, it is important to note the observed trend of an increasing average yearly anticholinergic burden among individuals who are prescribed at least one medication with anticholinergic activity. This increasing trend was also confirmed in a study conducted in the UK (24). Consistent with our study, the pooled average yearly anticholinergic burden reported in that study ranged between 5 and 6 from 2012 to 2015 and showed an increasing trend from 1991 to 2015. These findings emphasize the need to optimize pharmacotherapy by focusing on reducing the anticholinergic burden in individuals who are receiving multiple medications with anticholinergic properties.
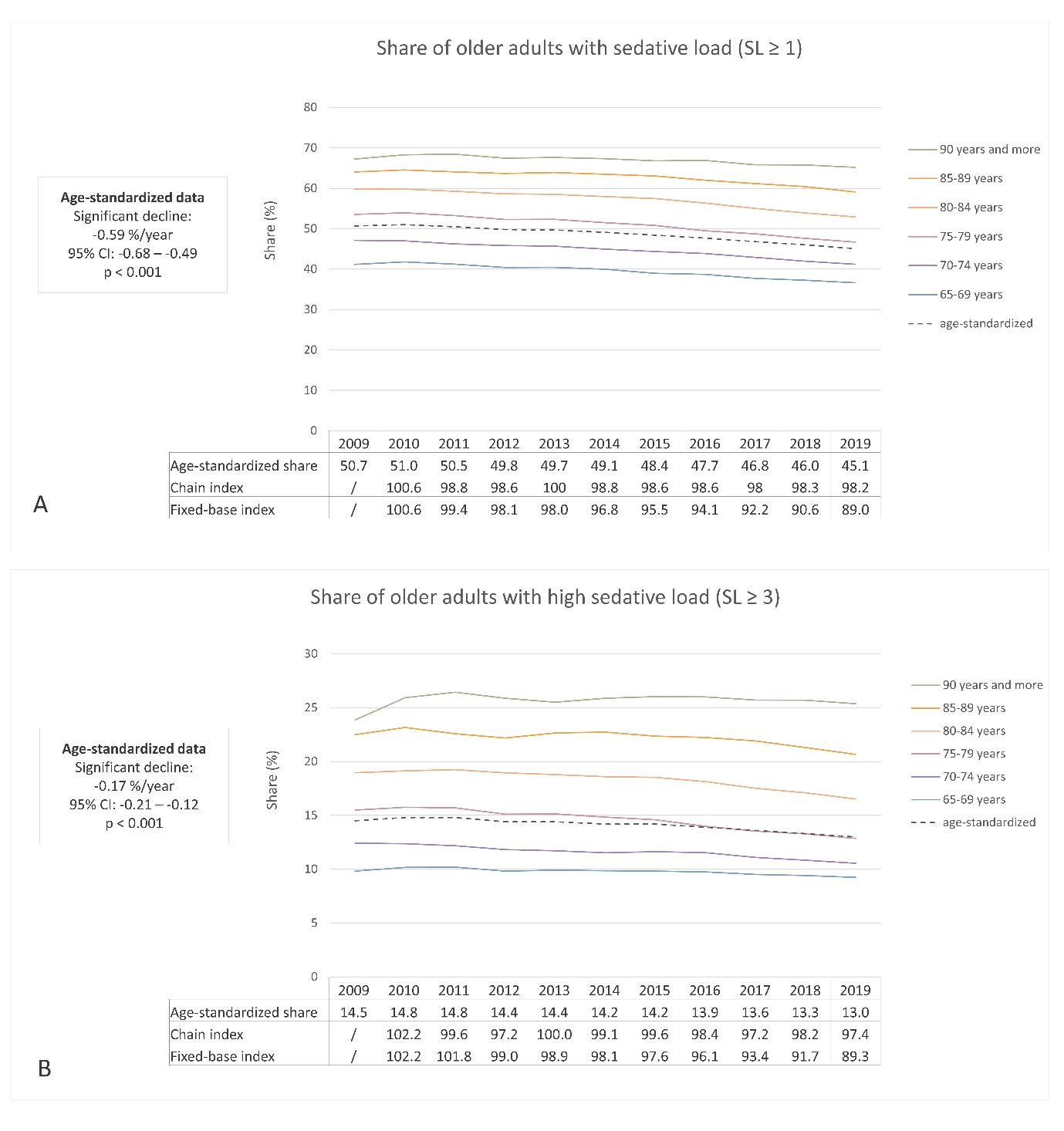
Fig. 1. Ten-year trend in sedative load (SL) among older adults in Slovenia: a) the share of older adults with at least one medication with sedative activity (SL ≥ 1); b) the share of older adults with a high sedative load (SL ≥ 3).
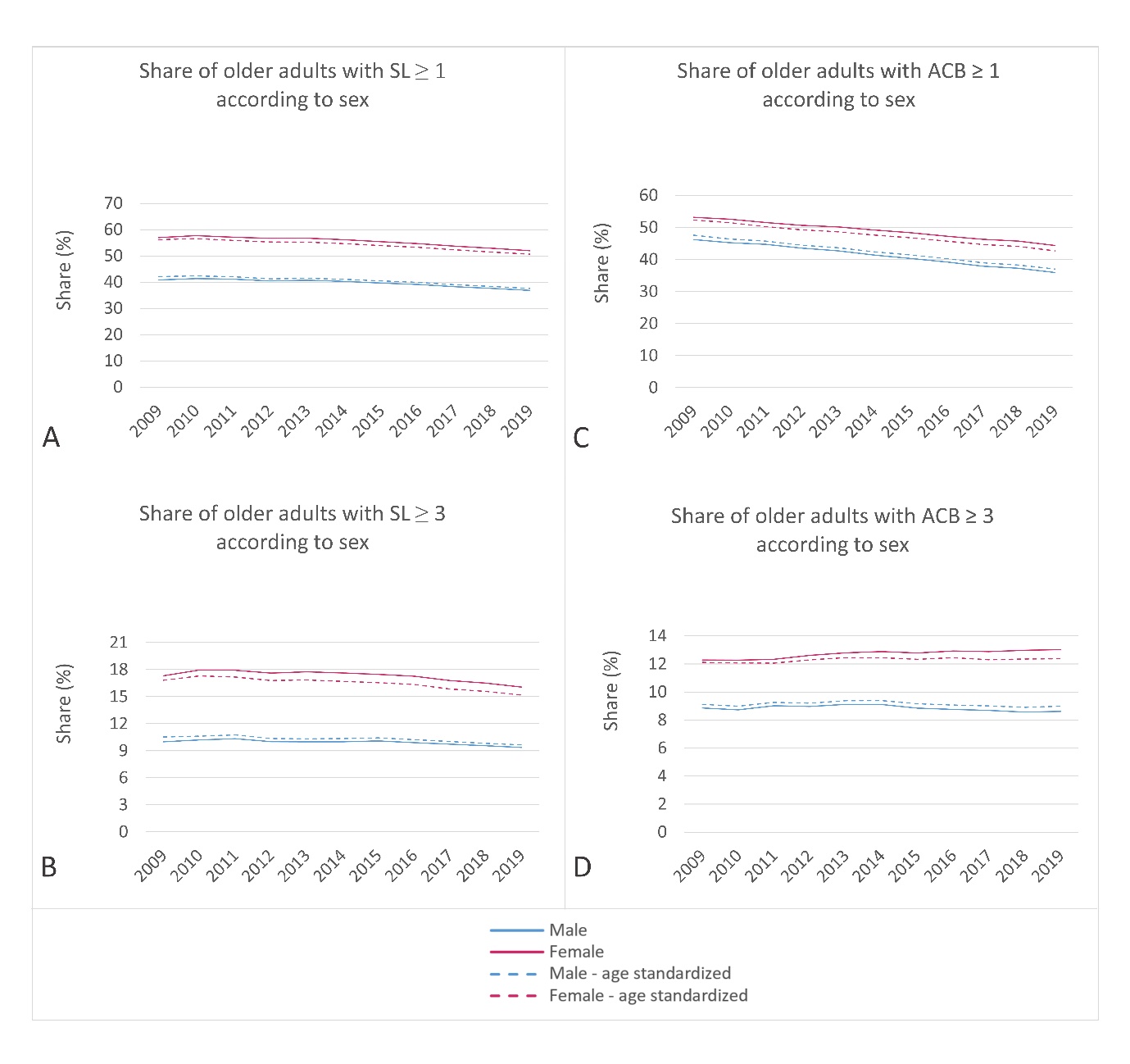
Fig. 2. Ten-year trend in: a) and b) sedative and c) and d) anticholinergic burden among older men and women in Slovenia. The upper charts present the share of older adults with at least one medication with sedative or anticholinergic activity (SL ≥ 1; ACB ≥ 1); the lower charts present the share of older adults with a high sedative load (SL ≥ 3) or a clinically significant anticholinergic burden (ACB ≥ 3).
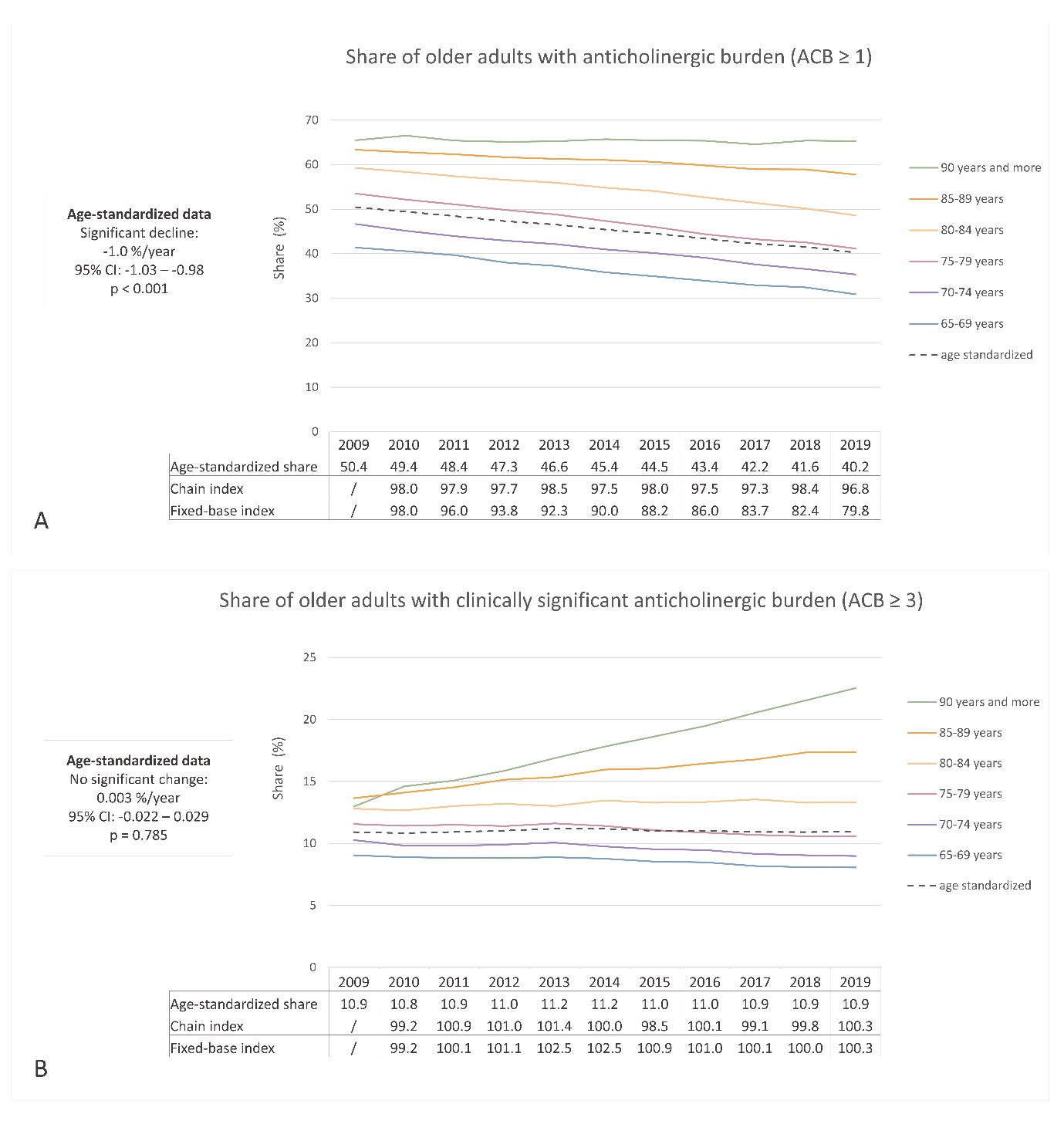
Fig. 3. Ten-year trend in anticholinergic burden (ACB) among older adults in Slovenia: a) the share of older adults with at least one medication with anticholinergic activity (ACB ≥ 1); b) the share of older adults with a clinically significant anticholinergic burden (ACB ≥ 3).
Most commonly prescribed medications
Table I presents the top five most commonly prescribed medications with sedative and anticholinergic activity. Among medications with stronger sedative effects, benzodiazepines, specifically zolpidem (27.5 %), alprazolam (23.5 %), and bromazepam (21.0 %), dominated the top three positions. Regarding medications with an ACB score of 3, the two most frequently prescribed medication groups were antipsychotics and medications for urinary diseases. The three most commonly prescribed medications in this category were quetiapine (antipsychotic, 41.2 %), trospium (for urinary problems, 21.2 %), and paroxetine (antidepressant, 10.2 %).
Other European studies have also identified antipsychotics and antidepressants as the most common medication classes with sedative or anticholinergic burden (25, 32). While some other countries report the widespread use of opioids (26, 27), opioids are not especially prevalent among the outpatient population of older adults in Slovenia.
Finally, although we observed decreasing trends in the prescription of sedative and anticholinergic medications that are currently included on most validated scales, it is essential to be aware of the potential introduction of new medications with anticholinergic or sedative effects in the future. Emerging medications need to be closely monitored in the future to ensure that their impact on the sedative and anticholinergic burden is properly assessed.
Table I. Top five most commonly prescribed medications with sedative or anticholinergic activity
a Percentage calculated relative to all persons with medications from that specific group.
Strengths and limitations
When interpreting the findings of this study, it is important to consider both its strengths and limitations. One of its key strengths is the inclusion of a large study population consisting of all older adults from Slovenia, making it one of the largest studies examining trends in sedative and anticholinergic exposure. Additionally, this study focuses on community-dwelling older adults, which distinguishes it from similar studies that have been conducted in nursing homes or similar settings. To mitigate the risk of overestimation, we further computed the prevalence of high sedative load (SL score ≥ 3) and clinically significant anticholinergic burden (ACB score ≥ 3). However, one of the major challenges in this study pertains to the definitions of anticholinergic and sedative medications themselves. There is currently no consensus on which scales best capture the characteristics of anticholinergic or sedative medications. In this study, we utilized the ACB scale, which is widely recognized and validated and has also been confirmed in a systematic review (40). For measuring sedative burden, we employed the SL model, which has also been validated, with higher scores being associated with adverse outcomes. Given that alternative tools exist for measuring sedative or anticholinergic burden, direct comparison of absolute numbers with other studies is limited. Nevertheless, the comparison of trends remains valuable in understanding changes over time. To ensure the reliability and comparability of information on this topic, periodic updates and consensus among researchers and experts are necessary. Meeting this need will aid in the development of more reliable and standardized measures for assessing anticholinergic and sedative burden, benefiting both pharmacoepidemiological studies and clinical practice.
CONCLUSIONS
The declining trend in the use of sedative and anticholinergic medications among older adults is encouraging and potentially reflects heightened awareness of adverse events linked to the sedative and anticholinergic effects of medications. The increasing trend of a clinically significant anticholinergic burden observed in the population above 85 years should be addressed in future interventions. This research has the potential to inform healthcare professionals, policymakers, and other stakeholders in implementing strategies to promote the safe and effective use of medications, reduce unnecessary medication exposure, and minimize the risk of adverse health outcomes among older adults.
Funding. – This work was financially supported by the Slovenian Research and Innovation Agency, Grant No. P1-0189.
Conflicts of interest. – The authors have no conflicts of interest.
Authors contributions. – Conceptualization, J.J., I.L. and M.K.; analysis J.J., I.L., M.K.; writing, original draft preparation, J.J.; writing, review and editing, I.L., M.K. All authors have read and agreed to the published version of the manuscript.
