Introduction
The most traded dairy products on the global market are butter, cheese and milk powder. The production of milk powder (MP) still remains relevant and, despite some disruptions in its export and import associated with COVID-19, it has a positive outlook for recovery and development by the end of 2021 (FAO, 2020; Zakharova, 2020; Baldwin et al., 2020).
Over the past 100 years, the industrial method of preserving raw milk is drying, i.e. moisture removal from the product (Baldwin et al., 2020). To obtain MP products, various drying methods are used. Spray drying is based on almost instantaneous evaporation of moisture from atomized finely dispersed droplets of pre-condensed milk when they come into contact with circulating hot dry air. During freeze drying (also known as lyophilization) moisture is removed from previously frozen milk under reduced pressure (vacuum) and gentle temperature mode (Ratti, 2021; Refstrup et al., 2011; Deshwal et al., 2020). The most common method for MP production is continuous spray drying (Baldwin et al., 2020). Freeze drying allows to obtain high quality products with long shelf life while maintaining structural integrity and minimal degradation of milk constituents (Fyfe et al., 2011; Castro-Albarrán et al., 2016; Yao et al., 2016; Turovskaya et al., 2018; Deshwal et al., 2020).
MP is in great demand in various sectors of food industry. It is used for the production of a wide range products, the condition of which directly depends on the quality of MP and its technological characteristics (Baldwin et al., 2020; Galstyan et al., 2019). The formation of such key technological properties of MP as heat stability and cheese making properties has a complex multifactorial nature (Simone et al., 2019; Tyulkin et al., 2018; Kruchinin et al., 2020), due to genetic and paratypical methods of obtaining raw milk of proper quality and desired composition, including protein, and production methods of influencing on it (Kruchinin et al., 2020; Magan et al., 2019; Sharma et al., 2012).
The protein part of cow raw milk consists of casein (about 80 %) and serum (about 20 %) fractions, which are encoded by six main protein genes: αS1-casein ( CSN1S1), β-casein ( CSN2), αS2-casein ( CSN1S2), κ-casein ( CSN3), α-lactoalbumin ( LALBA), β-lactoglobulin ( LGB) (Gurses et al., 2016; Mahmoudi et al., 2020; Maletić et al., 2016). In recent decades the influence of CSN3 gene on cattle milk yield, milk composition and technological properties have been widely studied. So far 14 polymorphic variants of this gene have been identified: A, A1, B, B2, C, D, E, F1, F2, G1, G2, H, I, J. The most significant and prevailing in terms of occurrence are three of its polymorphic genotypes AA, BB and AB, formed by a combination of alleles A and B, differing in amino acids in positions 136 and 148. In allele A, threonine and asparagine are located in these positions, in allele B-isoleucine and alanine respectively (Mahmoudi et al., 2020; Lukač et al., 2015; Awad et al., 2016; Djedovic et al., 2015). Numerous studies have revealed a significant prevalence of the frequency of occurrence of allelic variant A over variant B, while a tendency towards a decrease in daily milk yield and an increase in the mass fraction of protein in the milk obtained from allelic variant A to variant B have been noticed. Heterozygous genotype AB occupies an intermediate position between homozygous genotypes AA and BB (Comin et al., 2008; Zambrano-Burbano et al., 2010; Neamt et al., 2017). In the cow milk with allelic variant A of CSN3 gene a reduced content of total protein is observed, whereas during coagulation the duration of the process increases with the formation of weaker gels. For milk from cows with a predominance of allelic variant B for CSN3 gene, better coagulation properties were confirmed, consisting in a shorter duration of protein coagulation with the formation of a denser casein clot, as well as in obtaining a larger amount of cottage cheese or cheese with a higher protein content, which has a positive effect on the compositional quality of finished goods (Tyulkin et al., 2018; Mahmoudi et al., 2020; Lukač et al., 2015; Djedovic et al., 2015; Frederiksen et al., 2011).
At the same time, some researchers do not clearly interpret the relationship of genotypes with the technological properties of milk. For example, (Mahmoudi et al., 2020; Comin et al., 2008; Zambrano-Burbano et al., 2010) the authors also presented the results of studies based on meta-analyses that did not reveal clear and identical dependencies between CSN3 gene polymorphism and technological characteristics. In their opinion, this is due to the small number of samples, differentiation of animal breeds, insufficient volume of production output, difficulty in results comparison due to significant differences caused by the influence of a complex of genotypes and inconsistency of experimental data, etc.
Nevertheless, for the rational processing of dairy raw materials the vast majority of scientists consider appropriate to use the milk of cows with the dominance of A allele for k-casein gene for manufacturing of products undergoing high-temperature processing, and to send for cheese making the milk of cows with the prevalence of B allele due to high mass fraction of dry substances (due to the high fat and protein content in it) (Mahmoudi et al., 2020; Gilmanov et al., 2020).
In this regard, additional scientific studies of the effect of polymorphic variants of the k-casein gene on such technological properties of MP as heat stability and cheese making properties are needed.
The objective of the work was to study the relationship between the genotypes of lactating cows for CSN3 and the ratio of its allelic variants with the technological properties of milk obtained using spray and freeze drying.
Materials and methods
Milk samples
Milk was obtained from cows of Holstein breed kept on the Mukhametshin farms (Republic of Tatarstan, Russia) and Russian State Agrarian University - Moscow Timiryazev Agricultural Academy (Moscow, Russia). Using DNA analysis of the biomaterial, 68 animals were genotyped for the κ-casein gene, of which 33 cows were identified with AA genotype, 21 - AB, 14 - BB. The occurance of allele A for the k-casein gene was 64 %, and allele B - 36 %. The design of the experiment is shown in Figure 1.
The milk obtained from genotyped cows was cleaned 1-2 hours after milking, cooled to 4±2 °C and sent to the laboratory, where it was heated to 40±5 °C, separated and skim milk samples (AA 0, BB 0, AB 0) were obtained.
In order to maximize the preservation of the native state of the skimmed milk components, it was spray (AA 1, AB 1, BB 1) and freeze drying (AA 2, AB 2, BB 2) without heat treatment and condensation. The exclusion of these stages from the process of obtaining skimmed MP was based on the data (Lin et al., 2018; Schuck et al., 2013; Dumpler et al., 2020; Sobti et al., 2019), which reported that the main changes in the structure and functional properties of milk, both positive and negative, occur during heat treatment and condensation.
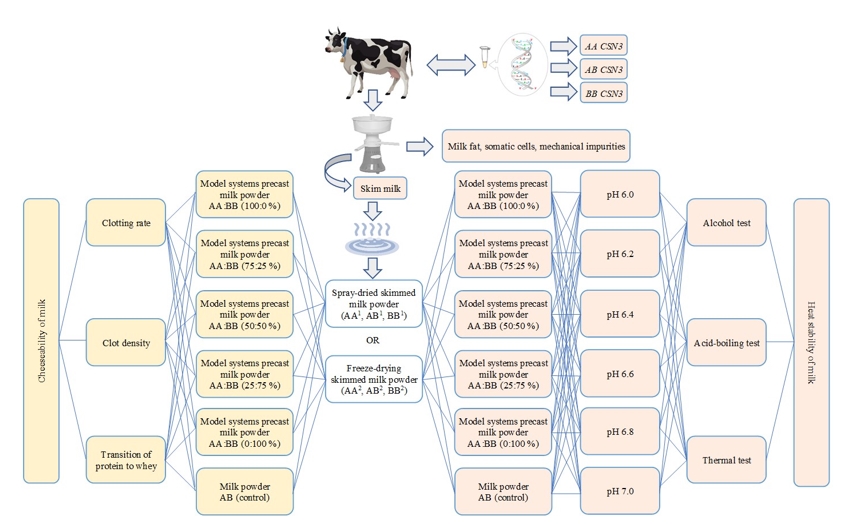
Figure 1. The design of the experiment
Spray drying was carried out on a laboratory setup (BIORUS-8000, Russia) using a two-flow spray nozzle and a unidirectional air and milk flow under the following modes:
temperature of milk fed for drying - 40±1 °С
temperature of air entering the drying zone - 150±1 °С
outlet air temperature - 70±1 °С
air flow rate - 70±2 m 3/h.
Freeze drying was carried out using a TG-50 batch unit of periodic action (Hochvakuum, Germany) under the following modes:
milk freezing temperature - minus 23±2 °С
residual pressure in the chamber - from 10.0 to 13.3 Pa
heating plate temperature - 33±2 °С
the duration of the process - 24±2 hours.
The mass fraction of moisture in the spray and freeze dried skimmed milk was 3.0±0.5 %.
Samples of skimmed MP were stored in a refrigerator at a temperature of 4±2 °C in a hermetically sealed container until used in further studies.
On the basis of dried samples, model dry systems were prepared, ranked according to the ratio of homozygous AA and BB genotypes (100:0 %; 75:25 %; 50:50 %; 25:75 %; 0:100 %, respectively), which fundamentally imitate the combined MP.
Before the study 9 g of a model sample of skimmed MP was dissolved in distilled boiled water with a pH of 6.5±0.1 at 40±2 °C, bringing the total volume to 100 ml. Milk reconstitution was carried out using a laboratory magnetic stirrer at a speed of 500 rpm. The reconstituted milk was left for 15 minutes to remove air trapped during stirring and stabilize the milk system. Then the milk was cooled to 20±2 °C.
Before determining the heat stability, a special preparation of the reconstituted samples was carried out, contributing to a more complete understanding of the mechanism of protein destabilization and making it possible to minimize the effect of the values of active or titratable acidity on its stability when exposed to various methods used in the assessment of heat stability. For this, the pH was determined in the reconstituted samples and a model series of samples was made for each sample with pH values in the range from 6.0 to 7.0 (step 0.2) by potentiometric titration with 1 N HCl or 1 N NaOH.
Methods
Standardized and generally accepted methods of physical and chemical control of dairy products were used for research.
The total dry matter content was determined by the thermogravimetric reference method described in ISO 6731:2010[IDF 21:2010].
The fat content was determined using the Gerber acid method according to ISO 2446:2008[IDF 226:2008], ISO 11870:2009[IDF 152:2009].
The total protein content was determined according to ISO 1871:2009, ISO 8968-1:2014[IDF 20-1: 2014] using a protein analyser Kjeltec-2400 Auto Analyzer (Foss Electric, Denmark) by measuring the total nitrogen by the Kjeldahl method with its subsequent recalculation per protein using a conversion factor of 6.38.
The content of casein and serum proteins was determined using the reference method according to ISO 17997-1:2004[IDF 29-1:2004] by acid precipitation of casein and measuring the total nitrogen in the filtrate.
The lactose content was determined by an enzymatic method using the pH difference according to ISO 26462:2010[IDF 214:2010].
The freezing point was determined by a reference method using a Cryoscope (Advanced Instruments, USA) according to ISO 5764:2009[IDF 108: 2009].
The titratable acidity was determined by titration with 0.1 N NaOH solution in the presence of 1 % alcoholic solution of phenolphthalein indicator and expressed in Terner degrees (°T).
The pH was determined by a potentiometric method using a laboratory pH meter (InoLab pH Level 1, Germany).
Ca content was determined by titrimetric method according to ISO 12081:2010[IDF 36:2010].
The content of somatic cells was determined by fluorescence microscopy using a DCC analyser (DeLaval, Sweden); the microscopic method described in ISO 13366-1:2008[IDF 148-1:2008] was used as a reference method.
Genotyping of animals by the k-casein gene was performed using PCR-RFLP analysis. The process of isolating DNA from biological material of animals was carried out in accordance with the instructions for the set "DNA-sorb-SM" (Central Research Institute of Epidemiology, Federal State Budgetary Institution of Supervision of Consumer Rights Protection and Human Welfare, Russia) (Vafin et al., 2021). Amplification was carried out on a Tertsik thermocycler (Russia) with reaction mixture volumes of 20 μL (SibEnzim, Russia) containing 2 μL of dNTP mixture (0.25 mM each), 2 μL of Taq DNA polymerase buffer (1 ×), 0.2 μL Taq DNA polymerase (1 unit), 0.4 μL of primers JK5 (5 /-ATCATTTATGGCCATTCCACCAAAG-3 /) and JK3 (5 /-GCCCATTTCGCCTTCTCTGTAACAGA-3 /), 2 μl of DNA sample in the following modes: ×1: 94 ºС - 4 min; ×35: 94 ºС - 10 s, 63 ºС - 10 s, 72 ºС - 10 s; ×1: 72 ºС - 7 min; storage: 4 ºС. PCR samples (10 μL) were treated with 5 units of restriction enzyme HinfI in SE-buffer "O" (SibEnzyme, Russia) with incubation at 37 °C for 12 hours. The incubated PCR-RFLP samples were mixed with buffer for loading samples onto an agarose gel (4× Gel Loading Dye, Blue, ZAO "Evrogen", Russia) in ratio of 3:1. Coloured amplifiers were applied to the wells of a 2 % agarose gel prepared by melting of 2 g of agarose (Biotechnology Grade, Amresco, USA) in 100 ml of Tris-acetate electrode buffer (500 mL 1 × TAE buffer, 15 μL 1 % ethidium bromide solution). Detection was carried out by horizontal electrophoresis using a SE-2 camera (Helikon, Russia) and an Elf-4 power supply unit (DNA-Technology, Russia) (Gilmanov et al., 2020).
The determination of heat stability by an alcohol sample is based on the detection of denaturation of milk proteins in the presence of C 2H 5OH. 2 cm 3 of the reconstituted sample was added to a Petri dish, 2 cm 3 of an alcohol solution of a certain concentration (68 %, 70 %, 72 %, 75 %, 80 %, 85 %, 90 %) was added and the dish was stirred in a circular motion. After 2 min. the absence or presence of protein coagulation, manifested in the form of flakes, was visually fixed.
Determination of heat stability by an acid-boiling sample is based on the use of a complex impact of heat and acid as a denaturing protein factor. 0.5 cm 3, 0.8 cm 3 and 1.2 cm 3 of 0.1 N HCl solution were added, 10 cm 3 of reconstituted milk was added and mixed in three glass heat-resistant vials. The vials were placed in a boiling water bath at a temperature of 100±1 °C, kept for 3 min and cooled to a temperature of 20±1 °C. The contents of the vials were transferred to Petri dishes and visually assessed the stability of proteins in the samples, characterizing it by the amount of added acid. According to this method, milk is considered heat-resistant if it withstands acid-boiling impact with the addition of more than 0.8 cm 3 of acid.
The determination of heat stability by a thermal sample is based on determination of the duration of the absence of coagulation of protein fraction of milk exposed to heat at a temperature of 130±1 °C. For testing a UKT-150 ultrathermostat (BioPishcheMash, developed at All-Russian Dairy Research Institute, Russia) filled with glycerine was used.
The cheese making properties of the reconstituted samples were determined using a method based on assessing the ability of milk to coagulate under the influence of rennet and microorganisms of milk not exposed to temperature (rennet-fermentation test) in accordance with the method described in (Vafin et al., 2021). The study was carried out using a milk-clotting enzyme derived from microbial chymosin CHY-MAX M 2500 IMCU (Chr. Hansen, Denmark). The enzyme weighing 0.32 g was dissolved in 100 mL of distilled water at a temperature of 30±1 °C and kept for 30 min with constant stirring on a magnetic stirrer (300 rpm). 90 cm 3 of reconstituted skim milk, heated to a temperature of 38±1 °C, was measured into a glass and 3 cm 3 of an enzyme solution was added. The sample was incubated at 38±1 °C until a dense clot was formed. A visual assessment of the quality of the resulting clot was done, fixing the duration of rennet clotting, and the class of milk quality was established:
I (good) - the curd has a smooth surface, elastic to the touch, without eyes in the longitudinal section, floats in a transparent serum that does not stretch;
II (satisfactory) - the coagulum is soft to the touch, with single eyes (from 1 to 10 pieces), torn, but not swollen;
III (unsatisfactory) - the coagulum is spongy, soft to the touch, has numerous eyes, swollen, floated up or instead of a clot a flocculent mass is formed.
After classification of milk quality, the content of the glass was filtered through a lavsan fabric. The filtrate (serum) was thoroughly mixed and the protein content was determined.
Results and discussion
The qualitative and quantitative composition of the source skimmed milk
Analysis of the parameters of skimmed milk obtained from cows with polymorphic genotypes for CSN3 showed the levelling of the factors of the mass fraction of fat and somatic cells in the process of sample preparation (Table 1).
Table 1. Physicochemical composition of skimmed milk samples
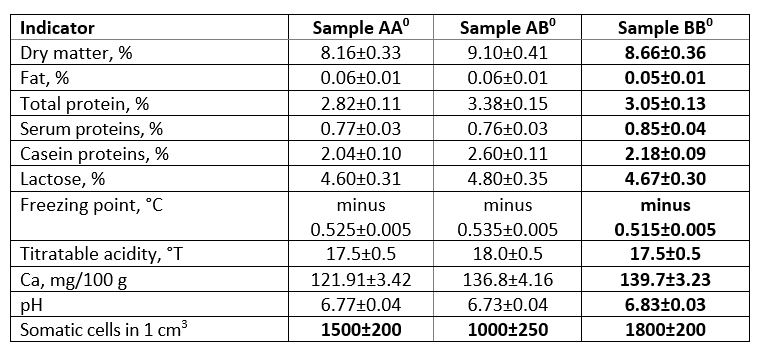
The difference in the mass fraction of protein between samples AA 0 and BB 0 was insignificant (2.82 % and 3.05 %, respectively) in contrast to sample AB 0, where the mass fraction of protein was at the level of 3.38 %. Considering the fractional composition of the protein of homozygous samples AA 0 and BB 0, a constant trend should be noted that the ratio of serum and casein fractions of proteins remains unchanged at the level of 27:73 %. The ratio of these fractions in the heterozygous AB 0 sample was 23:77 %. Sample AB 0 was characterised by an increased content of the mass fraction of lactose and dry substances in general, therefore, in further studies, this sample was considered as a reference.
k-casein genotype influence on heat stability of milk powder
One of the most important technological properties of MP is resistance to thermal effects after hydration (Kruchinin et al., 2020; Deshwal et al., 2020). Currently, scientists have not created a single agreed universal methodology for determining the milk heat stability, which is due to the lack of a thorough and deep understanding of the complex mechanism of milk system variability under temperature exposure, since it has a multifactorial nature and is associated with the protein cluster of milk, the concentration and ratio of mineral salts, acidity etc. (Kruchinin et al., 2020; Chandrapala et al., 2010; Huppertz et al., 2016; Dumpler et al., 2020). In this regard, to study the stability of casein micelles in reconstituted samples of skimmed milk by freeze and spray drying with k-casein polymorphism, an integrated approach was applied and from the whole variety of methods, the three most common ones were used, namely alcohol, acid-boiling, thermal samples (Kruchinin et al., 2020; Sikand et al., 2010).
The results of studying the heat stability of samples using an alcohol test are presented in Table 2.
Table 2. Heat stability by alcohol test of reconstituted model milk systems
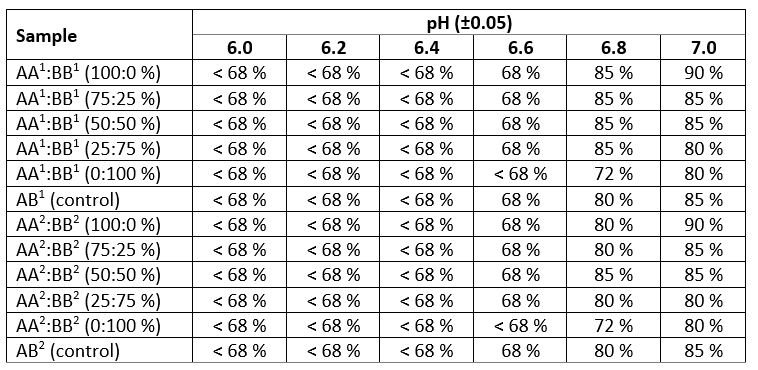
The analysis outlined in Table 2 of the research results showed that lowering the pH below 6.6 leads to a decrease in heat stability and visible denaturation of the protein when adding the lowest concentrated alcohol solution (68 %) to all milk samples without exception. A change in the pH value to the alkaline side led to an increase in the milk heat stability. At the same time, there was a tendency to an increase in the milk heat stability with an increase in the milk content of cows with a homozygous AA CSN3 genotype in model systems, reaching a maximum value at pH 7.0 (the sample withstood the addition of a 90 % alcohol solution) with 100 % prevalence. Such heat stability of the samples may be associated with the milk genotypic origin (Mahmoudi et al., 2020). The milk of cows with AA CSN3 genotype contained, although not much, a smaller amount of protein, including casein fractions (by 7-8 %), in comparison with BB CSN3 milk with practically equal values of lactose (within the method error). However, this difference was sufficient to ensure the stability of the protein fraction. Model systems with a predominance of milk of AA CSN3 genotype (more than 50 %) are less susceptible to disruption of the hydration membrane as a result of exposure to 85 % and 90 % alcohol solution at pH 6.8 and 7.0. A comparison of the results of studies of reconstituted milk samples by freeze and spray drying showed almost identical values, which probably indicates the lack of objectivity of the method for determining heat stability by an alcohol test to identify factors of negative or positive impact that depend on the method of milk drying and affect the heat stability of the protein fraction.
In the course of further studies, the heat stability of the reconstituted model milk systems was analysed by the method of an acid-boiling sample (Table 3).
Table 3. Heat stability by acid-boiling sample of reconstituted model milk systems
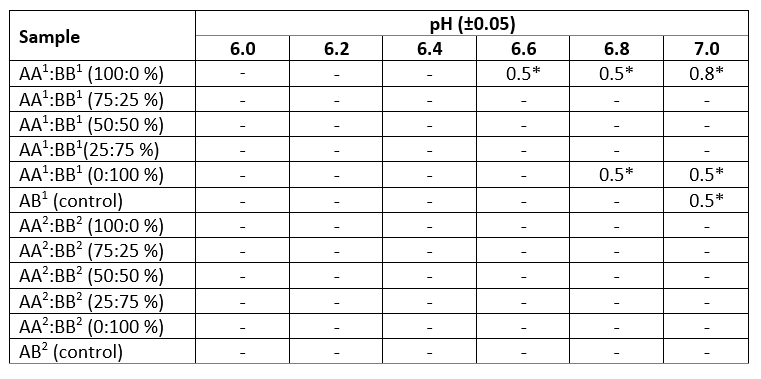
« - » - negative result, i.e. the sample failed to withstand the addition of a minimum amount of 0.1 N HCl (0.5 cm 3)
* Quantity of 0.1 N HCl, which the reconstituted milk withstood, cm 3
Analysis of the samples subjected to an acid boiling test revealed some differences in the heat stability of model line milk according to CSN3, spray dried, which is clearly seen at pH values above 6.6 (Table 3). Sample AA 1:BB 1 (100:0 %) with pH 6.6 and 6.8 withstood the addition of 0.5 cm 3 of 0.1 N HCl and remained stable during heat treatment. There was also no coagulation of this sample at pH 7.0 even as a result of the addition of 0.8 cm 3 of 0.1 N HCl. At a pH of 6.8 and 7.0, the addition of 0.5 cm 3 of 0.1 N HCl followed by heating withstood the sample AA 1:BB 1 (0:100 %). Similarly, when adding 0.5 cm 3 of 0.1 N HCl, sample AB 1 (control) retained its colloidal stability. None of the samples of model freeze-drying milk systems withstood the acid-boiling test even with a minimal amount of acid. Thus, according to the method used, only the sample AA 1:BB 1 (100:0 %) at pH 7.0, obtained by the method of spray drying, can be considered thermally stable. The data obtained indicate that as applied to the studied model systems the method for determining heat stability from an acid-boiling sample is not entirely representative.
The next stage of research was the study of the heat stability of reconstituted skimmed milk samples by the thermal test method, as the most common technique with greater reliability of results, as well as allowing to directly analyze the stability of MP under the influence of high temperatures without additional use of denaturing substances (Kruchinin et al., 2020; Sikand et al., 2010). The research results are presented in Table 4.
Table 4. Heat stability by thermal test of reconstituted model milk systems (±0.1 min.)
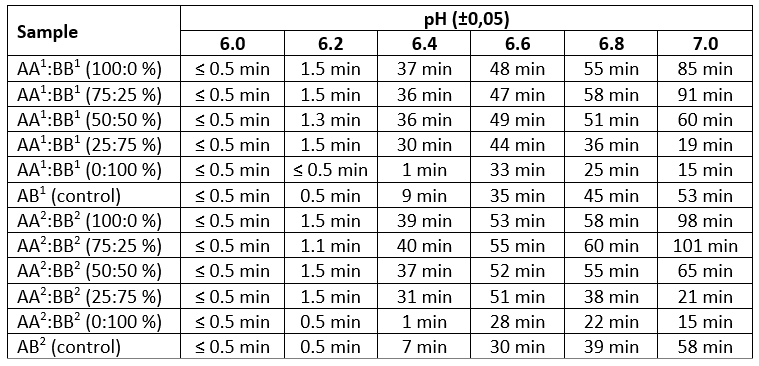
The research results presented in Table 4 showed that samples of spray and freeze dried milk have similar dependences of changes in heat stability both in the pH gradient and with an increase in the model systems of the content of milk of cows with AA CSN3 genotype. All samples could not withstand high-temperature exposure at pH 6.0 and 6.2. With an increase in pH to 6.4, an increase in the duration of heat stability occurred in all samples, particularly dramatic (by 20-30 times) in samples with a predominance of AA CSN3 genotype. Only samples with 100 % of milk content of cows with BB CSN3 genotype (1 min) and control samples (9 and 7 min) subjected to spray and freeze drying (respectively) were destabilized in a minimum time. Samples in the pH range from 6.6 to 7.0 withstood high-temperature processing, sufficient for the production of most types of dairy products, including sterilized. The observations obtained in the course of experiment confirm the results (Dumple et al., 2020) on the increase in the stability of casein micelles with increasing the pH. The authors claim that k-casein is mainly attached to the outer surface of micelles in the form of disulfide-bound oligomers and forms negatively charged sites that create electrostatic and steric barriers preventing aggregation with increasing temperature. A decrease in the pH leads to a weakening of the overall negative charge of the micelle, resulting in a reduction in the duration of heat stability.
Samples with a predominance (from 50 % to 100 %) of milk obtained from cows with AA CSN3 genotype were more heat stability. It should also be noted that even the minimal presence of such milk (25 %) maintained the protein cluster of model systems in a stable state, reaching maximum values for AA 1:BB 1 (44 and 36 min) and for AA 2:BB 2 (51 and 38 min) at pH 6.6-6.8, which proves the effect of genotypic differences in k-casein on the milk technological properties.
Analysis of data revealed higher stabilization qualities of the protein when heated in pH ranges from 6.4 to 7.0 in freeze dried samples compared to spray drying (by 3-10 %) in dairy systems with milk from cows with AA CSN3 genotype with a fraction of 25 % to 100 %, which is due to the sparing temperature regimes of processing casein and whey fractions during lyophilization compared to spraying. However, with 100 % replacement for milk from cows with BB CSN3 genotype the results were levelling.
Summarizing the conducted studies on the heat stability of the samples it should be noted that the results of the acid-boiling test do not correlate with the results of the alcohol and heat tests. This indicated the absence of grounds for its application to these findings. At the same time alcohol and heat tests are indicative and in most cases are correlated with each other. However, only the use of a thermal test made it possible to reveal differences in the heat stability of model samples.
Influence of the k-casein genotype on the cheese making properties of milk powder
At the next stage of research, experiments were carried out to determine the cheese making properties of model milk systems. The ability of dairy raw material, including powder milk after its reconstitution, to form an elastic and dense coagulum under the action of milk-clotting enzymes should be considered as the most important factor in the production of cheese and cottage cheese. The research results are presented in Table 5.
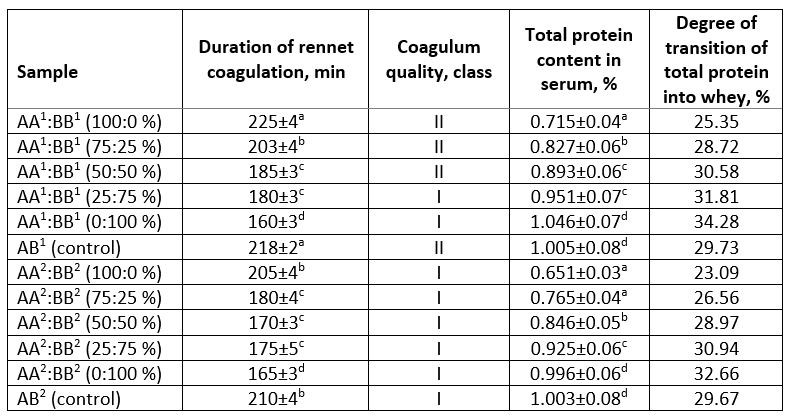
Table 5. Cheese making properties according to the rennet-fermentation sample of reconstituted model milk systems
a, b, c, d Мeans followed by different lowercase letter in column differ significantly by ANOVA; significance level of 5%
Analysis of the experimental data showed that with an increase in the proportion of milk from cows with BB CSN3 genotype from 0 % to 100 % in model systems the duration of rennet coagulation decreases for all samples, regardless of the drying method. The period of clot formation decreased by 40 % and 24 % in the samples of spray and freeze drying, reaching practically the same (within the margin of error) minimum values of 160 and 165 min (respectively) with maximum replacement. The regularities of an increase in the protein transition of serum with an increase in the milk content of cows with BB CSN3 genotype in model systems were also noted. Most likely, this fact is associated to the size of the casein micelles forming the coagulum. Milk with smaller casein micelles size forms a curd with a higher density and hardness than milk with larger micelles size. However, the higher content of small micelles appears to contribute to increased protein loss when separating the serum from the curd. The results obtained are consistent with studies (Bijl et al., 2014) which showed that milk with BB CSN3 genotype has an average casein micelle size less than that of AA CSN3 genotype (173.0±9.7 nm and 201.2±10.3 nm, respectively).
At the same time, there are differences in the quality of clots obtained from spray and freeze dried milk (Figure 2).
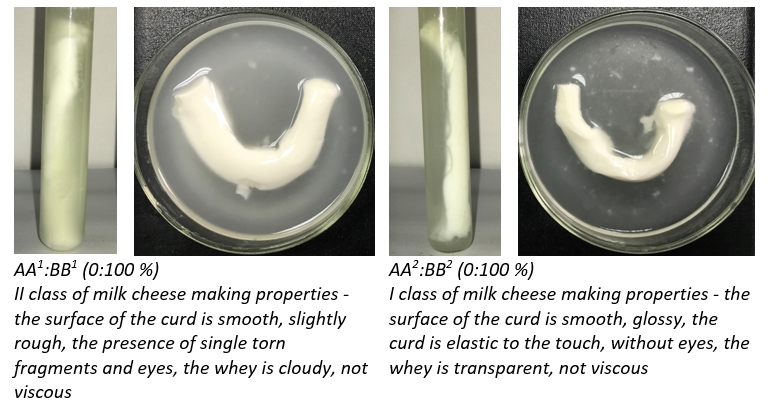
Figure 2. Quality of clots obtained from model samples by spray and freeze drying methods
When using freeze drying, coagulum of all model samples were assigned to class I, while during spray drying only samples AA 1:BB 1 (25:75 %) and AA 1:BB 1 (0:100 %) corresponded to this category which is consistent with the results of Zambrano-Burbano et al. (2010) on a greater resistance to heating and freezing of milk with BB CSN3 genotype, as well as a shorter duration of coagulation with the formation of clots of a denser structure and a higher yield of cottage cheese and cheese than milk with AA CSN3 genotype.
Conclusions
The present study confirms the dependence of the technological characteristics of model dairy systems on the milk genotypic origin and reveals a correlation between the polymorphism of CSN3 gene and the heat stability and milk cheese making properties obtained by various methods of dehydration.
The most representative method for determining the heat stability of milk proved to be a thermal test, whereby the most heat-resistant (36-91 min for AA 1:BB 1 and 37-101 min for AA 2:BB 2) were samples with a predominance (from 50 to 100 %) of milk obtained from cows with genotype AA CSN3, at pH from 6.4 to 7.0. Even the minimal presence of such milk (25 %) maintained the protein cluster of the model systems in a stable state (19-44 min for AA 1:BB 1 and 21-51 min for AA 2:BB 2), which allows to obtain products using high-temperature processing. Also, in dairy systems with a fraction of 25 % to 100 % of milk from cows with AA CSN3 genotype obtained by freeze drying, higher (by 3-10 %) protein stabilization qualities were revealed when heated in the pH range from 6.4 to 7.0 compared to spray drying.
The analysis of the results of cheese making properties showed that with an increase in the proportion of milk from cows with BB CSN3 genotype in model systems from 0% to 100%, the duration of rennet coagulation for all samples, regardless of the drying method, decreased. Also, when using freeze drying, the coagulum of all model samples were assigned to the highest I class of milk quality in terms of cheese making properties, while only samples AA 1:BB 1 (25:75%) and AA 1:BB 1 (0:100 %) corresponded to this category during spray drying.
The model studies presented in this article made it possible to confirm the effect of genotypic differences in k-casein fractions on the technological properties of milk and logically prove the relationship of heat stability and cheese making properties of reconstituted spray and freeze dried milk with the genotype of lactating cows for k-casein. Additional research for a more complete understanding of the cumulative effect of CSN3 gene polymorphism and such technological stages of MP production as heat treatment and thickening (using the traditional evaporation method or various baromembrane methods) on the technological properties of reconstituted milk are of interest.
Utjecaj genotipa k-kazeina na toplinsku stabilnost i prikladnost mlijeka u prahu za proizvodnju sira
Sažetak
U ovom istraživanju je ispitivan učinak polimorfizma gena za k-kazein na tehnološke karakteristike mlijeka u prahu dobivenog sušenjem raspršivanjem (AA 1: BB 1) i liofilizacijom (AA 2: BB 2). Korištene su standardne i općeprihvaćene metode fizikalno-kemijskih analiza mliječnih proizvoda, kao i metode za procjenu toplinske stabilnosti i prikladnosti mlijeka za proizvodnju sira. Najveću stabilnost naspram toplinskih tretmana pokazali su uzorci mlijeka u kojima je prevladavalo mlijeko krava s genotipom AA CSN3, pri pH od 6,4 do 7,0 (36-91 minuta za AA 1: BB 1 i 37-101 minuta za AA 2: BB 2). U modelnim sustavima s 25 % do 100 % mlijeka krava s genotipom AA CSN3 dobivenim liofilizacijom utvrđena je su veća (za 3-10 %) termostabilnost proteina u rasponu pH od 6,4 do 7,0 u usporedbi s uzorkom dobivenim sušenjem raspršivanjem. Analiza rezultata vezanih uz prikladnost mlijeka za proizvodnju sira pokazala je da se s porastom udjela mlijeka krava s genotipom BB CSN3 od 0 % do 100 % u modelnim sustavima, trajanje koagulacije sirilom smanjuje za sve uzorke, bez obzira na metodu sušenja mlijeka. Utvrđeno je da su korištenjem liofilizacije koagulumi svih uzoraka svrstani u najvišu kvalitetu mlijeka u pogledu prikladnosti za proizvodnju sira, dok su kod uzoraka dobivenih sušenja raspršivanjem u ovu kategoriju bili svrstani samo oni koji se sastoje od min. 75 % mlijeka dobivenog od krava s genotipom BB CSN3.
Ključne riječi: mlijeko u prahu; k-kazein; toplinska stabilnost; prikladnost mlijeka za proizvodnju sira
