Introduction
The role of colostrum for a new born during the first few days of life is critical due to providing passive immunity. Dromedary colostrum differs from dromedary milk since it contains low amounts of lactose and fat, but a high level of proteins, especially immunoglobulins, serum albumin and lactoferrin (Jrad et al., 2014). Dromedary colostrum and milk protein composition and structure differs from those of milk and colostrum proteins of the other mammals. Interestingly, dromedary colostrum and milk are closer to human milk than any other ruminant milk since they are rich in lactoferrin and free of allergenic β-lactoglobulin, the major bovine whey protein (El-Hatmi et al., 2015).
Dromedary colostrum and milk are a rich source of antioxidants (Jrad et al., 2014). Antioxidants may positively affect human health by protecting the body against oxidative stress which is involved in many diseases like cardiovascular diseases, cancer, diabetes and Alzheimer (Zuo et al., 2015). Consumers are becoming more and more aware of the relation between diet and disease and greatly concerned about functional foods and dietary supplements as an alternative to medication therapy (Yu et al., 2015). Colostrum and milk-based products have received much attention. Indeed, several investigations have recommended the use of colostrum for passive immunization of animal and human beings, improvements in gastrointestinal disorders, athletes’ performance and combatting oxidative stress (Borad et al., 2020; Chiang et al., 2017). Therefore, it is of interest to create a colostrum and milk bank for new born that cannot be fed by their own mothers immediately after birth and for the preparation of products that can be used as feed supplements for human.
Milk is highly perishable owing to the action of spoilage bacteria since it is an excellent medium for their growth (Perin et al., 2019). Colostrum is an unutilized product in the dairy industry because of its poor heat stability owing to immunoglobulins, which are highly thermo-labile proteins (Borad et al., 2020). Different methods have been developed for the preservation of colostrum and milk ensuring the safety and enlarging the consumption spectra. However, dried formulation is the most preferred form to preserve the immunotherapeutic virtues. They are easy to preserve, transport, reconstitute and administer. The drying process could expand applications of colostrum and milk, or even make them available globally (Ho et al., 2019).
Freeze-drying or lyophilisation is recognized as an excellent dehydration process for heat sensitive products. This process minimizes the degradation reactions and maintains adequate physical, chemical, and biological stability of the product during long-term storage at ambient temperature (Ibrahim and Khalifa, 2015).
Until now, few researchers have reported some information about the physicochemical and the sensory properties of freeze-dried dromedary milk powder. However, to the best of our knowledge, no studies are dedicated to freeze-dried dromedary colostrum powder. The antioxidant activities of freeze-dried dromedary colostrum and milk using various in vitro tests have never been investigated. Therefore, the aim of the present work is to prepare dromedary skim colostrum and milk powder by the freeze-drying method and to analyse their physicochemical and antioxidant properties.
Materials and methods
Materials
Colostrum and milk were obtained from ten multiparous dromedaries (Camelus dromedarius) belonging to the experimental station of the Livestock and Wildlife Laboratory (Arid Lands Institute, Medenine, Tunisia). Colostrum samples were collected within 24 h post-partum during the year 2017. Dromedary milk samples were collected between the second and third month post-partum during the year 2017. All reagents used were of analytical grade.
Preparation of freeze-dried dromedary skim colostrum and milk
Dromedary colostrum and milk samples (200 mL) were defatted by centrifugation at 5000 g for 30 min at 4 °C. Thereafter, dromedary skim colostrum and milk were immediately placed in especially glass bottles for freeze-drying and stored at -80 °C for 24 h. The frozen glass bottles were then freeze-dried at -50 °C for 48 h under vacuum (0.05 mbar) using a freeze-dryer (Christ Gamma, Germany) according to the manufacturer’s instructions.
Chemical analysis
The contents of moisture, total protein and ash were measured according to AOAC (2000) methods. Oven dry method was used for the determination of the free moisture content. Total protein was determined by the Kjeldahl method, while ash content was measured by ignition at 550 °C in an electric Muffle furnace. Lactose content was determined using phenol-sulfuric acid method (Masuko et al., 2005). Mineral content was analysed using the atomic absorption spectrophotometry (Shimadzu AA-6800, Shimadzu, Germany).
Colour measurement
Colour parameters were determined using a Chroma Meter CR-400/410 (Konica Minolta, Japan). The results were expressed in L*, a* and b* values, in which L* is a measure of lightness, a* represents the chromatic scale from green to red and b* represents the chromatic scale from blue to yellow. The Chroma Meter was calibrated with standard plate prior to performing the measurement. From L*a*b* colour system, whiteness of powders was calculated using the following equation (Ho et al., 2019).
Whiteness = 100 − [(100 – L*) 2 + a* 2 + b* 2] 1/2
Bulk density
Bulk density and tapped bulk density were evaluated as described by ISO 8967/IDF 134:2005 method. Samples were allowed to flow freely up to the 100 mL mark into a pre-weighted 100-mL graduated cylinder. The mass of 100 mL powder was calculated. Tapped bulk density was determined thereafter by manually tapping the cylinder about 50 times from a height of 10 cm on a solid marble surface to evaluate the final volume until a steady volume was obtained. The bulk density and tapped bulk density were then estimated using the following equations:
Bulk density (g/ml) = powder weight (g)/ vessel volume (mL)
Tapped bulk density (g/mL) = powder weight (g)/ vessel volume after tapping (mL)
Hausner ratio (HR) were calculated as follows: HR = tapped bulk density/bulk density
Protein solubility
Protein solubility was determined according to the method of Tsumura et al. (2005). Samples were dispersed in distilled water at 10 mg/mL and the pH of the mixture was adjusted to different pH values from 3.0 to 9.0 with 2 N HCl or 2 N NaOH solutions. The mixtures were stirred for 10 min at room temperature (25 °C) and then centrifuged at 8000 g for 10 min. Protein content in the supernatant was determined by the Kjeldahl method. Protein solubility was calculated as follows:
Protein solubility (%)= (Supernatant protein content)/ (Sample protein content) ×100
Antioxidant activity
DPPH radical-scavenging activity
The DPPH (2,2-diphenyl-1-picrylhydrazyl) radical-scavenging activity was determined as described by Bersuder et al. (1998) with slight modifications. A volume of 1.0 mL of each sample at various concentrations (1.0-7.0 mg/mL) was added to 1.0 mL of an ethanol solution of DPPH (125 µM). The mixtures were incubated for 60 min in the dark. For the control, distilled water was used rather than the sample. The absorbance of the resultant solution was read at 517 nm using a UV-Visible spectrophotometer (Cecil CE 2041, Cambridge, UK). The DPPH radical-scavenging activity was calculated as follows:
DPPH radical-scavenging activity (%) = [(A control – A sample)/A control] ×100
Where A control and A sample represented the absorbance of the control and the sample reaction, respectively.
ABTS radical-scavenging activity
The ABTS (2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical-scavenging activity was conducted using the method of Re et al. (1999) with slight modifications. Samples (250 µL) at different concentrations (0.25-1.0 mg/mL) were mixed with 1.0 mL of diluted ABTS solution. The mixture was incubated at room temperature for 10 min in darkness. The absorbance was measured at 734 nm using a UV-Visible spectrophotometer (Cecil CE 2041, Cambridge, UK). The control was performed using distilled water. The ABTS radical-scavenging activity was calculated as described for DPPH analysis.
Ferric reducing antioxidant power
The ability to reduce ferric iron was determined using the method of Wu et al. (2003) with slight modifications. An aliquot of 1.0 mL of each sample at different concentrations (1-20 mg/mL) was mixed with 1.0 mL of phosphate buffer (0.2 M, pH 6.6) and 1.0 mL of 1 % (w/v) potassium ferricyanide solution. The mixtures were incubated at 50 °C for 20 min. Then, 1.0 mL of 10 % trichloroacetic acid was added and the reaction mixtures were centrifuged for 10 min at 3000 g. Thereafter, a volume of 1.0 mL of the supernatant was mixed with 1.0 mL of distilled water and 0.2 mL of 0.1% (w/v) ferric chloride. After 10 min reaction, the absorbance was measured at 700 nm using a UV-Visible spectrophotometer (Cecil CE 2041, Cambridge, UK).
Ferrous ion chelating activity
The ferrous ion chelating activity was measured by the method of Zhu et al. (2006) with slight modifications. A volume of 1.0 mL of each sample at different concentrations (1-10 mg/mL) was added to 1.0 mL of distilled water and 0.05 mL of a solution of FeCl 2 (2 mM). The mixtures were incubated for 30 s and the reactions were initiated by adding 0.1 mL of ferrozine solution (5 mM). After 10 min reaction, the absorbance was measured at 562 nm using a UV-Visible spectrophotometer (Cecil CE 2041, Cambridge, UK) and the chelating activity was calculated using the same equation for DPPH analysis.
Statistical analysis
Statistical analysis of results was conducted using XLSTAT (Addinsoft, 2019). Each experiment was performed in triplicate, and data were expressed as mean ± standard deviation. Statistical comparisons of the results were subjected to one-way analysis of variance (ANOVA) followed by Tukey’s test using a probability value of 5%.
Results and discussion
Chemical composition of dromedary skim colostrum and milk and powder
Chemical composition of freeze-dried dromedary colostrum and milk and powder is shown in Table 1. The results revealed that the moisture content of freeze-dried dromedary skim colostrum and milk was lower because of freeze-drying. The moisture content of skim colostrum powder (7.22 %) was higher than that of skim milk powder (4.16 %). Likewise, the protein content in skim colostrum powder (65.10 %) was greater compared to skim milk powder (44.62 %). Interestingly, freeze-dried dromedary skim colostrum is an important source of proteins. El-Hatmi et al. (2007) reported that dromedary colostrum contain higher amount of lactoferrin, serum albumin and immunoglobulins compared to dromedary mature milk. However, skim milk powder possessed higher lactose and ash content compared to skim colostrum powder. The analysis of mineral content revealed that calcium level, which is necessary to bone growth of the new born, was greater in dromedary skim colostrum powder than dromedary skim milk powder. Similar results were found by Jrad et al. (2015). In addition, skim colostrum powder contained higher amounts of magnesium compared to skim milk powder. However, this latter contained higher level of potassium, sodium and phosphorus than skim colostrum powder. The iron level was not significantly (p > 0.05) different between freeze-dried skim colostrum and milk powder. The Changes in the composition of the freeze-dried colostrum and milk can be explained as a function of the freeze-drying process. These changes coincided with changes in moisture content (Ibrahim and Khalifa, 2015).
Table 1. Chemical composition of dromedary skim colostrum and milk and powders
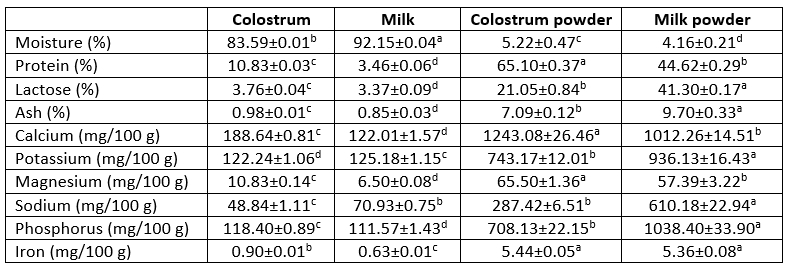
Averages ± Standard deviation (SD) of three replicates
a-dValues within the same row with different superscripts differed significantly (p<0.05)
Colour parameters of dromedary skim colostrum and milk and powder
Colour of any food product is directly related to its overall acceptability by the consumers. Colour measurement of freeze-dried dromedary skim colostrum and milk and powder is presented in Table 2. There was no significant difference in the lightness L* between skim colostrum and milk powder. However, fresh skim milk was lighter than fresh skim colostrum. Fresh skim colostrum and powder were redder than fresh skim milk and powder whereas the latter ones were whiter than fresh skim colostrum and powder. Skim milk powder was yellower than skim colostrum powder while it was the inverse when colostrum and milk were fresh. Chemical composition of skim colostrum and milk powder influence widely their colour. Indeed, dromedary colostrum is richer in lactoferrin, an iron-binding protein (Jrad et al., 2019), which may give it redder colour while dromedary milk is richer in casein (Jrad et al., 2014), which may explain the higher whiteness of dromedary milk powder. The differences in colour between fresh and freeze-dried dromedary colostrum and milk are attributed to the freeze-drying process.
Table 2. Colour parameters of dromedary skim colostrum and milk and powders
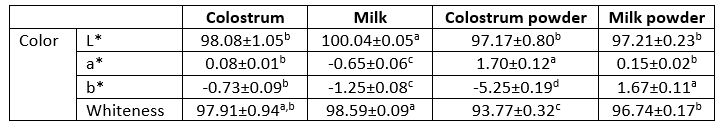
Averages ± Standard deviation (SD) of three replicates
a-dValues within the same row with different superscripts differed significantly (p<0.05)
Table 3. Physical properties of freeze-dried dromedary skim colostrum and milk powder

Averages ± Standard deviation (SD) of three replicates
a-bValues within the same row with different superscripts differed significantly (p<0.05)
Physical properties of freeze-dried dromedary colostrum and milk
The bulk density and tapped bulk density of freeze-dried dromedary skim colostrum and milk are shown in Table 3. The significantly lower bulk and tapped bulk density for freeze-dried skim colostrum compared to freeze-dried skim milk could be attributed to the difference in their composition and the size distribution of particles. High bulk and tapped bulk density is desirable since it implies reduced packaging, storage and transport costs of powder (Singh Banjare et al., 2019). Hausner ratio is an indicator of the cohesion of the milk powder. Freeze-dried dromedary skim colostrum and milk possessed a Hausner ratio of 1.37 and 1.23, respectively. Based on these findings, skim colostrum and milk powder showed fairly free flowing ability indicating the efficiency of freeze-drying process as indicted by Sulieman et al. (2014), who reported that a Hausner ratio of 1 to 1.25 indicates free flowing ability of powder particles, ratio of 1.25 to 1.4 indicates fairly free flowing powder and ratios greater than 1.4 are cohesive and do not flow well.
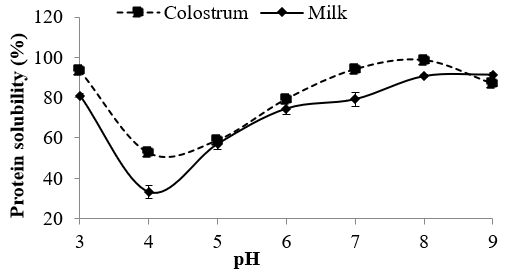
Figure 1. Protein solubility of freeze-dried dromedary skim colostrum and milk powder
Protein solubility
Solubility of proteins is one of the most important functional attributes required for use in many functional applications. Solubility of freeze-dried dromedary skim colostrum and milk powder at different pH values is depicted in Figure 1. Protein solubility of both skim colostrum and milk powder was minimum at pH 4.0 (52.37 % and 33.11 %, respectively), which is near to the isoelectric point of dromedary caseins. Protein solubility at pH 4.0 for dromedary colostrum was significantly higher (p <0.05) than that of colostrum. This result may be explained by the fact that dromedary milk is richer in caseins compared to colostrum (Jrad et al., 2014). Protein solubility of dromedary skim colostrum and milk increased below and above pH 4.0. The solubility was maximum at pH 8.0 for dromedary skim colostrum powder (98.94 %) and at pH 9.0 (91.32 %) for dromedary skim milk powder. Therefore, skim colostrum and milk powder could be useful in the formulation food systems for various applications.
Antioxidant activity of freeze-dried dromedary skim colostrum and milk
In the current work, the antioxidant activity was assessed using various assays owing to the complexity of oxidative processes as well as the various antioxidant mechanisms by which antioxidant compounds may act (Brandelli et al., 2015).
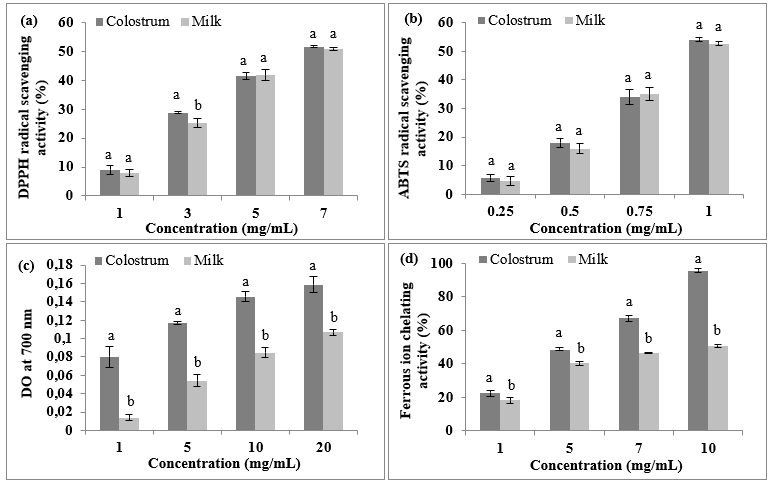
Figure 2. Antioxidant activity of freeze-dried dromedary skim colostrum and milk powder as a function of their concentrations: (a) DPPH radical-scavenging activity, (b) ABTS radical scavenging activity and (c) ferric reducing power assay and (d) Ferrous ion chelating activity
DPPH and ABTS radical-scavenging activities
The DPPH and ABTS radical-scavenging activities of freeze-dried dromedary skim colostrum and milk powder are shown in Figure 2a and Figure 2b, respectively. Increasing the concentration of dromedary skim colostrum and milk resulted in increased DPPH and ABTS radical-scavenging activities (p<0.05). The DPPH and ABTS radical scavenging activities of skim colostrum and milk powder were almost similar. However, Jrad et al. (2014) found a higher ABTS scavenging activity for dromedary milk compared to dromedary colostrum. The IC 50 values of dromedary skim colostrum and milk powder, defined as concentration of sample required to inhibit 50 % of the initial radical concentration, were 6.07±0.12 mg/mL and 6.67±0.19 mg/mL in the DPPH test and 0.97±0.02 and 0.95±0.01 mg/mL in the ABTS tests, respectively. The low IC 50 values displayed high radical scavenging abilities. The obtained results suggest that colostrum and milk can donate hydrogen or electrons able to react with free radicals and transform them to more stable products and consequently, preventing oxidative stress-related diseases. Several compounds like proteins, vitamins, enzymes and polyphenols are considered as antioxidant in milk (Khan et al., 2019). The difference in scavenging capacity of radicals could be attributed to their solubility and diffusivity. DPPH is dissolved in ethanol and may not easily diffuse to target peptides in aqueous solution. In contrast, ABTS, a water-soluble radical, could easily reach peptides in the aqueous solution. Hence, a report of higher activity with the ABTS test does not necessarily suggest a stronger DPPH-scavenging activity (Corrêa et al., 2011).
Ferric reducing antioxidant power (FRAP)
Figure 2c illustrates the FRAP of freeze-dried dromedary colostrum and milk powder at different concentrations. The reducing power of skim colostrum and milk powder exhibited a dose-dependent manner, the FRAP increased as the concentration of samples increased as for the DPPH and ABTS radical scavenging activity tests. Skim colostrum powder had significantly (p<0.05) higher reducing power than skim milk powder at all the tested concentrations. The observed difference in the FRAP could be related to the species-specific milk chemical composition, with particular regard to protein percentage between dromedary colostrum and milk powder. In fact, the FRAP might be attributed especially to cysteine amino acid (Mal et al., 2018).
Ferrous ion chelating activity
The ferrous ion chelating activity of freeze-dried dromedary colostrum and milk powder at different concentrations are depicted in Figure 2d. Dromedary skim colostrum and milk displayed ferrous chelating activity in a dose dependent manner. Skim colostrum, with higher ferric reducing power, had also higher ferrous ion chelating activity at all the tested concentrations compared to dromedary skim milk powder (p<0.05). IC 50 value of skim colostrum powder was 5.30±0.13 mg/mL while it was 8.23±0.25 mg/mL for skim milk powder. These results might be attributed to the richness of colostrum in lactoferrin (El-Hatmi et al., 2007). The chelating activity of dromedary skim milk found by Al-Shamsi et al. (2018) did not exceed 35 %. The difference in the chelating activity could be attributed to the difference in the chemical composition of dromedary colostrum and milk powder.
Conclusions
In this work, dromedary skim colostrum and powder were prepared through the freeze-drying process. The physicochemical and antioxidant properties of the powders were investigated. Freeze-dried dromedary colostrum and milk powder present an important source of proteins and minerals. Bulk density and tapped bulk density of skim colostrum and milk powder were within the acceptable level of dairy powders. Dromedary colostrum powder exhibited higher antioxidant activities in terms of ferric reducing power and metal chelating activity compared to dromedary milk powder. The differences in the antioxidant activities is due to the difference of the chemical composition of colostrum and milk powders. Therefore, freeze-drying process could be an effective method for producing dried powder from dromedary skim colostrum and milk with nutritional and antioxidant properties. Further studies should be conducted to investigate the changes in physicochemical composition and antioxidant activities of freeze-dried dromedary colostrum and milk powder with progression of storage period.
Fizikalno-kemijska i antioksidacijska svojstva liofiliziranog devinog kolostruma i mlijeka u prahu
Sažetak
Cilj ovog istraživanja bio je utvrditi fizikalno-kemijska svojstva i antioksidativnu aktivnost devinog obranog kolostruma i mlijeka u prahu obrađenog postupkom liofilizacije. Dobiveni rezultati pokazali su da je obrani prah kolostruma imao veći udio proteina u usporedbi s mlijekom u prahu, koje je s druge strane imalo veći udio laktoze i pepela. Analiza mineralnog sastava pokazala je da su koncentracije kalcija i magnezija veće u obrađenom prahu kolostruma, dok se koncentracija željeza nije bitno razlikovala između obranog kolostruma i mlijeka u prahu. Mjerenja parametara boje pokazala su da je devin kolostrum u prahu crveniji, ali manje žut i bijel od obranog devinog mlijeka u prahu. Nadalje, obrano devino mlijeko u prahu imalo je veću gustoću i veću nasipnu gustoću. Topljivost proteina u obranom kolostrumu u prahu bila je znatno veća nego u obranom mlijeka u prahu, i to u širokom rasponu pH (3-8). Antioksidacijska aktivnosti određivana je korištenjem različitih in vitro testova, uključujući 2,2-difenil-l-pikrylhidrazil (DPPH) i 2,2'azino-bis (3-etilbenztiazolin-6-sulfonska kiselina) (ABTS) reducirajuću aktivnosti radikala, kao i FRAP metodu te kapacitet keliranja željeza. Antioksidacijska aktivnost obje vrste ispitivanih uzoraka pokazala se ovisnom o ispitivanoj količini uzorka. Aktivnosti redukcije radikala DPPH i ABTS metodama bile su slične za obrani kolostrum i mlijeko u prahu, dok je aktivnost dobivena FRAP metodom te sposobnost keliranja željeza bila izraženija u obranom devinom kolostruma, bez obzira na ispitivanu koncentraciju. Stoga se postupak liofilizacije može upotrijebiti kao djelotvoran način proizvodnje praška iz obranog devinog kolostruma i mlijeka u prahu s očuvanom hranjivom vrijednosti i antioksidacijskim svojstvima.
Ključne riječi: liofilizacija; hranjiva vrijednost; topivost proteina; sposobnost redukcije radikala; sposobnost keliranja
References
https://doi.org/10.1007/s11746-998-0030-y
https://doi.org/10.1016/j.lwt.2019.108719
https://doi.org/10.1016/j.foodres.2015.01.016
https://doi.org/10.3390/molecules22030456
https://doi.org/10.1002/jsfa.4446
https://doi.org/10.1016/j.smallrumres.2006.04.001
https://doi.org/10.15567/mljekarstvo.2015.0302
https://doi.org/10.1016/j.foodchem.2019.05.122
https://doi.org/10.1007/s13594-013-0154-1
https://doi.org/10.1556/066.2015.44.0034
https://doi.org/10.1186/s12944-019-0969-8
https://doi.org/10.1111/jfbc.12660
https://doi.org/10.1016/j.ab.2004.12.001
https://doi.org/10.1016/s0891-5849(98)00315-3
https://doi.org/10.17113/ftb.57.03.19.6228
https://doi.org/10.1016/j.lwt.2004.06.007
https://doi.org/10.1016/s0963-9969(03)00104-2
