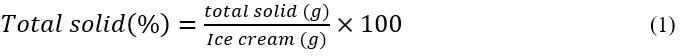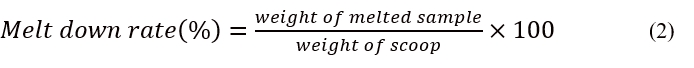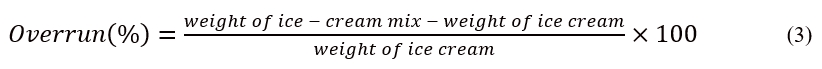Introduction
Ice cream, as a milk-based frozen food product involving freezed liquid mix with agitation, with ingredient constituents that provides its unique final structure and texture (Kambamanoli-Dimou, 2014;Deosarkar et al., 2015). Besides milk being the most important ingredient in ice cream production (Kambamanoli-Dimou, 2014), the higher fat content and sugar comprising half of its total solids make ice cream a promising energy source (Syed et al., 2018). With regards to allergy to bovine milk proteins occurring due to an immune response to cow milk dietary antigen, certain negative consequences on human health of sensitive population can be expected (Rangel et al., 2016;Zandona et al., 2021). Another concern is lactose intolerance, largely exacerbated by consuming animal milk, occurring due to inability to digest and absorb lactose, and arising because of decreased lactase expression after weaning (referred to as "lactase non-persistence") (Heine et al., 2017;Orr et al., 2020). In recent years, there is an increasing demand for plant-based milk substitutes, especially in ice cream production. In particular, plant-based milk is a source of fibres when substituted with animal milk (Sethi et al., 2016). Moreover, keeping the body, structure, and texture of ice cream requires optimising the right balance, which entails such constituent ingredients like fruit fibre, plant-based extracts, chunks, purees, pastes, and concentrates (Syed et al., 2018).
Bambara groundnut (Vigna subterranea (L.) Verdc) is among indigenous African crops cultivated in Africa. It is a highly nutritious plant that plays a crucial role in human diets (Murevanhema and Jideani, 2015). Most impoverished people in Africans homes use it as an essential source of protein in their diet because they cannot pay the high cost of animal proteins (Hillocks et al., 2012;Talabi et al., 2019). Due to its nutrient-dense nature, this legume is sometimes classified as a "complete food" due to its balanced macronutrient composition (Azman, 2019). Like soybean yoghurt, fermented Bambara groundnut milk (BGNM) may be a suitable probiotic bacterial strain carrier to the host considering its antioxidants, chemical and nutritional composition (Murevanhema and Jideani, 2015;Nti, 2009; Gonne, 2013). Containing high protein and amino acid contents, Bambara groundnut is an ideal food to complement most cereal-based diets (Alake, 2017). The total energy from Bambara groundnut grain consumption are among the highest compared to other legumes like pigeon peas (Cajanus cajan L.) and lentils (Lens culinaris L.) (Haleegoah et al., 2015). Bambara groundnut grain possesses some anti-nutritional factors (ANFs) that may limit its bioavailability (Unigwe et al., 2018).
Bambara groundnut is considered an underutilized legume (Adzawla et al., 2015;Bonthala et al., 2016;Karunaratne et al., 2015;Suhairi, et al., 2018;Nwadi et al., 2020). Furthermore, the crop seems to have attained a better status given its use to enrich a variety of foods (Nwadi et al., 2020). Bambara groundnut protein hydrolysate and peptide fractions could potentially serve as ingredients in the formulation of functional foods and nutraceuticals (Arise et al., 2017). As a cereal-legume-based (maize and Bambara groundnut) complementary food, Bambara groundnut serves as complementary feeding especially those formulated with locally available ingredients (Uvere et al., 2010;Attaugwu et al., 2016;Nwadi et al., 2020;Hayes et al., 2017). In addition, Bambara groundnut represent a good source of insoluble dietary fibre (Diedericks and Jideani, 2015). Bambara groundnut has been used in the production of many food products such as milk, yogurt, bread, biscuits, doughnuts, meat analogue, fura, fufu, pasta, and many extruded food (Nwadi et al., 2020).
Largely fluid-like, plant-based milk alternatives have emerged by reducing the particle size distribution of (plant) material (cereals, pseudo-cereals, legumes, oilseeds, nuts) extracted in water, followed by homogenization (Sethi et al., 2016). Examples of plant-based milk alternatives include corn milk, rice milk, soy milk, cowpea milk, Bambara groundnut milk (BGNM), etc. In addition to Bambara groundnut in Africa principally considered by farmers as a "famine food" (Tan et al., 2020), the seeds remain complete food given their chemical makeup, possessing such components as carbohydrate content of 49-63.5%, 15-27% protein, 4.5-7.4% fat, 5.2-6.4% fibre, and 3.2-4.4% ash (Majola et al., 2021). The potential of Bambara groundnut extract as milk substitutes have been shown as promising for the production of yogurt (Falade et al., 2015; Hardy and Jideani, 2020;Salami et al., 2020). Although BGNM appears increasingly applicable in yoghurt production, there are limited studies revealing its application in the production of ice cream. To supplement existing information, therefore, this current work investigated the quality indices of ice cream from dairy milk partially substituted with Bambara groundnut extract.
Materials and methods
Preparation and production of Bambara groundnut ice cream
The raw materials, which included full cream milk, vegetable oil, sugar, eggs, and Bambara groundnut, were purchased from designated market (Orie Orba Market, Udenu LGA, Enugu state, Nigeria). The making/production of BGNM, depicted inFigure 1a, was slightly modified from the milk substitute manufacturing method described byPahane et al. (2017). Briefly, Bambara groundnut seeds were manually sorted and cleaned with clean portable water, and afterwards, dehulled, followed by soaking for 12 h, whereby water was changed every 6 h. After 12 h of soaking, palm-rubbing approach was used for husk removal off the seed coat, then sieving, followed by wet milling. After, the beverage was extracted from the mash via Muslin cloth, allowed to stand briefly (~30 s) and filtered again. The BGNM has been pasteurized at 85 °C for 10 min and stored in a sterile container for further processing. All chemicals and reagents used in this study were of analytical grade standard.
The ice cream production was performed as depicted inFigure 1 b, following the method described byGoff and Hartel (2013) with slight modifications. This required the assembly of ingredients in the appropriate proportions, and subsequently mixed. Firstly, sugar (214.5 g), one whole egg (50 mL), and vegetable oil were added to dry full cream milk (195 g) reconstituted in 993.5 mL cold water (150 g). The ice cream mix was then made up to 1500 mL. The dosed ingredients were thoroughly mixed to obtain an ice cream mix with the correct composition. One litre of BGNM and one liter of reconstituted milk were measured out and used to produce the control samples. As shown inTable 1, five more ice cream mixtures were constituted with various volume-to-volume ratio of reconstituted milk and BGNM, that is, 90:10, 80:20, 70:30, 60:40, and 50:50, respectively. The different ice cream mixes were pasteurized at 85 °C for 10 min, then homogenized (3-5 min), thereafter, allowed to cool, and poured into the (ice cream) making machine (model BQL/216T, Garyton Industry Company Limited, Zhejiang, China) for the partial freezing and aeration processes. One half of frozen mix was transferred into polypropylene plates, adequately covered and labelled. The other half of frozen mix was placed in a blast freezer for further hardening.
| Sample | Reconstituted milk % | BGNM% |
|---|---|---|
| FCM1 | 100 | 0 |
| BGM2 | 0 | 100 |
| FBM3 | 90 | 10 |
| FBM4 | 80 | 20 |
| FBM5 | 70 | 30 |
| FBM6 | 60 | 40 |
| FBM7 | 50 | 50 |
FCM1 = Milk ice cream (control 1), BGM2 = Bambara nut milk ice cream (control 2), FBM3 = Ice cream from 10% Bambara nut milk: 90% milk, FBM4 = ice cream from 20% Bambara nut milk: 80% milk, FBM5 = ice cream from 30% Bambara nut milk: 70% milk, FBM6 = ice cream from 40% Bambara nut milk: 60% milk, FBM7 = ice cream from 50% Bambara nut milk: 50% milk
Analytical methods
Physicochemical analysis
Physicochemical components involved proximate composition, mineral contents (iron, calcium, and magnesium), pH, titratable acidity, total solids, viscosity, as well as over-run. The the determinations of proximate composition, as well as mineral contents (iron, calcium, and magnesium) followed the method described byAOAC (2010). The calibration curves of iron, calcium, and magnesium were established by Atomic Absorption Spectrophotometric (AAS) method.
The pH was measured using digital (pH) meter (Jenway model 3510, England, UK), where the electrode was gently stirred inside the tested sample. To determine the titratable acidity, four drops of phenolphthalein indicator were used, alongside standard 0.1N NaOH for titration to noticeable pink point, and resultant values expressed in terms of lactic acid (g/100 mL).
Total solids was determined using the method (33.2.44; 990.20) ofAOAC (2010) with slight modifications. The total solid was assessed gravimetrically by drying the samples to a constant weight in an oven at 105 °C. In a pre-dried weighing dish, ice cream samples were crushed with 20 g sea sand and a glass stick. The difference in weight before and after 4-5 h of drying at 105 °C gave the total solid content of the samples. The equation 1 below shows the calculation of total solids.
Viscosity was determined by way of Oswald type viscometer following the manual specifications. This employed 10 g portion of the ice cream. With some adjustments, the meltdown rate (%) was estimated using the approach described byMilani and Koocheki (2011). On top of a beaker, a plastic strainer with apertures of 1x1 mm was used to place a 25 g scoop of ice cream. The weight of the melted material collected in the beaker was measured after 60 min at 20±1 °C. The melting rate was calculated as a percentage of the weight of ice-cream initially scooped, as shown in equation 2 below:
Over-run was determined according toAkin et al. (2007) with slight modifications. Specifically, over-run depicted how much air was absorbed into the ice cream mix during freezing. The ice cream overrun was calculated by comparing the weight of equal volumes of ice cream mix and ice cream. The value of over-run was computed using Equation 3:
Determinations of phytic acid and tannins
The phytic acid was determined using the supernatant difference method described byThompson and Erdman (1982) with some modifications. In a flask containing 100.0 mL of 1.2 HCl and 10% Na2S04, two grams of tested samples were introduced. 10 mL of deionized water was added, then 12 mL of FeCl3 solution (2.0 g FeCl3.6H2O) and 16.3 mL of concentrated HCl per litre) with contents heated (75 min) and then chilled for 1 h at room temperature. Using solution of 0.6% HCl and 2.5% Na2S04, and the supernatant was decanted and discarded, thereafter 10 mL concentrated HNO3 quantitatively transferred with multiple tiny quantities of deionized water. Adding drops of concentrated H2SO4 followed by heating (30 min), and thereafter approximately 5 mL of 30% H2O2, heating continued, after which residue was dissolved in 15 mL of 3N HCl. The obtained solution was then centrifuged for 5 min at 1000 rpm. The supernatant was filtered using Whatman paper, from which the amount of total phosphorus was analysed using the method described byOnwuka (2005). From this supernatant, 1 mL of initial extract was diluted with 100 mL, and 2 mL of this was analysed. The phytic acid was deduced by difference in phosphorus values for the extract and for the post-precipitation supernatant, expressed by mg phytate/100 g.
The tannins were determined using the method described byPearson (1976) with slight modifications. This involved 10 mL distilled water added to one gram (1 g) of tested sample in a flask. The mixture was allowed to stand at room temperature for 30 min, with gentle shaking every 5 min. The mixture was centrifuged after 30 min. Separate 50 mL volumetric flasks were used to quantify 2.5 mL of supernatant and 2.5 mL of standard tannin solution. In each flask, 1 mL of Folin-Dennis reagent was added, followed by 2.5 mL of saturated Na2CO3. The solution was mixed up to the desired consistency and incubated at room temperature for 90 min. The absorbance was measured at 250 nm, and the tannin concentration was calculated using Equation 4.
Where, An = Absorbance of test sample, As = absorbance of the standard, C = Concentration of standard, W = Weight of sample used, Vf = Total volume of extract, and Va = Volume of extract used for the analysis.
Microbial analysis
Microbial determinations of total viable and coliform counts were performed as described previously (Murevanhema, 2012;Onwuka, 2005), and with slight modifications. To determine the total viable count, one gram of the sample was macerated and thoroughly mixed in 9 mL of diluent, and subsequently submitted to serial dilution. Thereafter, 15 mL sterile nutritional agar medium (Nutrient agar 500g Biolife, Italy) was poured over the inoculum and properly mixed. The colonies produced were counted and quantified as colony forming units per gram (cfu/g) after a 24-hour incubation period at 38 °C, using the pour plate method (Onwuka, 2005). To determine the coliform counts, the ice cream samples were applied directly or decimally diluted and plated with violet red bile agar (VRBA) (E & O Laboratories Ltd, Scotland, UK). The plates were incubated for 24±2 h at 32±1 °C. Confirmation of typical colonies from VRBA plates was made by transferring each of the colonies to 2% brilliant green bile (BGB) broth (E & O Laboratories Ltd, Scotland, UK) and incubating at 35 °C, using the pour plate method (Onwuka, 2005).
Sensory analysis
Sensory evaluation was performed using the method described byFalade et al. (2015) with some modifications. In particular, a group of 20 panellists conducted a sensory evaluation of the ice cream produced. The panellists were randomly chosen male and female students from the Department of Food Science and Technology. The controls were ice cream from reconstituted milk and from BGNM. The products were judged on a nine-point hedonic scale, with 9 indicating a high preference and 1 indicating a strong dislike. The sample was graded on four sensory attributes: flavor, color, consistency, and overall acceptability by the panelists. Each panelist was given clean warm water to rinse his or her mouth after each sample.
Statistical analysis
Data emergent from triplicate measurements were subjected to analysis of variance (ANOVA). Mean differences were resolved using the Duncan's New Multiple Range Test (DNMRT). Statistical significance was accepted at p<0.05. Statistical Product for Service Solution (SPSS) version 24.0 (IBM, Armonk, New York, United States) was used to run the data.
Results and discussion
Changes in physicochemical aspects
The proximate composition of ice cream produced from Bambara groundnut extract are as shown inTable 2. Moisture content ranged between 87.50 (least at sample FCM1) and 89.19% (peak at sample FBM7) across samples. Moisture content noticeably increased (p<0.05) in agreement withUmelo et al. (2014), except at samples FBM3 and FBM4 that resembled (p>0.05). The fat content ranged between 1.10 (least at sample FCM1) and 6.30% (peak at sample FBM7) across samples. Increased Bambara groundnut ratio reduced somewhat the fat content, which was probably associated with dilution effect. Moreover, milk fat and milk solid non-fat constitutes about 60% of the total solids of ice cream (Goff and Hartel, 2013). The fibre content resembled (p>0.05), like those reported for soybean-based ice cream (Kanika et al., 2015). As protein contents resembled (p>0.05) across samples except at FCM1, it appeared well above the values reported byLima et al. (2016). Bambara groundnut was expected to increase the protein contents (Arise, 2016), and modify the texture of ice cream (Umelo et al., 2014). Ash content ranged between 0.42 (least at sample FBM7) and 1.05% (peak at sample FCM1) with significant differences (p<0.05) across samples, despite resemblances between samples FBM3 and FBM4 (p>0.05). Carbohydrate content ranged between 0.79(least at sample FBM3) and 1.40% (peak at sample BGM2) with significant differences (p<0.05) across samples, except samples FCM1 and FBM3 and FBM4 that resembled (p>0.05).
Values are Means ±SD (standard deviation) of duplicate determination. Means on the same column with different superscripts are significantly different at p<0.05. Acronyms of samples,
FCM1 = Milk ice cream (control 1), BGM2 = Bambara nut milk ice cream (control 2), FBM3 = Ice cream from 10% Bambara nut milk: 90% milk, FBM4 = ice cream from 20% Bambara nut milk: 80% milk, FBM5 = ice cream from 30% Bambara nut milk: 70% milk, FBM6 = ice cream from 40% Bambara nut milk: 60% milk, FBM7 = ice cream from 50% Bambara nut milk: 50% milk
The mineral contents of ice cream produced from cow milk and Bambara groundnut extract are presented inTable 3. Zinc content ranged between 0.92 (least in sample FBM5) and 1.65 mg/100 g (peak in sample BGM2) with significant differences (p<0.05) across samples. Including Bambara groundnut extract in the ice cream formulation reduced the zinc content, with values lower than those reported by Yangilar (2016) for ice creams fortified with laboratory-prepared peach fibre. Magnesium concentrations ranged between 16.54 (least at sample FCM1) to 24.26 mg/100 g (peak at Sample FBM4), with significant differences (p<0.05) across samples. Magnesium aids muscle and neuron function, a healthy immune system, as well as the creation of energy and protein (National Institutes of Health, 2020). Potassium concentration ranged between 2.71 (least at sample BGM2) to 11.24 mg/100 g (peak at sample FBM5) with significant differences (p<0.05) between the samples. Bambara groundnut in the ice cream formulation increased the potassium concentration. Yangilar (2016) found that ice creams supplemented with laboratory-prepared peach fibre had similar magnesium but higher potassium levels. Iron content ranged between 0.62 (least at sample FBM7) and 5.53 mg/100 g (peak at sample FBM3) across samples. Including Bambara groundnut extract in the ice cream formulation reduced the iron content. Iron is responsible for transporting oxygen to the muscles and brain, and aids in the enhancement of focus and concentration (Ware and Marcin, 2018). Calcium ranged between 46.69 (least at sample BGM2) and 143.33mg/100 g (peak at sample FBM3) with significant differences across samples (p<0.05). The calcium values were also lower than the US RDA of 700 mg/day for children and 1000 mg/100 g for adults (WebMD, 2021).
Values are Means ±SD (standard deviation) of duplicate determination. Means on the same column with different superscripts are significantly different at p<0.05; Acronyms of samples, FCM1 = Milk ice cream (control 1), BGM2 = Bambara nut milk ice cream (control 2), FBM3 = Ice cream from 10% Bambara nut milk: 90% milk, FBM4 = Ice cream from 20% Bambara nut milk: 80% milk, FBM5 = Ice cream from 30% Bambara nut milk: 70% milk, FBM6 = ice cream from 40% Bambara nut milk: 60% milk, FBM7 = ice cream from 50% Bambara nut milk: 50% milk
The pH, overrun, viscosity, total solids, and total titratable acidity of ice cream produced from Bambara groundnut extract and dairy milk are presented inTable 4. The pH ranged between 5.20 (least at sample BGM2) and 6.61 (peak at sample FCM1) with significant differences (p<0.05) across samples, except the samples FBM4 and FBM5 that resembled (p>0.05). Yangilar (2016) observed similar pH (5.25-6.60) in ice creams fortified with laboratory-prepared peach fibre. Besides, the overrun ranged between 11.00(least at sample FBM4) and 73.50% (peak at sample FCM1), resembling earlier reports ofChoo et al. (2010) for of ice-cream formulated with virgin coconut oil. Knowing that overrun can influence the final structure of a given product, the presence of air in the ice cream provides a light feel and could influence both firmness and melting (Goffand Hartel, 2013).
Values are Means ±SD (standard deviation) of duplicate determination. Means on the same column with different superscripts are significantly different at p<0.05. Acronyms of samples, FCM1 = Milk ice cream (control 1), BGM2 = Bambara nut milk ice cream (control 2), FBM3 = Ice cream from 10% Bambara nut milk: 90% milk, FBM4 = ice cream from 20% Bambara nut milk: 80% milk, FBM5 = ice cream from 30% Bambara nut milk: 70% milk, FBM6 = ice cream from 40% Bambara nut milk: 60% milk, FBM7 = ice cream from 50% Bambara nut milk: 50% milk
Table 4 also shows that viscosity results ranged from roughly about 1.00x10-3 Pa s (least at sample FBM3) to about 2.25x10-3 Pa s (peak at sample FBM7), somewhat in agreement with previously reported data (Yangılar, 2016;Hwang et al., 2009). Viscosity increased with inclusion of Bambara groundnut in the formulation. Within the production process, viscosity is key in ensuring body and texture of the ice cream product (Goff and Hartel, 2013). According to results presented inTable 4, the total solid content ranged between 10.98 (least at sample FBM7) and 12.35% (peak at sample FCM1) with significant differences (p<0.05) across samples. Total solids reduced with increased inclusion of Bambara groundnut extract. Total solids appeared lower than those (37.7 to 40.42%) reported byChoo et al. (2010) in virgin coconut oil produced ice cream. Also inTable 4, the total titratable acidity ranged between 0.05 (least at sample FCM1) and 0.11g/100mL (peak at sample FBM7) with significant differences (p<0.05) across samples. InFigure 2, the meltdown is presented with increases in the ice cream samples formulated with 100% Bambara groundnut compared to those partially substituted. Both heat transfer rate and ambient temperature affect how quickly the ice melts (University of Guelph, 2016). Low meltdown values appeared after 100 min, near to data published elsewhere (Choo et al., 2010).
Changes in phytic acid and tannins
Anti-nutrients (also called anti-nutritional factors) in many foods are poisonous substances that limit nutrient availability to the human body. Typically, plant anti-nutrients are classified based on their biosynthetic origin, chemical structure, as well as specific actions (Thakur et al., 2019). Anti-nutrients, specific to tannin and phytic acid contents of ice cream produced from Bambara groundnut extract are shown inFigures 3 and 4, respectively. The values ranged between 0.32-2.64 mg/100 g, and 2.97 to 63.85 mg/100 g, with significant differences (p<0.05) across samples. For both, tannin and phytic acid contents, Sample BGM2 showed a peak, whereas FBM3 showed lowest values. Both tannin and phytate contents increased with inclusion of Bambara groundnut extract (Soetan and Oyewole, 2009). Phytic acid seemed undetected in sample FCM1, which might be because the ice cream was produced from dairy milk. Generally, freshly harvested legume seeds can possess high phytic acid content, given by their enriched phytates, which capably reduces the overall availability of certain minerals. However, the processing steps that enable their utilization can reduce the high phytic acid contents (Enujiugha and Ayodele‐Oni, 2003).
Changes in microbiological aspects
The microbial counts of ice cream are presented inTable 5. Total viable count ranged from 1.2x103 to 8.7x102CFU/mL all of which were within the acceptable public health safety standards (Cabral, 2010). Sample FBM5 showed a lower microbiological count than the other samples. Indeed, total viable count is well-known to provide a holistic view of microbial proliferation within a given food product, which would involve bacteria, yeast, or mould species. Herein also, no coliforms were found in any of the ice cream samples. Importantly, coliforms could involve gram-negative rod-shaped bacteria with similar biochemical features, such being capable of fermenting lactose to generate acid and gas in 48 h at 35 °C (Bartram and Pedley, 1996). Raw milk, meat, poultry, and other raw foods frequently contain coliforms in small levels. Coliform count appears routine and straightforward hygienic indicator that ascertains general microbiological quality, as well as pointer to heat treatment failure(s) (Bartram and Pedley, 1996).
| Sample | Total viable count (CFU/mL) |
|---|---|
| FCM1 | 8.7x102±7.07 |
| BGM2 | 2.7x103±35.36 |
| FBM3 | 2.3x103±0.00 |
| FBM4 | 2.0x103±0.00 |
| FBM5 | 1.2x103±0.00 |
| FBM6 | 4.3x102±7.07 |
| FBM7 | 5.4x102±0.00 |
Values are Means ±SD (standard deviation) of duplicate determination. Means on the same column with different superscripts are significantly different at p<0.05;
FCM1 = Milk ice cream (control 1), BGM2 = Bambara nut milk ice cream (control 2), FBM3 = Ice cream from 10% Bambara nut milk: 90% milk, FBM4 = Ice cream from 20% Bambara nut milk: 80% milk, FBM5 = Ice cream from 30% Bambara nut milk: 70% milk, FBM6 = Ice cream from 40% Bambara nut milk: 60% milk, FBM7 = Ice cream from 50% Bambara nut milk: 50% milk
Changes in sensory scores
Mean sensory scores of ice cream produced from Bambara groundnut extract are presented inTable 6. The sensory attributes involved colour, taste, smoothness, mouth feel, sweetness, aftertaste, and overall acceptability aspects, which obtained various ranges across the ice cream samples. For instance, colour score appeared most acceptable at sample FBM4, and least at BGM2, despite the resemblances (p>0.05) between FCM1 and FBM6, as well as FBM5 and FBM7, which seemed comparable to previously reported data ofChoo et al. (2010). Taste score appeared most acceptable at sample FBM4, and least acceptable at sample BGM2, despite resemblances (p>0.05) between FCM 1 and FBM 3, as well as FBM 6 and FBM7. Smoothness score appeared most acceptable at sample FBM 4 and least at sample BGM2, with resemblances (p>0.05) between samples FCM1 and FBM3, FBM5, FBM6 and FBM7. Mouth feel appeared most acceptable at sample FBM4, and least sample BGM2, with resemblances (p>0.05) between FCM 1 and FBM 3; FBM 5 and FBM 6. Sweetness scores appeared most acceptable at sample FBM 4 and least at sample BGM 2, with resemblances (p>0.05) between FCM1, FBM3 and FBM4, as well as FBM 5, FBM 6 and FBM 7. Aftertaste scores significantly differed (p<0.05) between the samples, most acceptable at sample FBM 4, and least at sample BGM2, also comparable to data reported byChoo et al. (2010). Overall acceptability resembled (p>0.05) between samples FCM 1, FBM 3 and FBM 4; FBM 5, FBM 6 and FBM 7.
Values are Means ±SD (standard deviation) of duplicate determination. Means on the same column with different superscripts are significantly different at p<0.05
FCM1 = Milk ice cream (control 1), BGM2 = Bambara nut milk ice cream (control 2), FBM3 = Ice cream from 10% Bambara nut milk: 90% milk, FBM4 = Ice cream from 20% Bambara nut milk: 80% milk, FBM5 = Ice cream from 30% Bambara nut milk: 70% milk, FBM6 = Ice cream from 40% Bambara nut milk: 60% milk, FBM7 = Ice cream from 50% Bambara nut milk: 50% milk
Conclusion
Quality indices of ice cream from dairy milk partially substituted with Bambara groundnut extract have been investigated. Plant milk combined with Bambara groundnut extract, whilst enhancing the protein and carbohydrate of ice cream, lowered the fat content. The use of Bambara groundnut extract resulted in increased concentrations of some micronutrients particularly calcium, iron, potassium and magnesium. Nutritional, physicochemical, and sensory properties of ice cream samples with dairy milk mixed with Bambara groundnut extract significantly differed (p<0.05), although those of 30% Bambara groundnut extract resembled (p>0.05) to the milk control. Anti-nutrient content of Bambara groundnut appeared not to adversely affect the ice cream. From the sensory point of view, the most acceptable appears to be sample FBM4, specific to such parameters like colour, taste, smoothness, mouthfeel, sweetness, after taste and overall acceptability. This current work demonstrated that a desirable ice cream can be produced by partially substituting dairy milk with up to 30% Bambara groundnut extract. The direction of future work should be subject this Bambara groundnut extract formulated ice cream to different refrigerated packaging/storage conditions to test which one obtains more favourable quality attributes.