Introduction
Mastitis can range in intensity and have several underlying reasons (interactions between animal, agent, and environment), with subclinical mastitis being the most common. Subclinical mastitis causes a decrease in milk production and a decline in milk quality without showing any indication of visual inflammation or alterations to the milk (El-Khabaz et al., 2022). The illness shortens the productive longevity of animals and alters the milk content. Subclinical mastitis is the cause of approximately 85 % of mastitis-related milk production losses (Gurbulak et al., 2009). During inflammation, activity of enzymes linked to the inflammatory response were increased (Yagci, 2008).
One of the main issues with dairy production, which results in considerable financial losses, is recommended to be resolved by the early diagnosis and proper adequate treatment of mastitis. Consequently, many investigations were performed in the early detection and control of the subclinical form of mastitis. Several laboratory tests can be utilized to identify the biochemical alterations brought on by this disease in the blood and other body fluids (Youssif et al., 2020).
Recently, biochemical tests are among the most used techniques for determining a disease's progression. To treat SCM and implement preventive and control measures, it is crucial to identify the effects of the condition, particularly on the biochemical parameters in serum and milk. Numerous biochemical tests on cows with subclinical mastitis were carried out, and various enzymes activities were examined to aid the diagnosis (Kandemir et al., 2013). In animals with subclinical mastitis the blood serum and milk enzymes activity such as ALP and ALT could be related to SCM (Batavani et al., 2003; Cetin et al., 2005; Yuksel et al., 2009).
Depending on their primary location, chemical structure, and principal function in milk, enzymes may be present at different stages of the milking process. The existence of enzymes in milk can result from processes, which include the active secretion from the apical regions of the mammary gland epithelium, spontaneous diffusion of low-weight enzymes from plasma, or the release of enzymes from somatic cells in secreted milk, frequently leukocytes or other macrophages (Kocić et al., 2010).
It was discovered that injured cells produced more ALP than normal cells (Turgut, 2000). According to Wada et al. (2002), animals with mastitis had a higher serum ALP activity than mastitis-free animals. Animals with mastitis had serum and milk ALP concentration that was 15 times higher and six times higher ALT concentration in comparison to mastitis free animals (Tripathi, 2000). The LDH and ALP enzymes can be used as indicators for SCM since it causes a significant increase in activity of both enzymes (Katsoulos et al., 2010; Gera and Guha, 2011; Guha et al., 2012).
There is a limited knowledge about the alterations in milk ALT, AST, GGT, and ALP activity during the SCM illness. These enzymes' activity in cows' milk and blood serum was observed (Liu et al., 2013). The enzymes found in mammary cells were derived from blood enzymes, and dairy cow udder health has been tracked by measuring the activity of these enzymes in milk (Fox and Kelly, 2006). The detection of enzyme activity in milk has received more practical attention, and numerous enzymes have been suggested and identified as trustworthy markers for the early identification of SCM (Katsoulos et al., 2010). GGT is crucial for the production of milk proteins because it transfers amino acids from the blood to the mammary glands (Mohamed, 2014).
Animal AST activity has been massive investigated (Kaneko et al., 2008). Because blood aminotransferases function as a catalyst in the metabolism of amino acids and carbohydrates, their activity is crucial. Their enhanced activity in cells or alteration in cell structure can lead to changes in the blood (Milinković-Tur et al., 2005). One of the crucial enzymes for the breakdown of proteins is ALT. It is crucial to the middle stage of metabolism for both glucose and amino acids (Ray et al., 2008).
There is an insufficient reporting about these milk and serum enzymes and their association with each other as an early tool to diagnose SCM animals, particularly in the Middle East. Therefore, the current study was planned to monitor and evaluate the impact of SCM with its different degrees of severity based on CMT scores on the following enzymes; LDH, ALP, GGT, AST, and ALT in milk and blood of dairy Holstein cows and to address the usefulness of selected enzymes as an early biomarker in subclinical mastitis diagnosis.
Materials and methods
Ethical approval
The research procedures were approved by the Institutional Animal Care and Use Committee with document serial number (Vet-CU 12/10.2021/367), Faculty of Veterinary Medicine, Cairo University, Egypt.
Study period and location
This study was conducted in May 2022. Samples were collected from a dairy farm located on the Misr-Alexandria desert road in Egypt.
Animals and nutrition
Thirty multiparous lactating dairy cows were enrolled in this study. The age range was 7-9 years. The weight range was 600-750 kg and the body condition score (BCS) of 3.5- 4.5. The selected cows for this study were apparently healthy, free of clinical mastitis and other noticeable udder lesions, with no history of health issues throughout this production season. The cows were classified into following four groups according to California mastitis test (CMT) score (Nesma et al., 2020) - group 1 SCM-free, CMT score = 0, group2 weak positive, CMT score = +1, group 3 positive, CMT score = +2, and group 4 strong positive, CMT score = +3. Cows were kept in loose sheltered barns. Regular vaccination and deworming programs were conducted. Animals were fed a total mixed ration (TMR) using conserved plants throughout the year. Diet was formulated to meet the cows’ energy requirements during the lactation period according to the NRC of dairy cattle (2001) software program. The composition and the type of offered diet adapted to their respective productive stage to provide at least 1.66 Mcal/kg NEl, metabolizable energy 2.85 (Mcal/kg) and crude protein (CP) 17.5 % DM. The diet was offered twice daily and had ad libitum access to water with an average feed intake of 38 kg/ head/ day. The physical composition of the total mixed ration is demonstrated in (Table 1).
Table 1. The physical composition of daily feed intake of dairy cows (amount/ head/day)
Milk and blood samples collection
All quarter milk and blood samples were collected at the early lactation (4-6th week postpartum), and all cows were sampled in the study. The sampling of milk was conducted after sanitizing the teats and the first stream of milk was discarded. Two hundred and fifty ml of milk was taken and kept in an aseptic container, then reached the laboratory in a secured ice vessel within 24h. One blood sample was collected from each animal. Blood samples were collected via coccygeal vein puncture and kept in a plain tube for serum separation. Serum was stored under a temperature of -20 °C till use.
Milk samples analysis
The California Mastitis Test was done in the laboratory using an index kit (Friesoythe, Cloppenburg, Germany) based on a reagent destroying the somatic cell membranes in milk (Schalm et al., 1971; Youssif et al., 2021). The quarters CMT score = 0 samples were gathered for each cow.
The whey detachment was achieved by cooled centrifugation (Bioevopeak, US) at 10000 RPM/ 10 min. Milk samples were used to estimate activities of milk enzymes LDH, ALP, GGT, ALT, and AST. These were assessed by Hitachi Roche, 902 Automatic Analyzer, Japan using enzymatic specified test kits - Spinreact, Spain for LDH, Beacon Diagnostics, India for
ALP, Spectrum diagnostic, Egypt for GGT, and Vitro Scient, Germany for ALT & AST by following manufacturers' instructions as Andjelić et al. (2022) previously described.
Serum samples analysis
Serum samples were used to measure the activity of serum enzymes LDH, ALP, GGT, ALT, and AST. These parameters were estimated spectrophotometrically using enzymatic kinetic specified test kits (Spectrum diagnostic, Egypt) and following manufacturers’ instructions as Ježek et al. (2017) and Djokovic et al., (2019) previously described.
Data analysis
The obtained data were analysed using completely randomized one-way ANOVA by SPSS program version 16.00. The measured parameters were expressed as mean value ± SE. The Pearson correlation coefficient (r) values were calculated between milk enzymes with each other, serum enzymes each other, and milk & serum enzymes with each other to determine the significant association between these enzymes with each other in SCM animals. The regression analysis (R2) test was performed to evaluate the relationships between milk & serum enzymes for each group separately. A probability "P" value of <0.05 was assumed as statistically significant.
Results and discussion
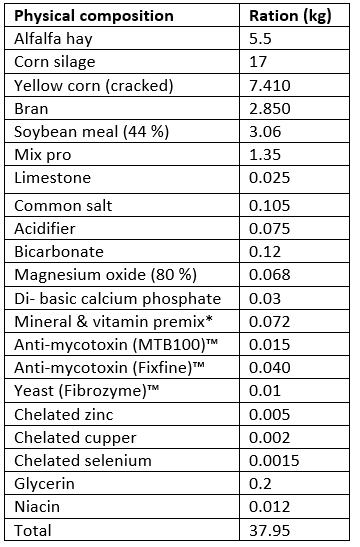
The activities of milk enzymes in different groups, as determined by the CMT score, are presented in Table 2. The milk LDH, ALP, and GGT activities in SCM groups were significantly higher (p<0.05) in groups 3 and 4 compared to group 2 and the control group (group 1). Additionally, their highest activities were recorded in animals belonging to group 4. The LDH activity was significantly higher in group 2 compared to the control group. There were no significant differences in milk AST and ALT values among all investigated groups.
The activities of blood serum enzymes in different groups, as determined based on the CMT score, are presented in (Table 3). The mean blood serum LDH and ALP activities in SCM groups were significantly (p<0.05) higher than SCM-free group. ALP blood activity was significantly (p<0.05) higher in group 4 compared to group 2. The blood GGT activity was significantly higher (p<0.05) in group 4 compared to other groups. There was no significant difference in blood serum ALT and AST values among all groups.
Table 2. Milk enzymes activities from subclinical mastitis free cows and those with subclinical mastitis based on CMT score
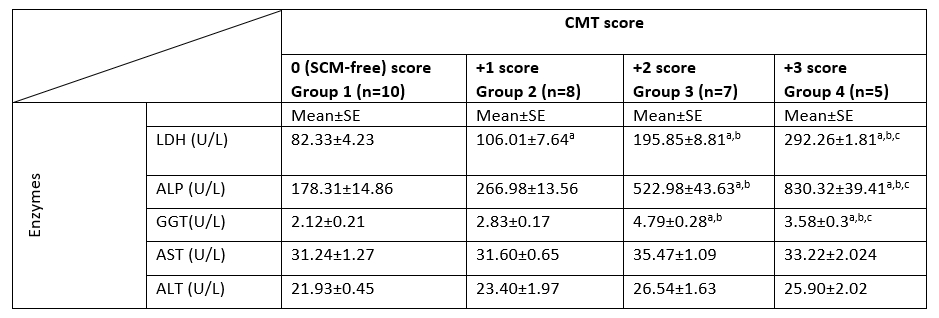
LDH, lactate dehydrogenase; ALP, alkaline phosphatase; GGT, Gamma Glutamyl transferase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CMT, California mastitis test; n, number of samples; SE, Standard Error; U/L, international unit per liter.
a: significant difference in comparison to group 1 (p<0.05).
b: significant difference in comparison to group 2 (p<0.05).
c: significant difference in comparison to group 3 (p<0.05).
Values without superscript letters were non-significant between groups (p>0.05).
Table 3. Blood serum enzymes activities from subclinical mastitis free cows and those with subclinical mastitis based on CMT score
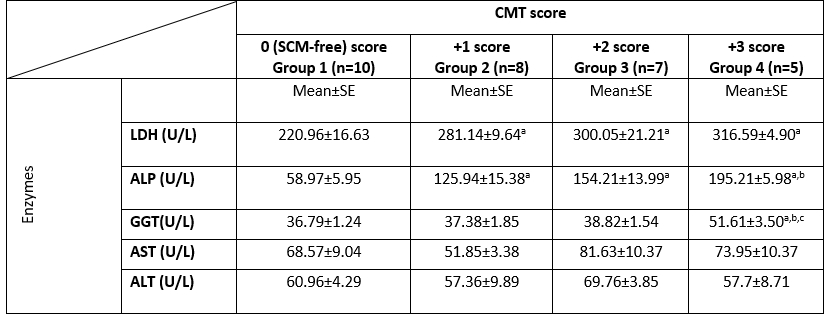
LDH, lactate dehydrogenase; ALP, alkaline phosphatase; GGT, Gamma Glutamyl transferase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CMT, California mastitis test; n, number of samples; SE, Standard Error; U/L, international unit per liter.
a: significant difference in comparison to group 1 (p<0.05).
b: significant difference in comparison to group 2 (p<0.05).
c: significant difference in comparison to group 3 (p<0.05).
Values without superscript letters were non-significant between groups (p>0.05).
The correlations between milk and blood enzymes in the examined SCM dairy cows are presented in Table (4). The correlation between milk enzymes with each other in the examined SCM animals showed a significant positive correlation between the GGT enzyme and the following enzymes (LDH, ALP, and AST). In addition, there was a significant positive correlation between milk LHD & milk ALP, and milk ALT and AST. The correlation between blood serum enzymes with each other in the examined SCM dairy cows showed a significant positive correlation between ALP enzyme and both enzymes (GGT and AST). The correlation between milk and blood serum enzymes with each other in the examined SCM dairy cows showed a significant positive correlation between milk LDH, ALP, and GGT enzymes and the blood serum enzymes (ALP and GGT).
Table 4. Pearson correlation coefficients between blood serum and milk enzymes in the examined SCM dairy cows
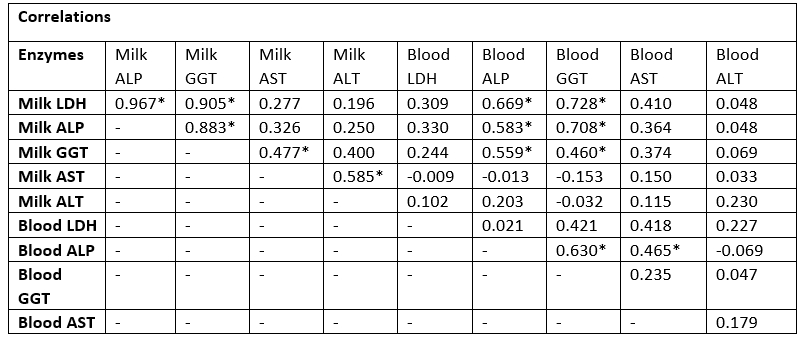
LDH, lactate dehydrogenase; ALP, alkaline phosphatase; GGT, Gamma Glutamyl transferase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Values equal to correlation coefficient (r); *Correlation with asterisk is only significant (p<0.05).
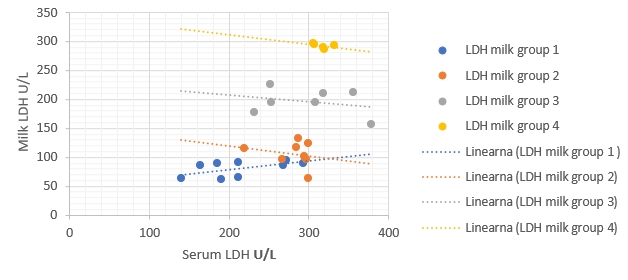
Figure 1. Regression of LDH enzymatic activity changes between milk (ŷ) and blood serum (x) in different groups. Regression equations: group 1:
[CHART]
y = 0.1494x + 49.311, R² = 0.34, p value = 0.07, group 2, y = -0.1705x + 153.94; R² = 0.05, p value = 0.61, group 3: y = -0.1118x + 229.4, R² = 0.07, p value = 0.56, group 4: y = -0.1683x + 345.56, R² = 0.20, p value = 0.44. Correlation is only significant when p<0.05
Figure 2. Regression of ALP enzymatic activity changes between milk (ŷ) and blood serum (x) in different groups. Regression equations: group 1: y = -0.1302x + 185.99, R² = 0.002, p value = 0.89, group 2: y = -0.4647x + 325.5, R² = 0.28, p value = 0.18, group 3: y = -5.8099x + 1418.9, R² = 0.50, p value = 0.078, group 4: y = -1.9803x + 1216.9, R² = 0.09, p value = 0.62. Correlation is only significant when p<0.05
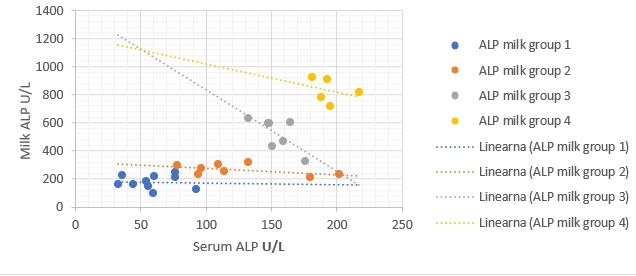
Figure 3. Regression of GGT enzymatic activity changes between milk (ŷ) and blood serum (x) in different groups. Regression equations: group 1: y = 0.0905x - 1.2109, R² = 0.27, p value =0.12, group 2: y = -0.0334x + 4.0765, R² = 0.13, p value = 0.37, group 3: y = -0.1445x + 10.397, R² = 0.64, p value =0.030*, group 4: y = -0.111x + 11.786, R² = 0.66 p value = 0.09. Correlation with asterisk is only significant when p<0.05
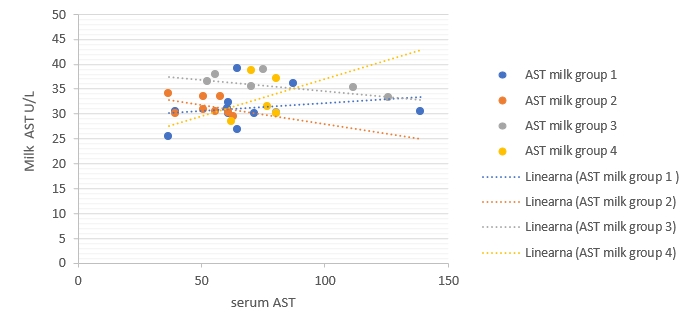
Figure 4. Regression of AST enzymatic activity changes between milk (ŷ) and blood serum (x) in different groups. Regression equations: group 1: y = 0.0314x + 29.084, R² = 0.05, p value = 0.53, group 2: y = -0.0753x + 35.503, R² = 0.15, p value = 0.338, group 3: y = -0.0455x + 39.19, R² = 0.19, p value = 0.33, group 4: y = 0.1498x + 22.14, R² = 0.067, p value = 0.67. Correlation is only significant when p<0.05
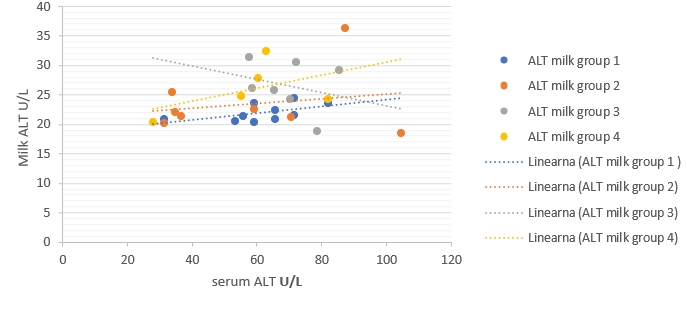
Figure 5. Regression of ALT enzymatic activity changes between milk (ŷ) and blood serum (x) in different groups. Regression equations: group 1: y = 0.0575x + 18.39, R² = 0.30, p value = 0.09, group 2: y = 0.0406x + 21.068, R² = 0.042, p value = 0.63, group 3: y = -0.1132x + 34.439, R² = 0.07, p value = 0.56, group 4: y = 0.1113x + 19.477, R² = 0.23, p value = 0.41. Correlation is only significant when p<0.05.
The regression models were established to compare enzymatic activity changes between milk and blood serum in each group separately in the current study (Fig. 1-5). The results showed that there was a significant (p<0.05) association between milk and blood GGT activity in group 3 (Fig. 3), and no significant (p>0.05) relationships had been detected for the other five enzymes in different groups.
Intramammary infections (IMI) are considered as a problematic animal health issue, modify milk composition, decrease the hygienic value of milk, affect the milk processing properties and cause enormous economic losses in dairy farming (Matei et al., 2010; Turk et al., 2017; Zheng et al., 2022). Additionally, animals with subclinical mastitis (SCM) are a constant source of infection within and among herds (Charaya et al., 2013). SCM is usually associated with apparently healthy milk and udder, but positive CMT (Stefanakis et al., 1995).
The bacteriological culture belongs to the established methods for IMI diagnosis, but an investigation is time-consuming, expensive, and not applicable in practice (Stuhr and Aulrich, 2010). Consequently, different parameters have been proposed by many authors, like the level of certain enzymes in milk and blood for the early detection of SCM, but until now no reliable parameter could be distinguished (Zhao and Lacasse, 2008; Singh et al., 2016). Also, the activity of enzymes in serum and milk has been broadly studied and involved as biomarkers for inflammation (Katsoulos et al., 2010).
For this study, the cows considered to have SCM were those milk samples that tested positive for CMT (Venturini et al., 2009). The CMT is still a simple routine procedure for the examination of SCM animals, especially in cattle (Babaei et al., 2007). The mean activity of LDH and ALP were significantly higher (p<0.05) in milk and blood from SCM cows compared to those of SCM-free milk and blood-based on the severity of CMT scores in this study. Accordingly, similar results were previously reported by Batavani et al. (2003). In contrast, Kalantari et al. (2013) observed a non-significant (p>0.05) increase in the activity of these enzymes in the blood serum of SCM dairy cows compared to healthy cows. LDH is an indicator of inflammatory conditions of the mammary gland. It is a cytoplasmic enzyme involved in carbohydrate metabolism that gets released into milk from ruptured mammary epithelial cells, phagocytes, and serum, subsequently changing the milk's physical and chemical properties during IMI (Singh et al., 2016).
Milk LDH activity has been established as a sensitive early marker for changes in mammary gland function, and ALP activity was reliable in SCM early detection (Babaei et al., 2007; Novac et al., 2022). Elevated milk LDH and ALP activities might be originated from mammary epithelial, leukocytes and interstitial cells damaged during inflammation, particularly from disintegrated leukocytes (Guha et al., 2012). In addition, the IMI increases the microcirculatory permeability of vessels by secretion of various chemical mediators such as prostaglandin, histamine, and free oxygen radicals from inflammatory cells (Atroshi et al., 1996), which leads to a breach in the blood-milk barrier selectively damaged by bacterial toxins (Narenji Sani et al., 2018).
The increased levels of these enzymes in blood serum can be linked with tissue damage in mammary parenchyma (Qayyum et al., 2016). A higher level of serum alkaline phosphatase is indicated for infection and plays a vital role in the pathogenesis of disease in infected animals (Hussain et al., 2012). The GGT activity was significantly higher in milk and blood from sub-clinically mastitis cows compared to those of SCM-free milk based on the severity of CMT scores. In line with our results, Matei et al. (2010) reported a considerable increase in GGT activity in milk serum from the sub-clinically infected bovine mammary gland. Variations in enzymatic activity in blood or milk can be originated from cell structure injury (Babaei et al., 2007). The higher level of ALP in SCM milk than in blood serum in the present study supports the theory that blood serum was not of the solitary origin of these enzymes in IMI, and it doubtless also originated from udder parenchymal cells and damaged leukocytes (Moawad and Osman, 2005).
Concerning the results of AST and ALT in milk and blood samples, it was found to be relevant to the results of the other investigators (Mosallam et al., 2014), who reported no changes in their blood serum activities in SCM compared to the control group.
Stojević et al. (2005) previously concluded that the activity of AST enzymes showed occasional irregular changes during pregnancy and lactation in dairy cows. In contrast to that, Kocić et al. (2010) noticed and recorded an increase in ALT and AST concentration in association with mammary gland inflammation. Also, our results are mismatched with Sarvesha et al. (2016), who reported a significant increase in serum AST levels in subclinical mastitis and clinical mastitis compared to the control.
The increased values of LDH, ALP, and GGT in milk and blood enzymes observed in the current study were correlated with the severity of the CMT score and these changes may be linked with tissue damage associated with IMI (Qayyum et al., 2016).
A significant positive correlation between blood serum ALP and both enzymes (GGT and AST) in SCM cows was noticed. In line with the present study, Abdel-maged et al. (2016) noticed a positive relationship between blood ALP and blood AST enzyme. The detected correlations among different milk enzymes observed in this study corroborated with an earlier observation by Guha et al. (2012). Additionally, these results were compatible with the study of Abdel-maged et al. (2016), who recorded a close association between the elevation of milk LDH and ALP due to mammary tissue destruction.
There was a significant positive correlation between milk LDH, ALP, & GGT enzymes and the blood serum enzymes (ALP and GGT) in SCM cows. This result was well-matched with the study of Liu et al. (2012), who recorded a strong positive association between milk and blood ALP and GGT.
The results of regressive models did not show a close relationship between enzymatic activities in the milk and blood samples of each group separately. This obtained results might suggest the existence of a statistical phenomenon called Simpson’s Paradox, where a correlation between two variables in a group emerges, disappears, or reverses when this group is divided into subgroups (Holt, 2016). Consequently, the alternative detection of these enzymes in milk for assessing dairy cows' health status still needs additional studies to be conducted on a higher number of dairy animals. The obtained results in the current study indicate that the detection of milk and blood enzymes may be a an useful tool for early detection of IMI. Making this promising method to be practical still needs additional laboratory investigations to confirm.
Conclusions
The alterations of milk and blood serum enzymes activities, LDH, ALP, and GGT were noticeably linked with the degree of subclinical mastitis based on the CMT score. The significant positive correlation between milk LDH, ALP, and GGT enzymes and the serum enzymes (ALP and GGT) in SCM cows confirmed the close association between these enzymes and subclinical mastitis in cows. The determination of these milk and blood enzymes activities seems to be an appropriate diagnostic and additional tool for the early detection of subclinical mastitis in dairy cows. Supplementary laboratory investigations are still essential to confirm the practicality of this promising method. The alternative detection of these enzymes in milk for the assessment of dairy cows' health status requires more future research. A further understanding of the relationship between the enzymes in milk and blood still needs more investigations on a larger number of cows with different levels of SCM to confirm a direct relationship between them.
Određivanje aktivnosti enzima u mlijeku i krvi holstein krava sa supkliničkim mastitisom
Sažetak
Cilj ovog istraživanja bio je odrediti aktivnosti enzima laktat dehidrogenaze (LDH), alkalne fosfataze (ALP), gama-glutamil transferaze (GGT), aspartat aminotransferaze (AST) i alanin aminotransferaze (ALT) u mlijeku i krvi holstein krava u laktaciji i utvrditi njihovu povezanost sa supkliničkim mastitisom (SCM). Jedan od ciljeva studije bio je, također, na temelju rezultata kalifornijskog testa na mastitis (CMT) procijeniti utjecaj stupnja SCM-a na aktivnost spomenutih enzima, kao i utvrditi učinkovitost njihove upotrebe kao alata za ranu dijagnostiku SCM-a. Trideset mliječnih holstein krava u laktaciji podvrgnuto je dijagnostici CMT-om i sukladno dobivenim rezultatima razvrstano u 4 skupine: kontrolna skupina 1 (bez SCM = 10, rezultat = 0), skupina 2 (slabo pozitivan = 8, rezultat +1), skupina 3 (pozitivan = 7, rezultat +2) i skupina 4 (jako pozitivan = 5, rezultat +3). Aktivnosti enzima LDH, ALP i GGT u mlijeku bile su značajno veće u skupinama 3 i 4 u usporedbi sa skupinom 2 i kontrolnom skupinom. Pri tom su najveće aktivnosti svih enzima zabilježene kod životinja iz skupine 4. Aktivnost LDH bila je značajno veća u skupini 2 u odnosu na kontrolnu skupinu. Srednje vrijednosti aktivnosti LDH i ALP u krvi u skupinama s potvrđenim SCM bile su značajno veće od kontrolne skupine. Aktivnost ALP u krvi bila je značajno viša u skupini 4 u usporedbi sa skupinom 2. Aktivnost GGT u krvi bila je značajno viša u skupini 4 u usporedbi s ostalim skupinama. Utvrđena je značajna pozitivna povezanost između aktivnosti enzima LDH, ALP i GGT u mlijeku i aktivnosti enzima u krvi (ALP i GGT) kod SCM pozitivnih krava. Rezultati su pokazali da supklinički mastitis uzrokuje izrazite promjene aktivnosti enzima u mlijeku i krvi zaraženih krava.
Ključne riječi: kalifornijski mastitis test; mliječne krave; enzimska aktivnost; supklinički mastitis
References
https://doi.org/10.37422/IJVS/20.020
DOI: https://doi.org/10.21708/avb.2021.15.2.9785
https://doi.org/10.2527/jas.2007-0302
https://doi.org/10.3390/cells11223658
