INTRODUCTION
The degree of mycotrophy of the main forest-forming species on Earth is one of the most important indicators of their growth and development. Over 8000 species of higher plants and 7000-10000 species of mushrooms form ectomycorrhizal relationships (Rossi et al. 2013). The most important symbiotic relationship between plants and fungi is mycorrhiza. Namely,Brundrett (2002) stated that about 80% of higher plants have mycorrhizal formations on the roots. In addition, around 83% of dicotyledons, 79% of monocotyledons and all gymnosperms have mycorrhizal life. Non-mycorrhizal plants appear on very dry or highly salty submerged areas, and habitats where soil fertility is either quite high or too low. However, mycorrhizal life has not been recorded among the members of the Cruciferae and Chenopodiaceae families,even under all environmental conditions (Harley 1975,Brundrett 1991,Marschner 1995). According to recent studies, mycorrhization has a close functional connection with the formation of the structure, diversity, and stability of plant communities (Püschel et al. 2007,Lambers et al. 2008, Veselkin2012a,2012b).
This symbiotic partnership allows participating in the circulation of nutrients, optimizing plant metabolism, activating mineral nutrition, and inducing resistance to drought, salinization, heavy metals, and pathogens (Rossi et al. 2013). There is consensus that these plant-fungal associations have profound impacts on nutrient cycling and vegetation dynamics in ecosystems, particularly in temperate forests (Taylor et al. 2016,Bennett et al. 2017,Jo et al. 2018).
The conservation of favorable living conditions in various regions of the world directly depends on the rational and careful use of forest resources. Forest ecosystems of central and northeastern Kazakhstan are one of the most important components of the Earth’s biosphere. In forest biogeocenoses, the leading role belongs to ectomycorrhizal relationships (Agerer 2008,Smith and Read 2008).
It is known that for the normal development of any type of tree, specialized strains of macromycetes fungi forming ectomycorrhizal (EcM) and rhizospheric associative microorganisms are necessary (Shubin 1990,Fitter and Garbaye 1994,Brundrett 2002,Shubin 2002,Roman et al. 2005,Cairncy 2005,Wu and Xia 2006,Polenov 2013). In the case of mutualistic relations, EcM mushrooms receive from 10% to 50% of organic carbon from plants and become competitive in the soil, and thus plants have the opportunity to use the underground communication network from the mycelium of EcM mushrooms and root systems of different tree species, along which metabolites, energy sources, cofactors, vitamins, hormones, toxins and possibly genetic information are exchanged. In this case, the integration of populations and even species, communities of plants in a single giant underground communication network of the mycelium of mycorrhizal fungi occurs.
The study of ecology and physiology of EcM has been mainly concentrated in Europe, North America and Australia (Read 1999,Finlay 2005,Smith and Read 2008,Polenov 2013). The studies on the mycobiota and ectomycorrhizal macromycetes within the Republic of Kazakhstan have been carried out byNam (1998),Abiev et al. (2000),Abiev (2015) andVeselkin et al. (2015). In addition, some pioneer studies regarding the identification and application of mycorrhizal macromycetes, particularly in Zailiysky Alatau region, were conducted byMeshkov et al. (2009a) andMeshkov (2010).
Due to the lack of the data on the mycotrophy, flora of mycorrhiza, and plant-fungi partnership in Kazakhstan forests, the aim of this research was to identify the ectomycorrhizas of the main forest-forming species inthe Irtysh River region of central and northeastern Kazakhstan.
MATERIALS AND METHODS
Study Area
This study was carried out on the right bank of the Irtysh River (latitude: N 51˚ 57' 56 92'', and longitude E 78˚ 50' 2 72'') of central and northeastern Kazakhstan in 2018 and 2019. Study sites include mixed forests of Scots pine and birch (Figure 1), with the presence of grassy vegetation in the protected and buffer zones (Anonymous 2009). The Irtysh River is an international river flowing through the territories of China, Kazakhstan, and Russia. Being 4,248 km long, the Irtysh River originates from the Altai Mountains in SinKiang, China, crosses the Chinese border flowing west through Zaysan Lake and northwest across eastern Kazakhstan (Huang et al. 2013). The Kazakhstan Altai covers the eastern part of the Altai range including the right sub-basins of the Irtysh River. This mountainous area is covered by forests consisting of spruce, larch, pine, birch, and aspen, while Pinus sibirica Du Tour occupies the top part of the mountain slopes (Meshkov et. al. 2009b,Sarsekova et al. 2016).
Deciduous trees are located on the edge of the meadow with a predominance of small-leaved Ulmus sp. with a shut-in crown forest density of 0.7. The species composition of plants is represented by 38 species of angiosperms and 1 species of gymnosperms belonging to 19 families. The largest number of species belongs to the family Rosaceae (4 species) and Compositae (9 species). Most of the examined species (21 species) are found in single specimens and 6 species are found very abundantly forming a dense grass cover. Both in open areas and under the canopy of trees, there is a litter layer whose thickness varies from 1-3 mm up to 7 mm under the trees. The moss, lichen and low shrub layer are absent within the study area (Anonymous 2009).
The climate is sharp continental and dry. This is due to the influence of dry and hot winds blowing from the Central Asian deserts in the summer. In winter, the territory of the pine forest is open to cold air flows coming from the North. The climate is characterized by cold and long winters (5.5 months), and short and hot summers with sharp temperature fluctuations in winter and summer (Anonymous 2009). The average annual air temperatures of the coldest month (January) and the warmest month (July) are 2.5°C and 30°C, respectively. The average duration of the warm period is 175 days, while frost-free days’ amount to 117 days. The precipitation average is 240–310 mm per year, of which 60–75% of the annual amount falls in May – September. In spring winds from the north and northeast, and in summer, south and southwest winds quickly dry out the soil. Relative humidity in the summer (at 1 p.m.) is about 40%, dropping down to 10% on some days, causing intense transpiration of plants and a large loss of moisture in the soil (Anonymous 2015).
In this region, the pine forest soil is zonal. On low surfaces among tape hogs’ meadow deep effervescent chernozem soils are developed, sometimes in a complex with solonets. In general, the soil profile is characterized by a slightly acidic to neutral reaction (pH of aqueous extract 6.5-7.0). Soils are washed from water-soluble salts. The mechanical composition is usually light, although there are layers of heavy and medium loam (Anonymous 2009).
Sampling and Identification of Macromycetes
The materials of macromycetes were collected in the central and northeastern part of Kazakhstan. Two methods of sampling were applied, including route and stationary research method. The routes covered various forest biocenoses — in pure or mixed forests of P. sylvestris L. with B. pendula Roth. Seven permanent multi-year transects with a total area of 1080 m2 were marked where the sampling of macromycetes was performed every 10-15 days during the seasons of 2018 and 2019. The collection was formed from the sampled material, and primary identification of voucher samples of fruiting bodies of macromycetes was performed. All the collected macromycetes were sampled as ectomycorrhizal fungi under P. sylvestris L., P. obovata Ledeb. and B. pendula Roth trees.
During the identification of fruiting bodies, standard methods were applied, using the available identifiers such as the resource www.indexfungorum, as well as the web-sites on "Mushrooms of the Kaluga Region" and "Mushrooms of the Novosibirsk Region". Micromorphological studies of characters were performed under the light microscope (Altami SMO745-T, St. Petersburg, Russia), at 400×–1000× magnification. To identify the natural color of the microstructures, the preparations were viewed in distilled water and in a 3-5% KOH solution. Hyaline structures were stained with a 5% aqueous solution of safranin, while the presence or absence of amyloid and dextrinoid structures was determined using Meltzer’s reagent. To determine the type of fungal symbiont in the ectomycorrhiza, the morphotyping method was used according toAgerer (2008). For these purposes, soil blocks (10×10×20 cm in size) were taken within the projection of the crown of mature trees, according to the method of concentric sampling (Smith and Smith 2011). Before sampling, the upper undecomposed layer of the litter was removed. Coniferous seedlings were extracted from the soil with the most intact root system. Moreover, DNA was isolated from the specimens that could not be identified using the conventional identification tools.
The samples were wrapped in the aluminum foil and stored at a temperature of 4°C. The roots were washed under the running water, cut into 3 to 5 cm segments, and ectomycorrhizal endings were separated under a magnifying glass with tweezers and scissors. Morphotyping of ectomycorrhiza was performed using a stereomicroscope in the binocular and trinocular version (Altami SMO745-T), equipped with an Altami UCMOSO3100KPA video camera. Based on the nature of branching, the color of the ectomycorrhizal ends, features of the mantle surface, the presence or absence of external mycelium and rhizomorph, and morphotyping were performed according to the DEEMY system (Agerer 2008). The data were entered into a specially developed checklist. The selected ectomycorrhizas were photographed and fixed in 70% ethanol for DNA isolation (Gardes and Bruns 1993). In determining the species affiliation of voucher samples of fruiting bodies, consultations were made by personal communication with leading mycologists from Russia, Sweden, and Norway during the XV International Meeting on Macromycetes held in August 2018 in Tomsk.
In Vitro Culture and Cultivation
For the mycelial development, Khudyakov-Voznyakovskaya, Hagem, and Wort-agar media optimized for the C/N ratio and thiamine was used (Kõljalg et al. 2013). Aseptic conditions were maintained during the superficial and deep cultivation of mycelium (Bukhalo 1988). At the same time, optimal cultivation modes have been optimized and used. In total, 25 strains were developed into in vitro cultures.
RESULTS
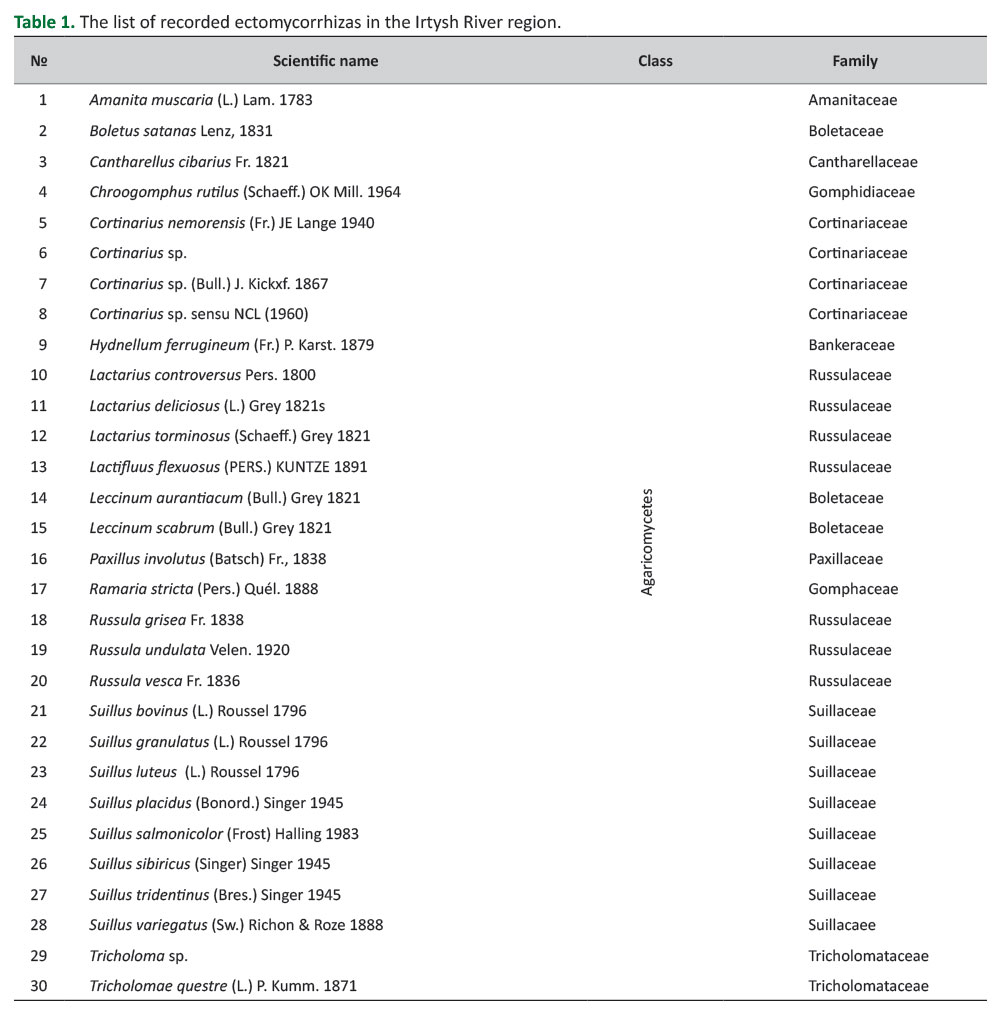
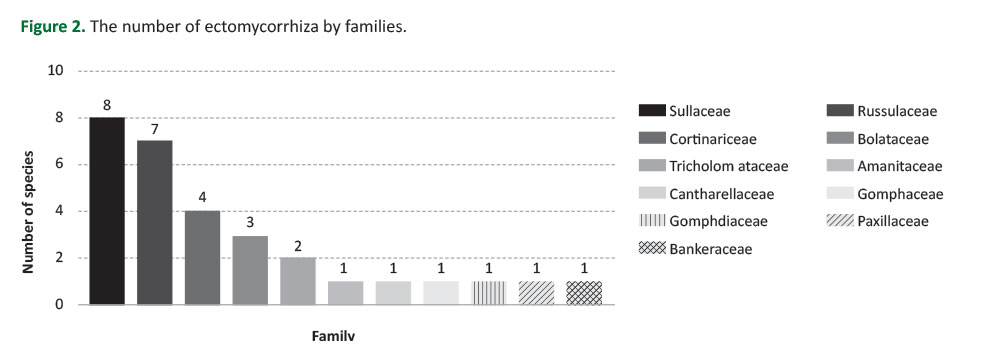
A total of 30 ectomycorrhizas belonging to Agaricomycetes were identified (Table 1). The distribution of 30 species into families was as follows; Suillaceae (8), Russulaceae (7), Cortinariaceae (4), Boletaceae (3), Tricholomataceae (2), Amanitaceae (1), Cantharellaceae (1), Gomphaceae (1), Gomphidiaceae (1), Paxillaceae (1), and Bankeraceae (1). The genus with the highest number of recorded species was Suillus (8) (Figure 2). The photos of some fungi identified in the research are shown inFigure 3.

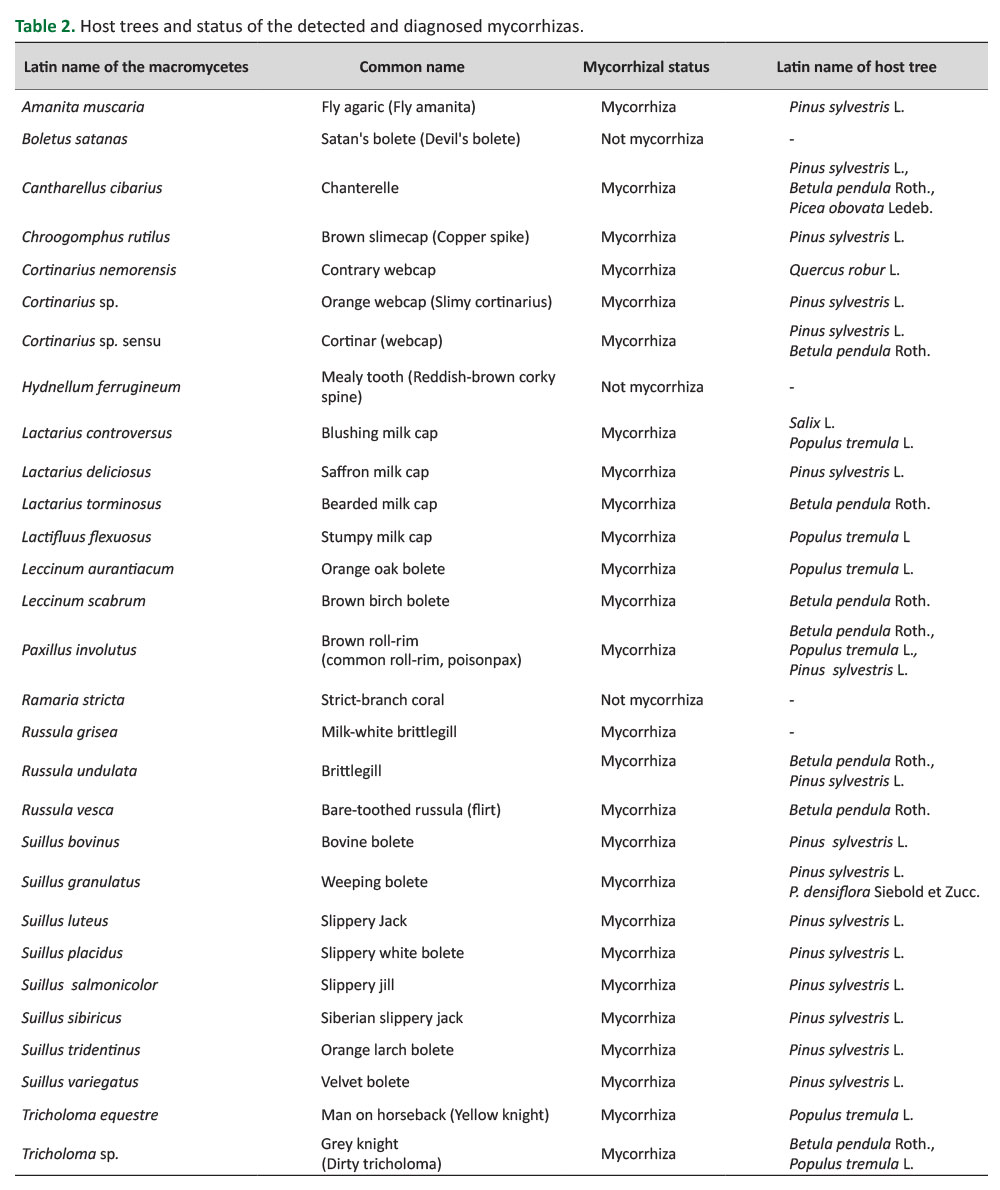
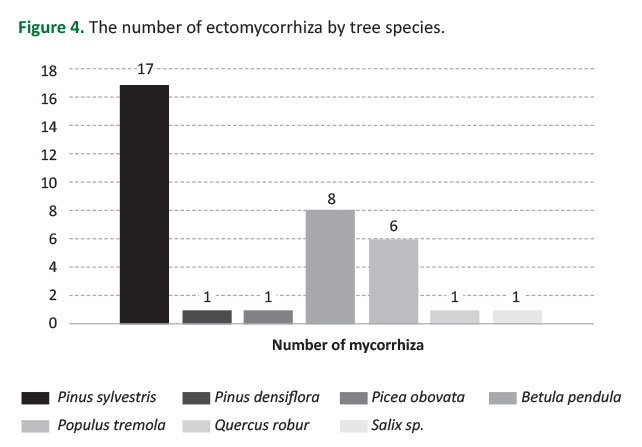
In the study area, three of the sampled tree species were coniferous and four were broadleaved. In total, 63.3% of all recorded mycorrhizae have established a symbiotic life with a coniferous host species (Table 2). Following the sampling and identification, 17 ectomycorrhizas were recorded under P. sylvestris, 8 under B. pendula, 6 under P. tremula, 1 under P. obovata, 1 under Q. robur, 1 under Salix sp., and 1 under P. densiflora. It should be noted that some species such as Paxillus involutus, Russula undulata, Cortinarius sp. and Cantharellus cibarius formed a symbiotic relationship with both coniferous and broad-leaved tree species (Table 2,Figure 4).
DISCUSSION
A rich diversity of 30 different mycorrhiza species was recorded in this study. Of these 30 mycorrhizae, 17 were determined under P. sylvestris, 8 under B. pendula, 6 under P. tremula, 1 under P. obovata, 1 under Q. robur, 1 under Salix sp., and 1 under P. densiflora. As determined within the scope of research, all Suillus species, Amanita muscaria and Chroogomphus rutilus associated a partnership with conifers, while the species of Leccinum, Tricholomaand Lactifluus flexuosus formed a partnership with hardwoods. In addition, Cantharellus cibarius, Paxillus involutus, the species of Cortinarius and Lactarius presented a partnership with both conifers and hardwoods on a broad spectrum.
Some macromycetes are selective in forming a partnership, while others may form a partnership in a very large spectrum. For example, of the genus Laccaria, Suillus form ectomycorrhiza on coniferous seedlings (early-stage fungi), fungi from the genera Russula, Boletus mycorrhizal roots of older conifers (late-stage fungi). For example, Amanita muscaria and Boletus edulis can form mycorrhiza with trees that are systematically distant from each other, such as conifers and hardwoods. There is a definite connection between the systematic groups of fungi and plants. Thus, fungi of the genus Gomphidius form mycorrhiza mainly with coniferous trees, while in Suillus sibiricus, mycoses with Pinus sibirica are more fixed than with Pinus sylvestris.Leccinum chromapes is confined to black birch (Betula dahurica Pall.) and does not form mycorrhiza with white birch (Betula pubescens Ehrh.) On the contrary, representatives of the genera Cortinarius, Inocybe, as well as Laccaria laccata, Paxiluus involutus, Amanita vaginata, and Hebeloma crustuliniforme possess a wide valence in relation to tree species (Singer 1938).
Valuable coniferous forests of the Irtysh River regions and Kazakhstan Altai have been exhausted by timber cutting and fires. The continuous felling of forests in the river basins of Bukhtarma and Uba in the East Kazakhstan region is responsible for the considerable loss of water in the Irtysh River. Because of the increasing demand for energy firewood, harvesting of these important forests has considerably increased (Meshkov et al. 2009b). Most ectomycorrhizal fungi cannot remain viable for a long time unless they find a host plant and establish mutually beneficial contact (Shemakhanova 1962,Shubin 1990,Timonen and Marschner 2005). The biodiversity of the fungi forming ectomycorrhiza with woody plants decreases rapidly due to clear-cutting operations and fires. The restoration of the natural diversity of this important component of forest biogeocenosis requires a long period of time if human-made and natural disturbances are severe in these forest areas. A number of researchers have shown that direct and indirect anthropogenic effects can very negatively affect the development of ectomycorrhizal mushrooms (Massicotte et al. 1998). The findings of this study are very important in this respect, in the sense of easier restoration of afforested areas.
Meshkov et al. (2009b) have emphasized that the priority should be given to forest rehabilitation on burned areas and lands where the forest was previously removed, including in the ribbon-like relict pine forests of the Irtysh region of the Kazakh Uplands (Akmola and Karaganda Provinces), plain forests of Kostanay Province. In addition,Meshkov et al (2009b) andSarsekova et al. (2016) recommended that in many parts of Kazakhstan, in the degraded forest areas, mycorrhizas should be used as a major improvement tool.
Applied aspects of the application of mycorrhization were investigated by Meshkov who was the first in Kazakhstan not only to define four species of macromycetes into the culture, but also to develop a technology for their scaling and application in the form of mycorrhized compost for reforestation in Zailiysky Alatau (Meshkov et al. 2009a,Meshkov 2010). Such practical projects should be increased and implemented as soon as possible. In this study, due to higher atmospheric and soil drought during the autumn-summer period in 2018 and 2019, no mass fruits of fungi were formed at the end of September. Following the rains in late September, the fruit-bearing macromycetes’ organs started to appear. Moreover, most of the recorded mycorrhizae (63.3%) were found under the coniferous host species (Table 2). However, this could be associated with the dominance of coniferous tree species in the studied region, and the most sampled hosts in this study belonged to conifers.Vaishlya et al. (2017) emphasized that coniferous trees are able to form symbiotic relationship with 200-300 species of ectomycorrhizal fungi. There are nearly 50 ectomycorrhizal fungi species which are capable of forming ectomycorrhizas with Pinus sibirica in Tomsk region of west Siberia.
Species of Suillus are found all over the northern hemisphere where members of the Pinaceae tree family can be found. Although a few species are distributed in mainly mountainous regions of tropical regions, most are limited to temperate areas (Singer 1986). The Russulaceae have a worldwide distribution, but there are differences among the distribution of genera. Russula is the most widespread, found in North, Central and South America (Buyck and Ovrebo 2002), Europe, temperate (Gorbunova 2014) and tropical Asia, Africa (Natarajan et al. 2005), and Australasia (McNabb 1973). It is the only Russulaceae genus that occurs in the Nothofagus zone of temperate South America (Singer 1953). Lactarius is mainly known from the north temperate zone, but some species also occur in tropical Asia and Africa. Lactifluus has a more tropical distribution than Lactarius, with most species known from tropical Africa, Asia, South America, and Australasia, but some also occurring in the north temperate zone (Verbeken and Nuytinck 2013).
In addition,Shi et al. (2016) stated that there are still critical gaps remaining in our understanding of biogeographic patterns of mycorrhizal associations, and our limited knowledge of the anthropogenic factors responsible for shifting plant-mycorrhizal distributions has hindered the efforts to predict the ecosystem feedbacks to climate change.
CONCLUSIONS
The species of ectomycorrhizae identified in the study and the knowledge about which tree species they form partnership with have great critical importance, especially for the propagation of ectomycorrhizal seedlings to be grown in the central and northeastern Kazakhstan regions where environmental conditions and anthropogenic effects are severe. As a result, ectomycorrhizas must be used as a major performance-enhancing tool in afforestation and restoration studies in the Irtysh River basin, under extreme ecological conditions and climate change effects.