Introduction
In recent years, functional food producers have been showing the tendency to diversify their product palette by creating a variety of low-fat and probiotic bacteria-containing foods. Several countries around the world have major commercial markets relating to this production. Cheese is one of those products specific to certain parts of the land. Due to their regional uniqueness, cheeses play a vital role in this sector (Aydogdu and Kok Tas, 2021). A demand for reduced-fat, low-fat, and fat-free cheeses has risen dramatically as well (Anvari and Joyner (Melito), 2019). Reducing fat in foods like cheese, on the other hand, creates some flavour and textural defects due to the lack of fat as a flavour compound solvent or the suppression of specific enzymatic reactions required for flavour compound synthesis (Mohamed, 2015). Fat substitutes are one of the most common strategies for preventing the taste and texture problems produced by fat reduction. Inulin is the most used fat substitute in dairy products. Inulin is a fructo-oligosaccharide and a soluble dietary fibre. Form the structural aspect, inulin is a fructan with a terminal glucose unit that's constituted of a mix of fructose polymers and oligomers with chain lengths ranging from 2 to 60 fructose units and is linked by β (2-1) bonds (Moghiseh et al., 2021). Compared to some other prebiotics, inulin can promote better fermentation and bacterial growth. It also promotes microbial activity during cheese ripening, bringing low-fat cheeses' quality and functional characteristics closer to those of full-fat cheeses (Zhang et al., 2021).
Due to its buffering activity in acidic conditions during digestion in the gastrointestinal tract and its protective function in the stomach, cheese is the most effective carrier for probiotic bacteria. At the start of ripening, cheese has a high total lactic acid bacteria count. Bifidobacterium spp. ( B. bifidum, B. longum, B. infantis, etc.), Lactobacilli spp. ( L. acidophilus, L. casei, L. paracasei, L. rhamnosus, L. plantarum, etc.) and other probiotic microorganisms are successfully used in different cheeses (Hammam and Ahmed, 2019).
Tulum cheese has a rich aromatic profile and is one of Turkey's most popular cheeses. "Tulum" is the goatskin that is used as a packaging material for the ripening of Tulum cheese (Demirci et al., 2021b). Cheeses ripened in an animal skin sack have a strong aroma, flavour and odor and a specific granulated or smooth texture (Kalit et al., 2020). Today, plastic packaging is being used for the ripening of Tulum cheese. It is crumbly, white or cream-colored and is dispersible in the mouth. It is buttery having a strong flavour with high fat content, and has semi hard texture (Hayaloglu et al., 2007).
Lactic acid bacteria diversity of traditional Tulum cheese ripened in goat skin (Demirci et al., 2021b), volatile aroma components (Atik et al., 2021), some properties of Erzincan Tulum cheeses produced with different probiotic cultures and materials in packaging (Akarca, 2020; Tomar et al., 2018), and the use of probiotic Enterococcus faecium and Enterococcus durum in İzmir Tulum cheese (Yerlikaya and Akbulut, 2019) have already been investigated. The microbiology, fatty acid composition, and volatile profile of traditional Sogle Tulum cheese (goat's skin bag) (Gursoy et al., 2018), some properties of low-fat Tulum cheeses (Oluk et al., 2014), and volatile aroma components of Divle Tulum cheese (Ozturkoglu-Budak et al., 2016) were also studied. However, no research has been identified on the use of inulin in the production of probiotic and low-fat Tulum cheese.
The goal of this study was to determine the survivability of probiotic bacteria during the ripening period (90 days) in low-fat and functional Tulum cheese produced by the addition of inulin and the inclusion of probiotic bacteria ( Bifidobacterium BB-12 and Lactobacillus acidophilus LA-5). Some chemical characteristics, volatile aroma components, and changes in sensory attributes were also investigated.
Materials and methods
Tulum cheese production
Raw cow's milk was semi-skimmed to adjust the milk fat content to 1.5 %. After that, it was pasteurized for 5 minutes at 72 °C and separated into three parts. The first part of semi-skimmed milk was prepared without any additives as inulin (control-YC). Inulin was added to the other two semi-skimmed milk groups at a rate of 1 % (Y1) and 2 % (Y2), respectively (50 oC). 1 % mesophilic starter culture (R-707, FD-DVS, Chr Hansen) and 1 % probiotic culture ( Bifidobacterium BB-12® and Lactobacillus acidophilus LA-5 ® (FD-DVS nu-trish ®, Chr Hansen) (>10 7) were added to each group (35±1 °C). For 15 minutes, milk was allowed to pre-maturate. Then, 0.02 % of 40 % CaCl 2 solution was added to the milk. Liquid rennet (Naturen Mandra 175, Chr Hansen) was added when the pH of the milk reached 6.50 (with Calculation of Yeast Strength). Fermentation took place for 60 minutes at 35±2 °C. The cut clot was filtered without pressure for the first 20 minutes after the coagulation process has been completed. After that, stepwise suppression was used. Curds were procured and chopped into 3-4 mm 3 pieces. In a chilly environment, the pressing process was conducted for about 14-15 hours. The curds were then chopped into smaller pieces and the salt was added (2.5 % w/w). The salted cheeses were mixed on a clot and dried until they reached around 65 % dry matter. Tulum cheeses were then squeezed into one-kilogram white plastic barrels with no air holes. Inulin added probiotic low-fat Tulum cheeses were ripened at 6±1 °C till they were analysed on the 1 st, 30 th, 60 th, and 90 th days. The study was performed in a triplicate (Figure 1).

Figure 1. Low-fat probiotic Tulum cheeses (YC: Control group (without inulin), Y1: 1 % inulin added group, Y2: 2 % inulin added group)
Physicochemical analysis
The pH value of the samples was determined using a WTW pH 315 (Weilheim, Germany) digital pH meter (AOAC, 1997).
Microbiological examination
Under aseptic conditions 10 g of Tulum cheese was added to 90 mL of sterile Ringer's (1/10) solution and 1 mL of this dilution was transferred into 9 mL of sterile Ringer's solution. Then, serial dilutions were prepared. The materials were microbiologically analyzed using the spread plate method. The MRS-NNLP (nalidixic acid, neomycin sulfate, lithium chloride, and paromomycin sulfate) agar medium was employed, which contained neomycin sulfate (100 mg/L), nalidixic acid (50 mg/L), lithium chloride (3000 mg/L), and paronomycin sulfate (200 mg/L) in Bifidobacterium BB12 count. The NNLP combination was mixed to sterile MRS (de Man, Rogosa, and Sharpe) agar (Merck, Germany) medium, which was sterilized with a 0.45 µm disposable sterile filter just before pouring into petri plates. Petri dishes were incubated for 72 hours at 37 °C in anaerobic jars. MRS-Sorbitol agar was used to count Lactobacillus acidophilus LA-5. Before adding sorbitol, MRS agar was sterilized, and 10 % (w/v) D-Sorbitol solution (10 mL) was added to MRS agar (90 mL) medium using a sterile 0.45 µm filter. Before adding sorbitol, MRS agar was sterilized, and 10 % (w/v) D-Sorbitol solution (10 mL) was added to MRS agar (90 mL) medium using a sterile 0.45 µm filter. Petri dishes were incubated for 72 hours at 37 °C in anaerobic jars (Dave and Shah, 1997). Anaerobic kits from Anaerocult (Merck, Germany) were used to create the anaerobic environment. The lactobacilli were counted using MRS agar. Petri dishes were incubated for 72 hours in an anaerobic jar at 37 °C. After 48 hours of aerobic incubation at 37 °C, lactococci were counted on M17 agar (Gardini et al., 1999).
Plate Count Agar (Merck, Germany) was used to determine the total number of mesophilic aerobic microorganisms. Petri dishes were incubated for 72 hours at 30 °C in an aerobic atmosphere (Anonymous, 1987). For yeast-mould counting, 1 mL of the prepared 1:10 dilution was inoculated into Potato Dextrose Agar (Merck, Germany) medium. For 4-5 days, the cultivated petri dishes were incubated at 25 °C. 1 mL of a 1:10 dilution was obtained and put into Merck brand EMB (Eosin Methylen-Blue Lactose Sucrose) medium for coliform detection. Petri dishes were incubated for 24-48 hours at 37 °C. According to Halkman (2005), colonies growing on petri plates were counted. Using a logarithmic transformation, the results are reported as log cfu/g.
Volatile composition determination by SPME-GC-MS
For the analysis of carbonyl compounds, Yang and Peppard's (1994) method was utilized. 3.0 g of frozen Tulum cheese samples were placed in a 15 mL silicone septal vial (Supelco 27159 ml transparent PTFE/Silicone septa Cap) for solid phase microextraction (SPME) analysis. The samples were first heated for 15 minutes at 45 °C in a heating block without fibre. Using a vial injection, the extraction process was carried out using CAR/PDMS fibre (75 µm Fused Silica, Supelco Ltd., Bellefonte, PA, USA). To absorb volatile compounds from the slide, it was left at 45 °C for 30 minutes. The GC-MS apparatus was used to desorb the volatile components to be extracted, which was done at 250 °C for 5 minutes. The volatile aroma components of the Tulum cheese samples were determined using a Shimadzu GC-2010 gas chromatography system and a Shimadzu MS-QP2010 mass spectrometry system (Shimadzu Corporation, Kyoto, Japan). The following are the conditions for the analysis: The temperature program was kept at 40 °C for 2 minutes on column Rx-5sil MS (30 m x 0.25 mm, i = 0.25 um film thickness; Restek, Bellefonte, catalogue No:13623, PA, USA). It was heated to 250 °C at a rate of 4 °C/min and hold at that point for 5 minutes, with injector and detector temperatures of 250 °C (detector voltage, 70 eV; carrier gas, He; flow rate of 1.61 mL/min). A GC/MS solution software was used to process the data and GC/MS analysis was performed in scanning mode in the range of 40-300 amu. The retention indices (RI) and mass spectra of volatile compounds were compared to analytical standards mass spectra libraries of Wiley-NIST, Tutor, FFNSC (Flavor and Fragrance Natural and Synthetic), and RI values were used to confirm the volatile components.
Sensory analysis
On the 1 st and 90 th days, sensory analyses of Tulum cheeses were conducted. A panel of eight panellists (6 females and 2 males) from the Department of Food Engineering conducted sensory analyses. They ranged in age from 25 to 50 years old and had a prior experience with Tulum cheese evaluation. Until sensory analysis, samples were kept at 4±1 °C. Three-digit numbers were chosen at random to code the samples. The hedonic scale system was used to generate the scores for the sensory analysis of cheese samples, with 9 points being the maximum and 1 point being the minimum. Before beginning to analyse the cheese samples, the panellists, who are experts in sensory analysis, were trained. The panellists tasted samples that were both extremely good and very awful in terms of sensory attributes. They were asked to serve as a reference for evaluating the original samples. Analyses were carried out twice a day, between 10:00 a.m. and 14:00 p.m., for around three days (Lawless and Heymann, 2010). Cross-sectional appearance features such as oily appearance, colour characteristics, and prominence of dietary fibre; structural features such as hardness, greasy-diet fibre feel in the mouth, adhesion/plumbing in the mouth; odour characteristics such as distinctive pleasant odour and acidic odour; and taste characteristics such as oily, pleasant, salty, rancid, and acidic taste were evaluated during the sensory analysis of the samples.
Statistical analysis
The research was carried out in three replications, using three parallel analyses. The SPSS 26.0 program was used to conduct statistical analyses on the samples. Analysis of variance (ANOVA) followed by Duncan multiple comparison tests were used to make paired comparisons of features found statistically significant in ANOVA (Duzgunes et al., 1987). Principal component analysis (PCA) on sensory analysis results (section-appearance, texture, odour, and taste) was used in this work to create a visual representation of the similarity or distance model between a group of objects and to reduce the quantity of the data.
Results and discussion
Physicochemical properties
The difference in pH values between the samples was determined to be statistically significant (p<0.05). Furthermore, it was discovered that throughout ripening, the pH values of the samples decreased, then increased somewhat. Due to the presence of lactic acid bacteria, which convert lactose to lactic acid during cheese manufacture, the pH decreases dramatically. The increase in pH during ripening could be caused by specific yeasts. Yeasts can raise the pH level by deamination amino acids to produce ammonia (Ozturkoglu-Budak et al., 2016). The biological conversion of fatty acids to methyl ketones was also cited as a contributing factor to the rise in pH. (Demirci et al., 2021b). Other investigations have reported a prolonged increase in pH in Divle Tulum cheese (Ozturkoglu-Budak et al., 2016) and traditional Tulum cheese (Demirci et al., 2021b).
Table 1. Microbiological properties of low-fat probiotic Tulum cheeses (log cfu/g)
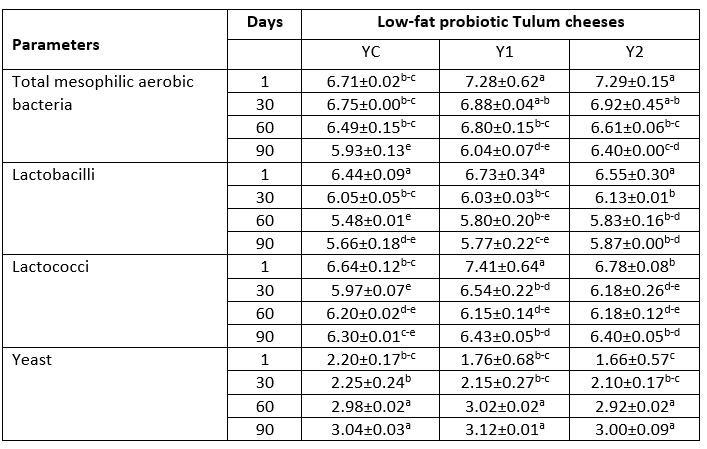
YC: Control group, without inulin Y1: 1 % inulin added control group, Y2: 2 % inulin added control group.
a-e: Lowercase letters indicate that the difference is statistically significant (p<0.05)
Results of microbiology analysis
The numbers of total mesophilic aerobic bacteria, Bifidobacterium BB-12, Lactobacillus acidophilus LA-5, lactobacilli, and lactococci were significantly changed by the addition of inulin (p<0.05) (Table 1). The overall amount of mesophilic aerobic bacteria in Tulum cheese samples decreased steadily over the 90-day ripening period. The total amount of mesophilic aerobic bacteria in the samples containing 1 and 2 % inulin was found to be greater than 10 6 cfu/g at the end of storage. It was also discovered that the sample containing 2 % inulin had the highest number of mesophilic aerobic bacteria, and the addition of inulin enhanced the total number of mesophilic aerobic bacteria.
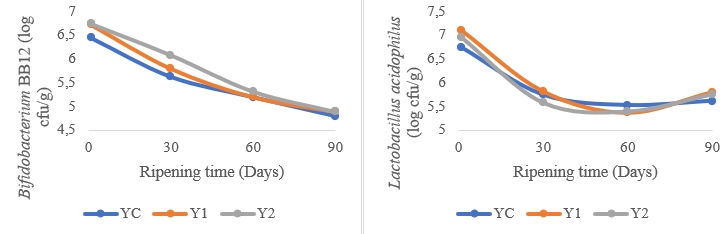
Figure 2. Changes in Bifidobacterium BB-12 and Lactobacillus acidophilus LA-5 log counts during the ripening phase of low-fat probiotic Tulum cheeses (YC: Control group (without inulin), Y1: 1 % inulin added group, Y2: 2 % inulin added group)
Figure 2 shows the changes in Bifidobacterium BB-12 and Lactobacillus acidophilus LA-5 log counts during the ripening stage of low-fat probiotic Tulum cheeses. At the start of ripening, the Bifidobacterium BB-12 count is in the range of 6.44-6.74 log cfu/g, with the sample containing 2 % inulin having the highest value. The number of Bifidobacterium BB-12 bacteria significantly decreased as they ripened. After ripening, inulin was expected to show a protective behavior as well as enough presence of Bifidobacterium BB-12, however, they were less than 5 log cfu/g. At the start of maturation, the sample containing 1 % inulin had the highest L. acidophilus LA-5 count of 7.10 log cfu/g. The sample containing 2 % inulin comes next (6.95 log cfu/g). The L. acidophilus LA-5 count of all samples decreased for 60 days during maturation, however it increased at day 90. At the end of the maturation, the number of L. acidophilus LA-5 was more than 5 log cfu/g. 5.0 to 7.0 log cfu/g viable cells are the recommended number of viable probiotics for health benefits (Hammam and Ahmed, 2019). Low-fat Tulum cheeses contained enough Bifidobacterium BB-12 and L. acidophilus LA-5 to promote customers' health after 60 days of storage and 90 days of storage, respectively. It has also been proposed that to get therapeutic advantages, the number of viable L. acidophilus should be larger than 5 log cfu/g (Gardini et al., 1999). L. acidophilus LA-5 survived better after 90 days of storage, maintaining a cfu/g over 5.5 log. The probiotic data from another study (Tomar et al., 2018) on Tulum cheeses containing L. acidophilus and Bifidobacterium animalis spp. is less than the results reported in this study. The addition of inulin to this scenario is thought to have a favourable influence on the survivability of these bacteria.
Higher populations of Bifidobacterium BB-12 and L. acidophilus LA-5 were found in the sample containing 1 % inulin as a result of maturation. However, it was discovered that increasing the inulin ratio to 2 % had little effect on probiotic bacteria growth towards the end of maturation. Inulin supported the survival of probiotic organisms during the processing and storage of dairy products, specifically by boosting or sustaining the viability of Bifidobacterium spp. and L. acidophilus (Cardarelli et al., 2008). Accordingly, adding inulin to low fat UF-soft cheese considerably boosted the viability of L. acidophilus and Bifidobacterium BB12 after 20 days of storage, after which, their numbers sharply fell. Furthermore, it was noted that the addition of 5 and 7 % inulin during the storage period was more effective than the addition of 1 and 3 % inulin (El-Baz, 2013). Cardarelli et al. (2008) discovered that the populations of Bifidobacterium animalis subsp. lactis and L. acidophilus in petit-suisse cheese containing inulin dropped after storage (28 days) but remained above 10 6-10 7 cfu g -1 throughout the shelf-life of the product. Another study found that adding inulin to synbiotic Fresh Cream cheese had no impact on microbial growth and viability (p>0.05) (Buriti et al., 2007). Ozer et al. (2005) discovered that adding 0.5 and 1.0 % inulin to yogurt had no effect on the development of S. thermophilus, L. bulgaricus, or L. acidophilus LA-5, but dramatically enhanced the growth of Bifidobacterium bifidum BB-02.
Mould and coliform growth were not observed in the samples within this study. At the end of the storage, yeast growth had increased. The yeast values found in this study were lower than those found in other probiotic Tulum cheese studies (Yerlikaya and Akbulut, 2019; Tomar et al., 2018). The microbiological quality of the milk utilized, as well as changes in hygiene and sanitation conditions during manufacturing, were linked to differences in yeast counts in previous similar investigations. The lactobacilli readings of the samples were in the range of 6.44-6.73 log cfu/g at the beginning of ripening and declined to the range of 5.66-5.87 log cfu/g on the 90 th day in the study. The number of lactobacilli increased as the rate of inulin addition increased toward the end of ripening. On the first day of storage, the number of lactococci varied from 6.64 to 7.41 log cfu/g, and by the end of storage, it was in the range of 6.30 to 6.43 log cfu/g. Lactococcus ssp. was a dominant species in probiotic Tulum cheese during ripening period according to Yerlikaya and Akbulut (2019).
Volatile aroma compounds
During the ripening of low-fat probiotic Tulum cheeses, 72 volatile components were determined (Table 2a and 2b). Alcohols (5), aldehydes (8), carboxylic acids (5), esters (4), hydrocarbons (25), ketones (17), terpenes (7), and other components have been identified (1).
Table 2a. Volatile aroma components of low-fat probiotic Tulum cheeses
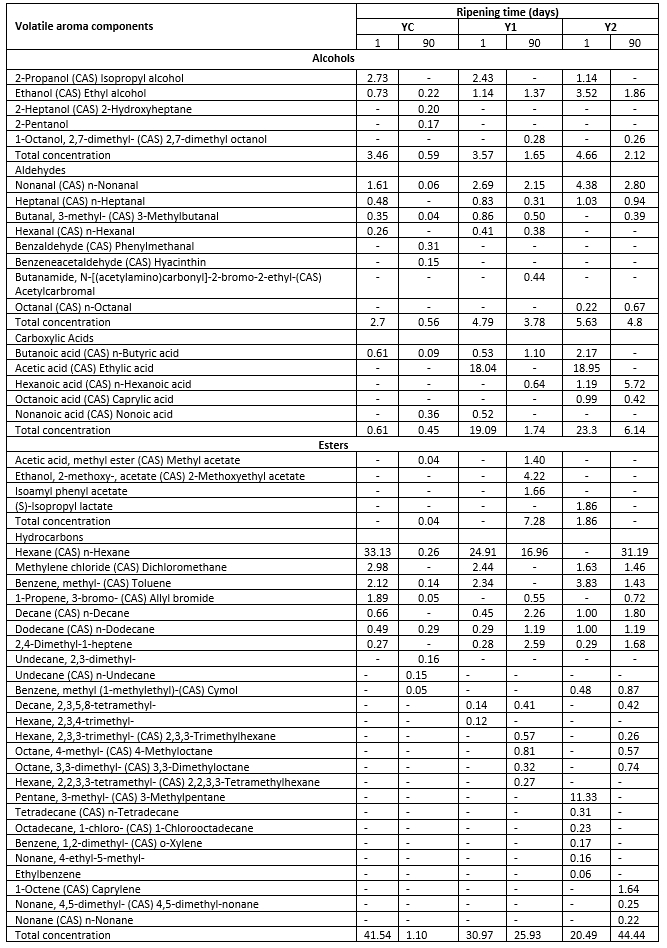
YC: Control group, without inulin Y1: 1 % inulin added control group, Y2: 2 % inulin added control group
Table 2b. Volatile aroma components of low-fat probiotic Tulum cheeses
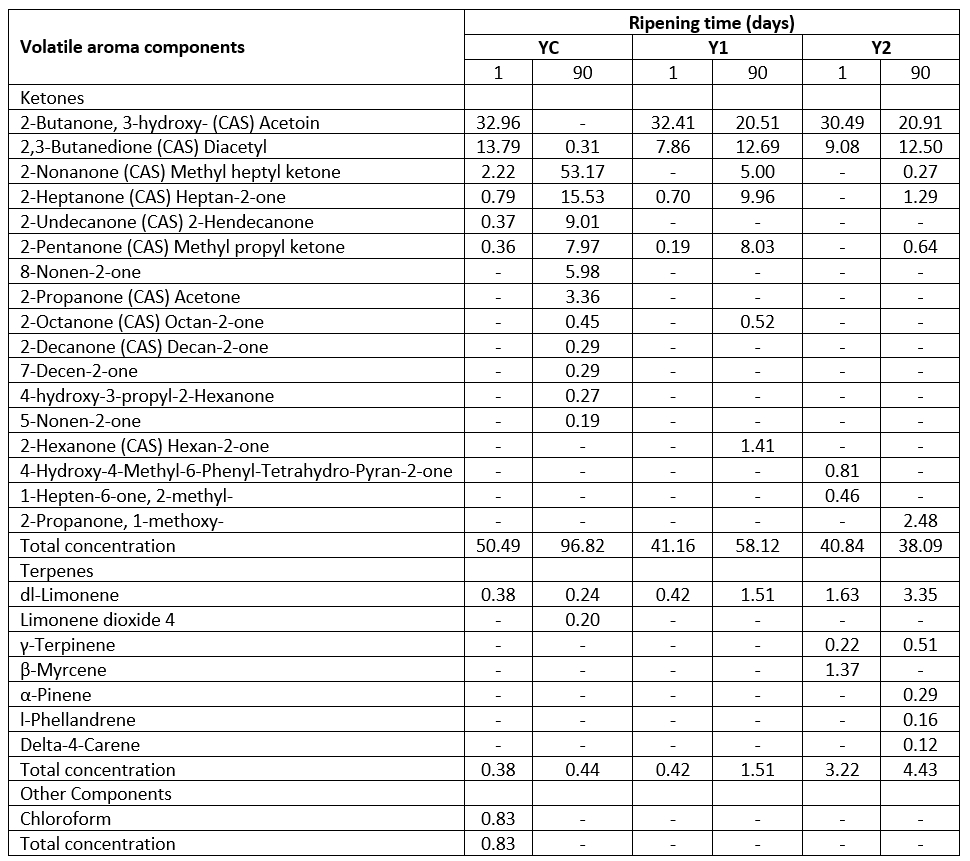
YC: Control group, without inulin Y1: 1 % inulin added control group, Y2: 2 % inulin added control group
Alcohols
Low-fat Tulum cheeses were found to contain a total of five alcohols. On the 90 th day of ripening, 2-heptanol and 2-pentanol were produced in the YC sample. These alcohols, on the other hand, were not produced in the inulin-added samples. Secondary alcohols including 2-heptanol and 2-pentanol result from the enzymatic reduction of methyl ketones. In aged cheese, 2-heptanol has been discovered as an essential component (Atik et al., 2021). On the first day of ripening, 2-propanol was found in all Tulum cheese samples. Tulum cheeses having 1 % and 2 % inulin generated 2,7-dimethyl octanol on the 90 th day. During ripening, all the samples included ethanol. As the amount of inulin increased, so did the ethanol concentration. The ethanol concentration of the YC and Y2 samples decreased as storage progressed. Ethanol is a key component in the production of esters. It is largely made up of lactose breakdown and alanine amino acid catabolism (Gursoy et al., 2018). Lactococcus spp. is also the principal source of ethanol in cheese due to citrate metabolism. Acid-producing mesophilic cheese starters have a limited capability for ethanol production (Karimi et al., 2012).
It was discovered that adding inulin to Tulum cheeses boosted the total alcohol percentage. At the start of ripening, the total content of alcohols ranged between 3.46 and 4.66 %, while it decreased (0.59-2.12 %) at the end of all samples The drop in alcohol content has been attributed to the formation of esters by the reaction of alcohol with acid (Demirci et al., 2021a). In comparison to prior Tulum cheese investigations, within the present one a lower alcohol concentration was determined (Atik et al., 2021; Ozturkoglu-Budak et al., 2016). A possible reason may be the use of pasteurized milk for Tulum cheese production. Hayalolu et al. (2007) found that using raw milk in Tulum cheese production increases alcohol concentrations, however, pasteurization of milk has a negative impact on the formation of alcohol in cheese.
Aldehydes
Most of the aldehydes impart herbaceous, grassy, and malty qualities to the cheese (Curioni and Bosset, 2002). Due to such low detection thresholds, aldehydes may add to the flavour of Tulum cheese (Hayaloglu et al., 2007). Aldehydes are the third key volatiles in Tulum cheeses. The aldehyde with the highest concentration in the samples was n-nonanal. At the end of storage, the concentration of n-nonanal decreased. The n-nonanal concentration was altered by the addition of inulin, and the ratio increased with the increase of the inulin ratio. At the beginning of storage, n-heptanal was identified in the YC sample, but not at the 90 th day. Inulin addition increased the quantities of n-heptanal in the samples. During storage, the n-hexanal ratio in the sample containing 1 % inulin fell from 0.41 to 0.38 %. In the sample containing 2 % inulin, no n-hexanal was identified. In a Tulum cheese sample having only 2 % inulin, n-octanal was identified, and its ratio increased from 0.22 to 0.67 % during storage. The linear chain aldehydes, n-nonanal, n-heptanal, n-hexanal, and n-octanal were determined in the samples. Green grass and floral fragrances distinguish them. Unsaturated fatty acids undergo β-oxidation, which results in the formation of such molecules, which aere essential for Grana Padano and buffalo Mozzarella's fragrance (Curioni and Bosset, 2002).
On the 90 th day of ripening, 3-methylbutanal was discovered in the Y2 sample. It has also been detected in YC and Y1 samples during storage and decreased with time. Further, 3-methylbutanal was produced at a faster rate in the Y1 sample than in the YC sample. 3-methylbutanal has a green malt odour, but at low doses it has a fruity and pleasant odour (Curioni and Bosset, 2002). Cheddar, Camembert, and Emmental cheeses have all been shown to contain 3-methylbutanal (Ozturkoglu-Budak et al., 2016). As a result, it's been determined that it's also applicable to low-fat probiotic Tulum cheese. Benzaldehyde and benzene acetaldehyde aldehydes were generated at the end of ripening in the YC sample, while acetylcarbromal aldehyde was formed in the Y1 sample. In aged cheeses like Camembert, trace quantities of benzaldehyde have been found. In cheese, benzaldehyde, an aromatic aldehyde, imparts a bitter almond flavour (Atik et al., 2021). In the present study, fluctuations in aldehyde concentration were detected during ripening, most probably because aldehydes usually do not accumulate in cheese and are rapidly converted to alcohols or acids because due to fatty acids catabolism, decarboxylation or deamination of amino acids (Dunn and Lindsay, 1985).
Carboxylic acids
The presence and concentrations of carboxylic acids in the present study differed by inulin addition and during ripening stages. It was observed that only butanoic acid was formed at the beginning of ripening in control group (YC) of Tulum cheese, and nonanoic acid was dominant at the end of ripening. When 1 % inulin samples were tested, it was discovered that volatile components of acetic acid, butanoic acid, and nonanoic acid were present at the start of ripening, while volatile components of butanoic acid and hexanoic acid were present at the end of ripening. Acetic acid was shown to be more dominant in the samples with 2 % inulin added, whereas hexanoic acid was predominant at the end of the ripening. Furthermore, it was discovered that the samples containing 2 % inulin had a larger concentration of carboxylic acid components during storage, resulting in the formation of octanoic acid, which was not detected in the other samples. However, it was determined that the total carboxylic acid concentration decreased as the inulin ratio decreased and the samples without inulin had the minimum value.
In low-fat Tulum cheeses, acetic, butanoic, and hexanoic acids were prominent, according to Oluk et al. (2014). Butanoic acid is a fatty acid found in dairy products. Butanoic acid plays a significant role in many cheese types, but too much of it causes rancidity (Curioni and Bosset, 2002). The butanoic acid component was not prominent in this investigation. Lactose is converted to lactate in all cheese types, which can subsequently be broken down by microbes into acetic and propanoic acids. Acetic acid is a significant volatile component that also plays a function in the flavour production of cheese. Sharp aromas are produced by free fatty acids with 4-12 carbons (Atik et al., 2021). The pungent cheesy odour is supposed to be caused by the hexanoic acid and octanoic acid components generated in Tulum cheeses. Furthermore, acetic acid was found to be prominent at the start of ripening in both Y1 and Y2 samples, contributing to the production of cheese flavour. On the 90 th day of storage, however, the presence of acetic acid could not be detected. Acetic acid was discovered to be the most dominating acid at the start of the ripening process of Tulum cheese, whereas octanoic acid, pentanoic acid, and hexanoic acid were discovered at the end of the ripening process (Demirci et al., 2021a). On the 120 th day of ripening, the acetic acid concentration of Divle Tulum cheeses decreased. Lactic acid bacteria are responsible for the production of acetic acid. Ozturkoglu-Budak et al. (2016) found a link between increased mould content and decreased lactic acid bacteria content as ripening progressed. The amount of acetic acid in cheese reflects bifidobacteria metabolism that results in production of acetic acid as a by-product of glucose metabolism via the fructose-6-phosphate shunt metabolic pathway (Karimi et al., 2012). The absence of acetic acid at the end of storage was linked to a decrease in Bifidobacterium BB-12 and lactic acid bacteria levels in this study.
Esters
Esters are useful scent molecules because they can cover aromas created by the short-chain free fatty acids, methyl ketones, and amines (Akarca, 2020). Esters in cheese give fruity and floral odors while reducing the pungency of fatty acids and the harshness of amines (Ziino et al., 2005). Tulum cheese samples contained methyl acetate, 2-methoxyethyl acetate, isoamyl phenyl acetate, and (S)-isopropyl lactate esters, according to this investigation. On the 90 th day of the Y1 sample, methyl acetate, 2-methoxyethyl acetate, and isoamyl phenyl acetate esters were discovered, while (S)-isopropyl lactate was detected on the first day of the Y2 sample. At the start of ripening, samples containing 1 % inulin were devoid of ester, but at day 90 of ripening, they had a higher ester concentration than other samples. The primary esters in low-fat Tulum cheese samples were 3-methylbutyl acetate, ethyl acetate, methyl butanoate, ethyl butanoate, ethyl capronate, and hexyl ethanoate, according to another study. It has been suggested that the existence of esters is linked to lactic acid bacteria's esterase activity (Oluk et al., 2014).
Hydrocarbons
The major volatile category of low-fat probiotic Tulum cheeses was hydrocarbons. There were 25 different types of hydrocarbons identified in this investigation. On the 90 th day of the Y2 sample (44.44 %), the highest rate of total concentration was discovered. On the first day of ripening, YC (41.54 %) and Y1 (30.97 %) samples were followed by YC (41.54 %) and Y1 (30.97 %) samples, respectively. The hydrocarbon ratio of the YC (0 % inulin) and Y1 (1 % inulin) samples declined at the end of the storage but increased in the Y2 (2 % inulin) sample. According to the results, n-hexane is the most prevalent hydrocarbon in Tulum cheese samples. n-hexane was found in the highest concentrations in samples YC (33.13 % 1 st day) and Y1 (24.91 % 1 st day and 16.96 % 90 th day) and Y2 (31.19 % 90 th day). 3-methylpentane (11.33 %) dominated on the first day in the Y2 sample. Other hydrocarbon components were found at lower concentrations, with variations across samples and during ripening. Dichloromethane, toluene, allyl bromide, n-decane, n-dodecane, and 2,4-Dimethyl-1-heptene, as well as n-hexane, are all present in all Tulum cheese samples.
In the study of Tulum cheese, Atik et al. (2021) o-xylene and styrene, Demirci et al. (2021a) styrene, toluene and p-cymene, Gursoy et al. (2018) styrene, Ozturkoglu-Budak et al. (2016) toluene, styrene, and 1,2-dichlorobenzene, Hayaloglu et al. (2007) determined toluene, pentane, hexane, octane, styrene hydrocarbons. On contrary, no styrene found in earlier investigations could be detected in the present study. However, in comparison to previous studies, more hydrocarbons were determined, which is most probably related to the inclusion of probiotics and inulin. Such conclusions are based on the finding that 15 types of hydrocarbons were detected in the samples with inulin added (Y1 and Y2) that were not found in the sample without inulin (YC).
Ketones
Ketones are the second most common volatile group in Tulum cheese samples. However, in terms of total concentration, ketones are the volatile aroma components discovered at the highest rate in the samples (except on the 90 th day of the Y2 sample). On the 90 th day of the YC (without inulin) sample, more ketone types (12 types) were generated, resulting in a total of 17 types of ketones. Also, with the addition of inulin, the ketone type was found to decrease at the beginning and the end of storage. The 2-nonanone component is prominent on the 90 th day of the YC sample (without inulin). 2-nonanone has the highest rate (53.17 %) among ketones, and is associated with flowery, fruity, and peach flavours. On the 90 th day, 2-nonanone developed, which was lacking on the first day of the inulin-containing samples. All the samples showed an increase in 2-nonanone. This component's rise with storage has been linked to fatty acids being converted to methyl ketones by microorganisms during lipolysis (Atik et al., 2021).
On the first day of the YC sample and during the storage of the other samples supplemented with inulin, the acetoin was prevalent (between 20.51 and 32.96 %). Diacetyl is another important ketone component found in Tulum cheese samples, and it is the second most abundant ketone (0.31-13.79 %) in the samples (except for the 90 th day of the YC sample). The breakdown of pyruvate, which is created because of the breakdown of lactose and citrate, results in the formation of acetoin and diacetyl components. Diacetyl is formed in small amounts, whereas acetoin is produced in much larger amounts. Diacetyl is a flavouring agent found in Dutch cheeses such as Quarg and Cottage cheese (Mc Sweeney and Sousa, 2000). In some cheese types, diacetyl can also be metabolized to acetoin, 2,3-butanediol, and 2-butanone molecules (Mc Sweeney and Sousa, 2000; Karimi et al., 2012). Diacetyl contributes to the flavour of cheese by giving it a creamy, buttery flavour (Karimi et al., 2012). Similar results were found in Tulum cheese studies, but there were less ketone types revealed (Gursoy et al., 2018; Akarca, 2020; Atik et al., 2021). When the overall concentration of the samples was evaluated, it was determined that the highest ketone concentration was in the YC sample without inulin, at the beginning and end of storage. This one was followed by Y1 and Y2 samples, respectively, and the ketone concentration decreased with the addition of inulin. In addition, while there was an increase in total ketone concentration in YC and Y1 samples at the end of storage, there was a decrease in Y2 samples.
Terpenes
All Tulum cheeses include dl-limonene, which is more dominating than other terpene components. In samples containing 2 % inulin, terpenes of γ-terpinene, β-myrcene, α-pinene, l-phellandrene, and delta-4-carene were detected. Only on the 90 th day of the YC sample included limonene dioxide 4. The terpenes identified in volatile extracts of roughage ingested by cows and cheese prepared from their milk were studied by Viallon et al. (1999). Limonene, γ-terpinene, β-myrcene, α-pinene, α- and β-phellandrene, and 3-carene components were detected in roughage and cheese samples. The volatile components that resulted from the roughage are the terpene components detected in Tulum cheese samples in this example.
In probiotic Tulum cheese, Akarca (2020) found limonene, α-pinene, α-phellandrene, p-cymene, cymol, β-pinene, and camphene components. Demirci et al. (2021a) found limonene, β-myrcene, p-cymene, α-pinene, and γ-terpinene in various traditional Tulum cheeses, whereas Gursoy et al. (2018) found limonene, γ-terpinene, limonene, and β-caryophyllene. The components of dl-limonene, α-pinene, and o-cymene were determined in another low-fat Tulum cheese study (Oluk et al., 2014).
Other components
In the study, 0.83 % chloroform was determined in the YC sample without inulin supplementation. It is not found in other Tulum cheese samples. Hayaloglu et al. (2007) detected chloroform in Tulum cheeses ripened in goat skin or plastic containers. In addition, it was reported that chloroform compound was determined in trace amounts in other cheese varieties such as Provola, Camembert, Parmigiano and Cheddar (Ziino et al., 2005).
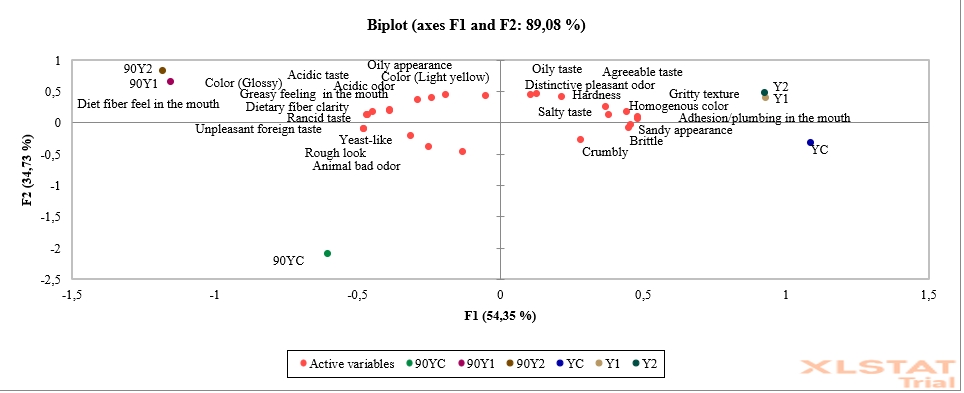
Figure 3. Principal component analysis (PCA) of low-fat probiotic Tulum cheese samples (YC: Control group (without inulin), Y1: 1 % inulin added group, Y2: 2 % inulin added group)
Sensory evaluation and principal component analysis (PCA)
Principal component analysis (PCA) was used to examine the sensory analysis results such as the cross-section appearance, texture, odour, and taste of low-fat probiotic Tulum cheese samples. In this study, the model designed for principal component analysis was carried out on 6 components (1st and 90th day storage samples of Tulum cheeses). These variables account for 89.08 % of the total variation. The first principal component accounts for 54.35 % of total variation, while the second is responsible for 34.73 % (Figure 3). The first basic component's eigenvalue was found to be 13.587, and the second component's eigenvalue was found to be 8.684. When Figure 3 is examined, it is seen that the YC sample is brittle, crumbly and sandy in appearance. It was discovered at the end of the storage that it had a yeast-like, rough look, and an unpleasant foreign taste cheese quality. At the beginning and the end of storage, the Y1 and Y2 samples had similar attributes. At the end of storage, dietary fibre qualities became apparent, and mouth oiliness and dietary fibre sensation increased in inulin supplemented samples. The samples appear to be oilier, glossy, and light yellow. It could also been noted that it had a more acidic taste and odour, and a rotten flavour. In comparison to the control group, the samples which contained inulin had a more acceptable taste, desirable hardness, gritty texture, and homogenous colour.
Conclusion
In this study, inulin was added to low-fat and probiotic Tulum cheese at two different percentages (1 and 2 %). While there were differences in pH and percent oil values of the samples during ripening, there were no differences in water activity or percent salt values, according to the findings of the study. The total number of mesophilic aerobic bacteria in the samples containing inulin was higher than 10 6 cfu/g at the end of storage. At the start of ripening, the number of Bifidobacterium BB-12 cells increased along with the rate of inulin addition. However, the sample containing 1 % inulin had the highest Bifidobacterium BB-12 count at the end of ripening, followed by the sample containing 2 % inulin. During storage, the number of L. acidophilus was higher in the sample containing 1 % inulin. The addition of inulin enhanced the development of probiotics, according to the findings. In addition, the most prevalent hydrocarbons, ketones, aldehydes, and terpenes are n-hexane, acetoin, and diacetyl, n-nonanal, and dl-limonene, respectively. The addition of inulin changed the type and concentrations of volatile aroma components, which differed towards the end of ripening. During the storage period, inulin addition had a good effect on numerous sensory attributes such as oily appearance, colour properties, dietary fibre clarity, hardness, greasy feeling in the mouth, rancid flavour, acidic taste, and odour. At the beginning and the end of storage, samples containing 1 % and 2 % inulin had identical sensory qualities. The addition of inulin to low-fat Tulum cheeses improved their volatile aroma components, microbiological qualities, and sensory properties. It was discovered that adding 1 % inulin to the mix boosted the development of probiotic bacteria even more.
According to these findings, inulin is a suitable material (particularly 1 % inulin) for producing low-fat Tulum cheese with functional and good sensory qualities, and functional food makers can use it successfully.
Funding
This study was supported by the Süleyman Demirel University Scientific Research Project Coordination Unit through project no FDK-2020-7409 and YÖK 100/2000 Doctoral Scholarship Programme.
Utjecaj dodatka inulina na preživljavanje probiotičkih bakterija i udjel hlapljivih spojeva u probiotičkom posnom Tulum siru
Sažetak
U ovom je istraživanju ispitivan utjecaj dodatka probiotičkih bakterija i inulina na mikrobiološku kvalitetu, hlapljive tvari arome i senzorska svojstva nemasnih Tulum sireva tijekom zrenja. Dodavanje inulina posnim Tulum sirevima utjecao je na povećanje udjela hlapljivih tvari arome, te na poboljšanje mikrobiološke kvalitete i senzorskih svojstava. Dodatak inulina pospješio je rast i preživljavanje probiotičkih bakterija. Ukupne koncentracije alkohola, aldehida, karboksilne kiseline, ugljikovodika i terpena povećavale su se proporcionalno s povećanjem količine dodanog inulina te uslijed dozrijevanja. Tijekom skladištenja inulin je povoljno djelovao na brojna senzorska svojstva sireva poput izgleda, teksture, okusa i mirisa. Dodatak 1 % inulina imao je povoljniji učinak na preživljavanje probiotika.
Ključne riječi: posni Tulum sir; inulin; probiotik; hlapljive tvari arome; senzorska svojstva
