Introduction
Soil organic acids are a water-soluble fraction of organic molecules in the rhizosphere. Organic acids (OAs) in soil may be produced by plants and microorganisms or are the result of organic matter decomposition (Adeleke et al. 2017). The concentration of monocarboxylic OAs in soil can reach up to 1 mM. Acetic acid (AA), a monocarboxylic OA, is the smallest OA and is less effective in mobilizing minerals than high molecular weight OAs. Half-life of AA acid can be a few days (Adeleke et al. 2017). AA, also known as ethanoic acid or vinegar, absorbs moisture; is corrosive to metals, and is widely used in industry and household cleaning. Once AA enters the cell, it dissociates into an acetate anion and a proton. Acetate is converted by acetyl-CoA synthetase (ACSS) to produce acetyl-coenzyme A (acetyl-CoA), an essential molecule produced in carbohydrate metabolism and histone acetylation (Pietrocola et al. 2015). Lynch (1977) reported that 0.1-1 mM acetic acid treatment stimulates root growth in barley seedlings. Recent papers have suggested that AA can protect plants against biotic (Chen et al. 2019) and abiotic stress (Utsumi et al. 2019) through jasmonic acid (JA) and abscisic acid (ABA) synthesis. AA treatment induces de novo JA synthesis and enhanced survival during drought stress is linked to histone acetylation (Kim et al. 2017). AA-treated plants show increased leaf water content and pigment levels, as well as upregulation of stress-response and stress-tolerance genes (Chen et al. 2019, Utsumi et al. 2019). AA supplementation ameliorates the toxic effects of seawater in mung bean by increasing uptake of Ca2+ and Mg2+ and decreasing uptake of Na+, enhancing antioxidant activity, water use efficiency and the contents of several metabolites e.g. soluble sugars and phenolics and flavonoids (Rahman et al. 2019). AA also enhances drought tolerance in maize and Arabidopsis through proline metabolism (Mahmud et al. 2023) and in soybean through improved antioxidant defence and photosynthesis and accumulation of soluble sugars and free amino acids (Rahman et al. 2021). However, besides these positive effects there are some reports about negative effects of AA on plants. In cassava plants, application of 20-50 mM AA induced wilting (Utsumi et al. 2019) while in maize seedlings root and shoot growth was inhibited by AA at doses as low as 10 mM (Allen and Allen 2020). In reed ( Phragmites australis) root growth was reduced at 0.3 mM, and entirely inhibited at 1.7 mM acetic acid (Armstrong et al. 1996). In Chlamydomonas reinhardtii AA can even cause cell death (Zuo et al. 2012).
In this study, the effects of AA treatment on early seedling growth were investigated in barley. For this purpose, root and shoot length, fresh (FW) and dry (DW) weight and water content (WC) of seedlings, as well as pigment and H2O2 content in leaves, and soluble and insoluble carbohydrate contents in roots were measured. Possible cytotoxic effects of AA treatment were investigated by measuring cell viability in roots by Evans Blue staining, which can penetrate into dead cells and therefore discriminates between viable and non-viable cells. Results of our study show that AA treatment inhibited growth and caused decreases in the levels of photosynthetic pigments e.g. chlorophyll, carotenoid, and pheophytin, while increasing the contents of anthocyanin, flavonoids, UV-absorbing compounds and UV-B marker. AA treatment caused the accumulation of H2O2 in leaves and decreased cell viability in roots. Insoluble carbohydrate content was enhanced by AA treatment, while soluble carbohydrate content decreased.
Plant material, growth conditions and treatment
Barley ( H. vulgare cv. Bornova-92) mature seeds were obtained from the Aegean Agricultural Research Institute (AARI, Izmir, Türkiye).
Ten seeds were placed between two filter papers in a 9-cm-diameter Petri dish containing 6 mL water for overnight imbibition at 6 °C and then germinated in the dark (16/8 h, 25/18 °C, 70 ± 5% humidity, darkness) for 3 days. Uniformly-germinated seedlings were subsequently placed on a filter paper containing 6 mL of the test solution containing AA for 2 days under a light intensity of 1400 μmol m-2 s-1 (16/8 h, 25/18 °C, 70 ± 5% humidity). At the end of the treatment, seedlings were rinsed, transferred to dishes containing 6 mL of water, and then further incubated for 48 h for recovery at the same growth conditions. Test solutions contained 0, 2.5 mM (0.0143% v/v) or 5 mM (0.0286% v/v) acetic acid (glacial, Merck). Plant samples were harvested immediately after AA treatment (day 0) or 2 days after AA was removed (day 2). Day 2 group represents recovery plants.
Measurement of seedling growth and water content
Seedlings were briefly soaked on filter paper and weighed to determine FW. To determine DW, seedlings were dried at 65 °C until the weight became constant. FW and DW were expressed as milligrams (mg). Water content was calculated according to the formula WC = ((FW−DW) / FW) × 100 and expressed as a percentage. Root and shoot lengths were expressed as centimetres (cm).
Determination of pigment content
To estimate the content of chlorophyll, carotenoid and pheophytin, leaves were homogenized in 80% acetone, incubated at -20 °C and, centrifuged at 6000 × g for 5 minutes at 4 °C. Supernatants were used to determine chlorophyll a and b, carotenoid and pheophytin levels. The absorbances of the extracts at 470, 655, 663 and 666 nm were measured in a glass cuvette (104.002-OS, Hellma) using Nanodrop (2000C, Thermo Fisher) due to the large volume of the samples and expressed as mg g-1 FW (Lichtenthaler 1987, Costa et al. 2006).
To estimate the content of UV-B-absorbing compounds, leaves were homogenized in a methanol : HCl solution (99:1) and centrifuged at 10000 × g for 15 min at 4 °C. To determine the levels of UV-B-absorbing compounds and flavonoids, the absorbances (A) of the supernatants were measured at 300 and 350 nm in a quartz cuvette (104.002-QS, Hellma) using Nanodrop. To determine the levels of anthocyanins, the absorbances of the supernatants were measured at 535 and 657 nm. Anthocyanin contents were calculated according to the formula ((A535 – (0.25 × A657)) using a molar extinction coefficient of 38000 L mol–1 cm–1. Flavonoid contents were calculated using a molar extinction coefficient of 20000 L mol–1 cm–1. Anthocyanin and flavonoid contents were expressed as µmol g-1 FW and µmol mg-1 FW, respectively. Content of other UV-B absorbing compounds measured at A300 was expressed as A300 g-1 FW (Cicek et al. 2012).
Determination of hydrogen peroxide and UV-B marker content
Leaves were homogenized in 0.1% trichloroacetic acid and centrifuged at 10000 × g for 15 min and the supernatants were collected. The absorbances of the supernatants at 440 nm were measured to determine UV-B marker content which was expressed as A g-1 FW (Cicek et al. 2012).
For the determination of H2O2, supernatants were mixed with 0.1 M Tris-HCl and 1 M potassium iodide and incubated at room temperature (RT) for 90 min. The absorbances were read at 390 nm and the H2O2 amounts of the unknown samples were estimated according to the standard curve (0-330 nmol) of H2O2 (Merck) and expressed as nmol g-1 FW (Cicek et al. 2012).
Evans Blue staining
Cell viability in roots was measured according to Baker and Mock (1994). Root samples were immersed in 0.25% Evans Blue stain for 20 min at RT. Then, roots were rinsed with water for 30 min, and 10 root tips (1 cm long) were incubated in 1% SDS : 50% methanol at 50 °C for 1 h. The absorbances of the methanol : SDS solution containing stain released from cells were measured at 595 nm.
Determination of carbohydrate content
Carbohydrate extractions were performed according to Sonjaroon et al. (2018). Briefly, roots were homogenized in 80% ethanol, incubated at 75 °C for 15 minutes and centrifuged at 6000 × g for 5 minutes. After the collection of the supernatant, the extraction was repeated twice. The supernatants were combined and used to measure soluble sugar content. The pellet phase containing ethanol-insoluble material was used for starch analysis.
Starch extraction was performed according to McCready et al. (1950) and Sonjaroon et al. (2018). Briefly, the pellet was dried at RT to evaporate EtOH completely and then dissolved in 52% perchloric acid at 6 °C for 30 minutes. After centrifugation at 2000 × g for 5 minutes and recovery of the supernatant, extraction was repeated twice. The combined supernatants were used to estimate starch content. Soluble sugar and starch (insoluble carbohydrate) contents were measured according to an optimized phenol-sulphuric acid method (Masuko et al. 2005). Fifty µL of the sample was mixed with 150 µL of H2SO4 and 30 µL of 5% phenol solution (a generous gift from Chembio, Turkey) incubated at 90 °C for 5 min and then cooled to RT. The absorbances were read at 490 nm. The glucose amount of the unknown samples was estimated according to the standard curve (0 - 100 µg) of glucose. For the estimation of starch, the concentration value was multiplied by 0.9. Soluble sugar and starch contents were expressed as µg mg-1 FW.
Statistical analysis
All experiments were conducted as independent triplicates. Each datapoint is the arithmetic mean of biological triplicates (N = 3) and the technical triplicates were also included in each experiment. Data were analysed by 2-Way ANOVA, and Tukey’s multiple comparison test using Graphpad Prism (version 8.0.1.244). The ANOVA included 2 independent variables as time (day 0 and day 2) and AA concentration (0, 2.5 and 5 mM).
Seedling growth and water content
AA treatment significantly (P ˂ 0.01) decreased root (Fig. 1A) and shoot (Fig. 1B) lengths. At day 0, immediately after AA treatment with 2.5 and 5 mM concentration for 2 days 33.79% and 45.42% decreases in root lengths, respectively, were observed. At day 2, in seedlings recovering for 2 days under non-AA condition, decreases were 37.07% and 45.15%, at 2.5 and 5 mM concentrations, respectively. For shoot lengths, AA treatment caused 13.73% and 12.63% decreases at day 0 and 18.47% and 22.70% decreases at day 2 for 2.5 and 5 mM concentrations, respectively.
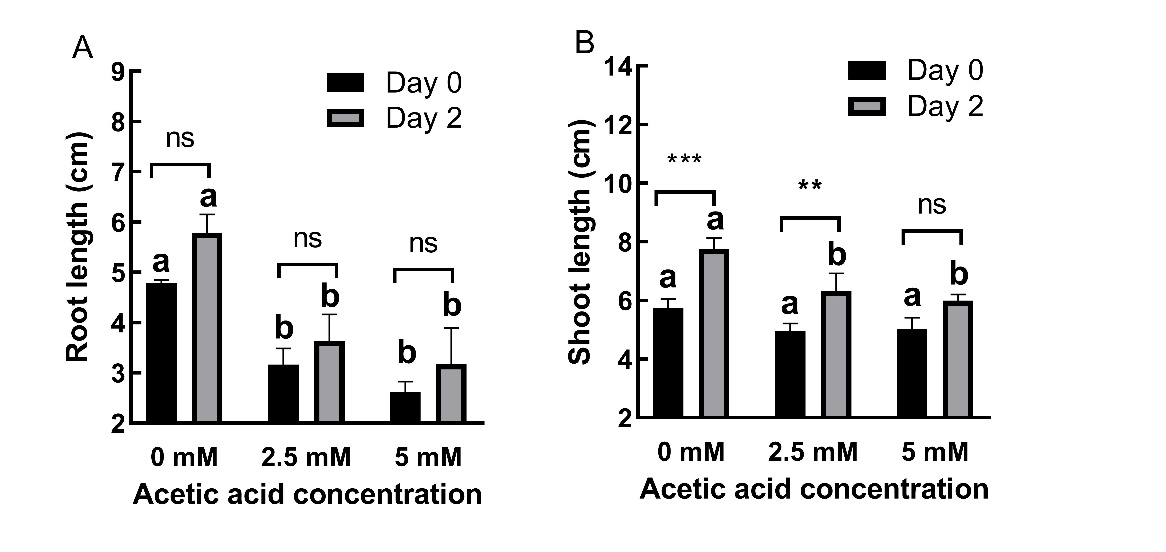
Fig. 1. Changes in root (A) and shoot (B) lengths in 2-day old barley seedlings under acetic acid (AA) treatment. Results are expressed as means ± standard errors (N = 3). Columns indicated by different letters are statistically different at the same timepoint (P ˂ 0.05). Data representing the same concentration at different timepoints are indicated as not significant (ns, P ˃ 0.05), * for P ˂ 0.05 or ** for P ˂ 0.05.
AA-treated plants could not restore shoot and root growth after AA was removed. The effects of AA on root and shoot length were time-independent (p ˃ 0.05).
AA treatment significantly decreased (p ˂ 0.01) FW (Fig. 2A) and WC (Fig. 2B) without affecting (p ˃ 0.05) DW (On-line Suppl. Figure 1A). AA-treated plants could not restore FW after AA was removed. At day 0, AA treatment caused 10.02% and 19.58% decreases in FW at 2.5 and 5 mM concentrations, respectively. At day 2, these decreases were 22.08% and 28.43%, respectively. AA treatment also caused 3.32% and 4.68% decreases in WC at day 0 and 4.48% and 6.04% decreases at day 2 for 2.5 and 5 mM concentrations, respectively.
The effects of AA on FW were time-dependent (P ˂ 0.01), while its effects on WC were not time dependent (P ˃ 0.05).
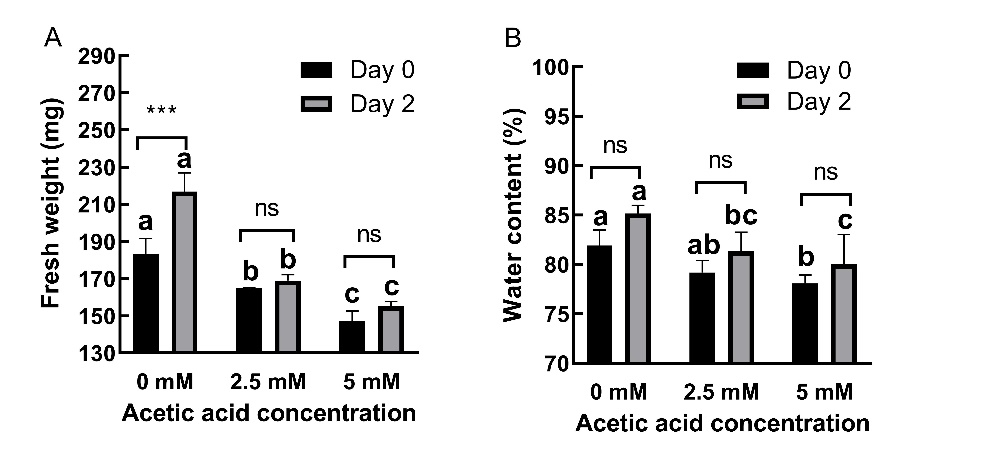
Fig. 2. Changes in fresh weight (A) and water content (B) in 2-day old barley seedlings under acetic acid (AA) treatment. Results are expressed as means ± standard errors (N = 3). Columns indicated by different letters are statistically different at the same timepoint (P ˂ 0.05). Data representing the same concentration at different timepoints are indicated as not significant (ns, P ˃ 0.05), * for P ˂ 0.05 or ** for P ˂ 0.05.
Chlorophyll, carotenoid and pheophytin content
AA treatment decreased chlorophyll a (Fig. 3A), total chlorophyll (Fig. 3B), carotenoid (Fig. 3C), and pheophytin (Fig. 3D) levels but significantly (P ˂ 0.01) at day 2, in the recovery group of plants.
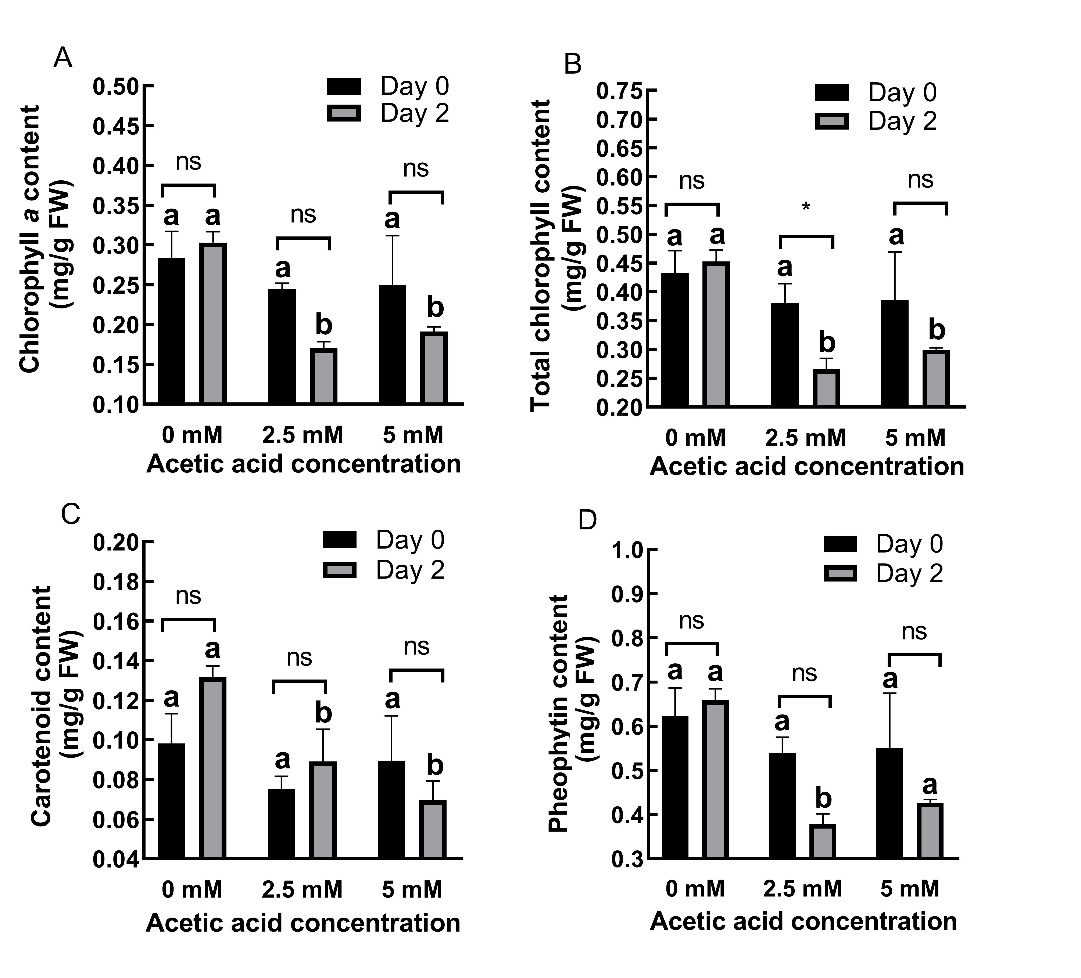
Fig. 3. Changes in chlorophyll a (A), total chlorophyll (B), carotenoid (C) and pheophytin (D) content in shoots of 2-day old barley seedlings under acetic acid (AA) treatment. Results are expressed as means ± standard errors (N = 3). Columns indicated by different letters are statistically different at the same timepoint (P ˂ 0.05). Data representing the same concentration at different timepoints are indicated as not significant (ns, P ˃ 0.05), * for P ˂ 0.05 or ** for P ˂ 0.05.
At day 0, AA treatment caused 13.77% and 12.29% decreases in chlorophyll a content at 2.5 and 5 mM concentrations, respectively. At day 2, these decreases were 43.82% and 36.89%, respectively. For total chlorophyll content, AA treatment caused 11.74% and 10.68% decreases at day 0 and 41.22% and 33.84% decreases at day 2 for 2.5 and 5 mM concentrations, respectively. At day 0, AA treatment caused 23.33% and 8.92% decreases in carotenoid content at 2.5 and 5 mM concentrations, respectively. At day 2, these decreases were 32.29% and 47.18%, respectively. For pheophytin content, AA treatment caused 13.54% and 11.70% decreases at day 0 and 42.51% and 35.21% decreases at day 2 for 2.5 and 5 mM concentrations, respectively. Chlorophyll b content (On-line Suppl. Fig. 1B) was not significantly affected (P > 0.05).
The effects of AA on photosynthetic pigments were found to be time-dependent (P < 0.05).
Content of anthocyanins, flavonoids, UV-absorbing compounds and UV-B marker
AA treatment mostly increased (P ˂ 0.01) content of anthocyanins (Fig. 4A), flavonoids (Fig. 4B), UV-absorbing compounds (Fig. 4C) and UV-B marker (Fig. 4D) in shoots. At day 0, AA treatment caused a 28.98% increase and a 6.89% decrease in anthocyanin content at 2.5 and 5 mM concentrations, respectively. At day 2, both concentrations caused increases (97.94% and 95.37%, respectively). For flavonoid content, AA treatment caused 73.89% and 54.61% increases at day 0 while at day 2, 10.02% and 18.65% decreases for 2.5 and 5 mM concentrations, respectively, were noticed.
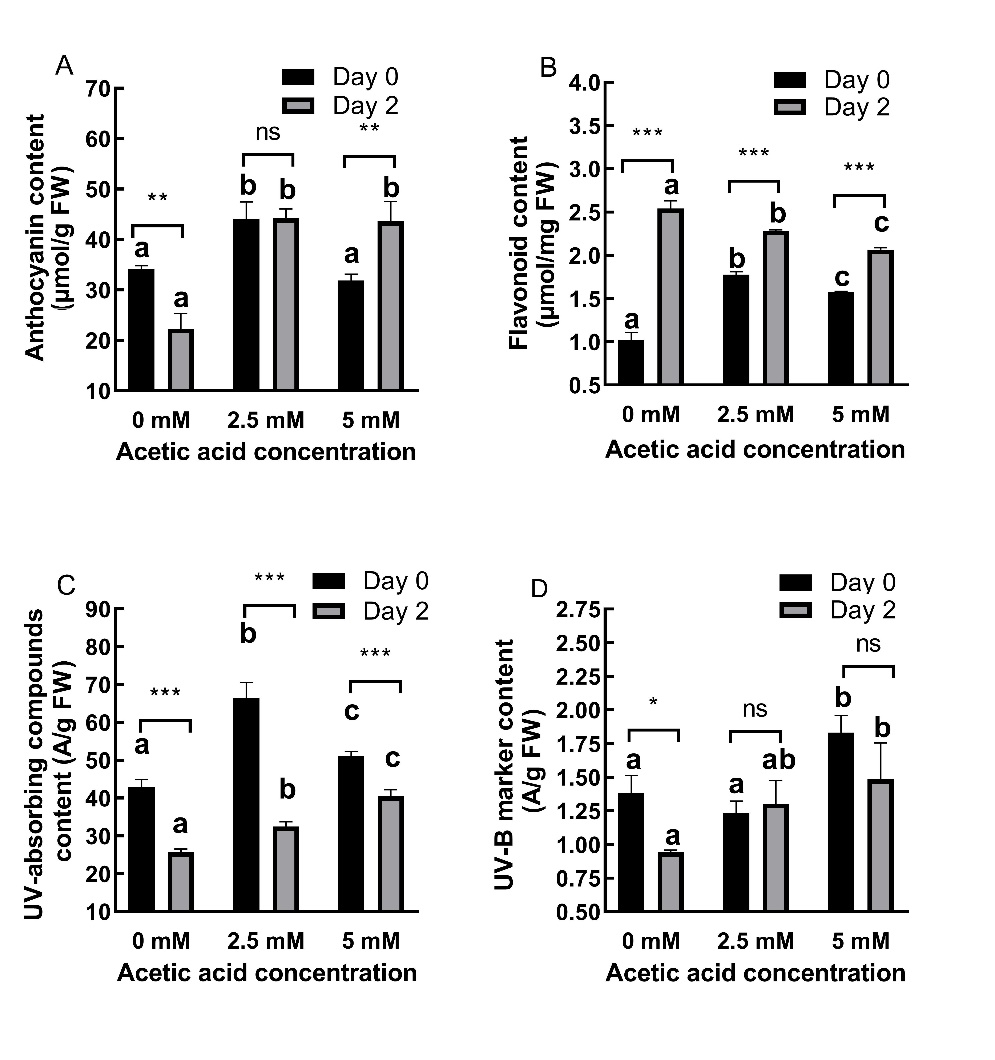
Fig. 4. Changes in anthocyanin (A), flavonoid (B), UV-absorbing compounds (C) and UV-B marker (D) content in shoots of 2-day old barley seedlings under acetic acid (AA) treatment. Results are expressed as means ± standard errors (N = 3). Columns indicated by different letters are statistically different at the same timepoint (P ˂ 0.05). Data representing the same concentration at different timepoints are indicated as not significant (ns, P ˃ 0.05), * for P ˂ 0.05 or ** for P ˂ 0.05.
For UV-absorbing compounds, AA treatment caused 54.80% and 19% increases at day 0 and 26.93% and 58.04% increases at day 2 for 2.5 and 5 mM concentrations, respectively.
At day 0, AA treatment caused an 11.06% decrease and a 32,28% increase in UV-B marker content at 2.5 and 5 mM concentrations, respectively. At day 2, both concentrations caused increases (38.14% and 57.66%, respectively).
AA-treated plants accumulated all pigments except flavonoids even after AA was removed. The effects of AA on UV-protective pigments were time-dependent (P ˂ 0.05).
H2O2 content and cell viability
AA treatment dramatically increased (P ˂ 0.01) H2O2 content in shoots (Fig. 5A) while in roots, increase in A600 (Fig. 5B), indicated reduced cell viability, and these effects persisted even after AA was removed. With respect to H2O2 content, AA treatment caused 44.22% and 29.71% increases at day 0 and 61.08% and 57.61% increases at day 2 for 2.5 and 5 mM concentrations, respectively.
AA treatment caused 81.76% and 65.09% increases in A600 values at day 0 and 105.96% and 100.89% increases at day 2 for 2.5 and 5 mM concentrations, respectively.
The effects of AA on cell viability were time-dependent (P < 0.01), while its effects on H2O2 content were not time-dependent (P > 0.05).
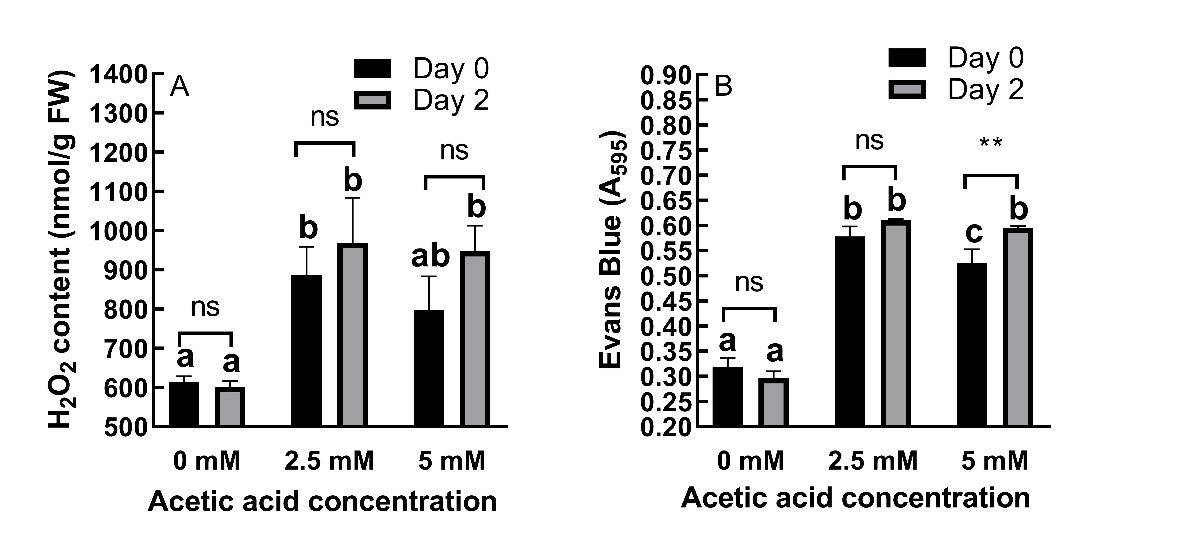
Fig. 5. Changes in H2O2 content in barley shoots (A) and cell viability measured by Evans Blue stain (B) in roots of 2-day old barley seedlings under acetic acid (AA) treatment. Results are expressed as means ± standard errors (N = 3). Columns indicated by different letters are statistically different ( P ˂ 0.05). Data representing the same concentration at different timepoints are indicated as not significant (ns, P ˃ 0.05), * for P ˂ 0.05 or ** for P ˂ 0.01.
Carbohydrate content
AA treatment had significant (P ˂ 0.05) effects on carbohydrate content in roots. At day 0, AA treatment caused a 26.66% increase and a 12.43% decrease in soluble carbohydrate content at 2.5 and 5 mM concentrations, respectively. At day 2, both concentrations caused decreases (18.83% and 52.54%, respectively) (Fig. 6A).
With respect to insoluble carbohydrate content, AA treatment induced 10.42% and 23.15% increases at day 0 and 29.78% and 19.76% increases at day 2 for 2.5 and 5 mM concentrations, respectively (Fig. 6B).
The effects of AA on soluble carbohydrate content were time-dependent (P ˂ 0.05) while its effects on insoluble carbohydrate content were not time dependent (P ˃ 0.05).
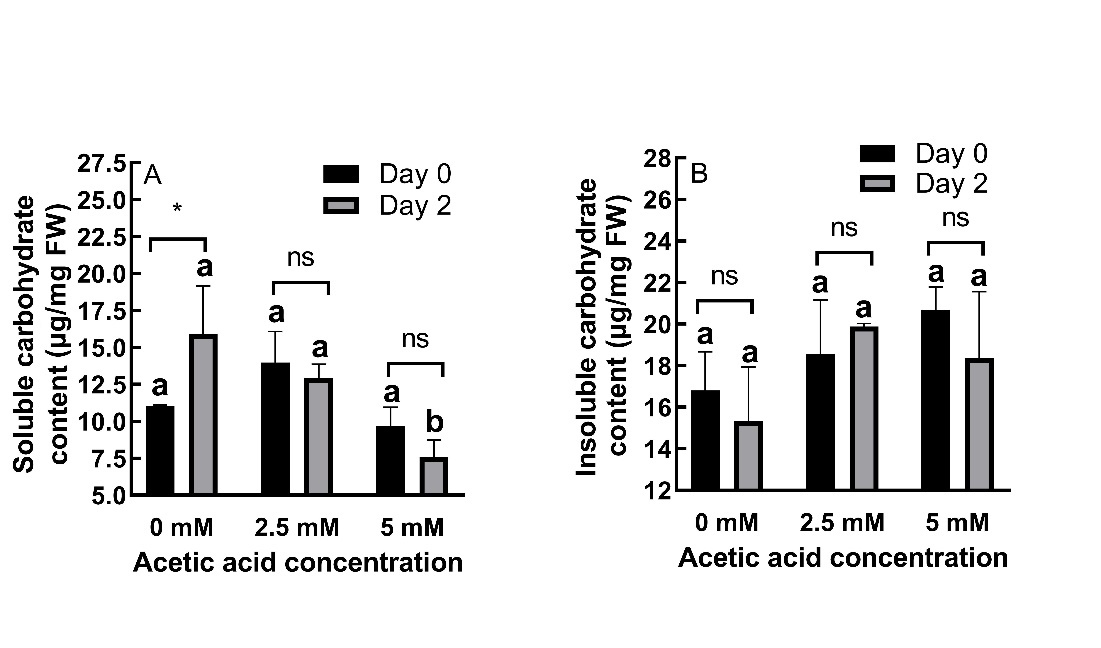
Fig. 6. Changes in soluble (A) and insoluble carbohydrate (B) contents in roots of 2-day old barley seedlings under acetic acid (AA) treatment. Results are expressed as means ± standard errors (N = 3). Columns indicated by different letters are statistically different (P ˂ 0.05). Data representing the same concentration at different timepoints are indicated as not significant (ns, P ˃ 0.05), * for P ˂ 0.05 or ** for P ˂ 0.01.
Discussion
Stress conditions inhibit plant growth in terms of root and shoot length, DW and leaf area (Rahman et al. 2019). Under stressful conditions plants synthesize various phytoprotectant metabolites that help them defend against stress (Parvin et al. 2022). These metabolites can also be applied exogenously e.g. salicylic acid (SA) and phenolic compounds and alleviate stress-induced decreases in yield and productivity by affecting several processes including plant growth, photosynthesis, membrane permeability, antioxidant systems and synthesis of various molecules (Parvin et al. 2022). OAs were also reported to accumulate in plants under stress as these molecules, besides being intermediates in carbon metabolism, are important metabolically active solutes for osmotic adjustment, the balance of cation excess or in coping with nutrient deficiencies and metal tolerance (López-Bucio et al. 2000). AA, an OA, was shown to confer tolerance in various plant species, including tomato, cassava, Arabidopsis, rapeseed, maize, rice and wheat against several stressors (Kim et al. 2017, Chen et al. 2019, Rahman et al. 2019, Utsumi et al. 2019, Rahman et al. 2021, Mahmud et al. 2023). AA-sprayed mung bean plants exhibited enhanced shoot and primary root length and shoot DW under salt conditions (Rahman et al. 2019). The same group later showed that foliar application of AA resulted in improvement of root biomass and leaf area in soybean exposed to drought (Rahman et al. 2021). Utsumi et al. (2019) reported the maintenance of leaf relative WC in drought-treated cassava plants. AA resulted in higher WC and elevated water use efficiency due to decreased stomatal conductance in drought stressed Cunninghamia lanceolate plants (Li et al. 2022). A recent paper by Mahmud et al. (2023) similarly stated that AA restored shoot and root growth in maize under drought stress. Interestingly, in our study AA treatment of barley seedlings inhibited plant growth, especially root growth and decreased FW and WC but did not affect DW. These detrimental effects mostly could not be repaired after the 2 day-recovery period. Thus, it can be inferred that, when applied directly to the young seedlings through roots, AA might be regarded as a stressor and the roots, being the first exposed to the effects of AA, are more affected than the shoots. Our results are different than those of most papers, which reveal the positive effects of AA (Kim et al. 2017, Li et al. 2022, Mahmud et al. 2023). However, these studies were performed on different species, with higher concentrations of AA and for longer periods and demonstrated its effects in plants growing in a culture or soil. In the present study, a shorter treatment duration (2 days) with lower concentrations (2.5 and 5 mM) of AA was tested on very young barley seedlings growing on moist filter papers. Lynch (1977) reported that the growth promoting effects of AA depend on pH and plant species. In an interesting study, Allen and Allen (2020) suggested that adjusting pH of the medium can compensate the harmful effects of AA in maize roots. They demonstrated that under unbuffered conditions, such as during germination on filter paper, acetic acid exists in the membrane permeable undissociated form which caused maize seedling root inhibition. Similarly, in our study the pH of the water with AA was not adjusted, which could explain the toxic effects of AA on barley seedlings, even at low concentrations.
Severe stress conditions cause degradation of chlorophyll, while low stress stimulates chlorophyll content, to allow plants to cope with stress (Agathokleous et al. 2020). Under stressful conditions, degradation of free chlorophyll is necessary to prevent cell damage (Takamiya et al. 2000) and chlorophyll content can reflect the damage caused by stress (Agathokleous et al. 2020). Exogenously applied AA reduced the impact of drought stress in cassava plants by increasing chlorophyll and carotenoid amounts (Utsumi et al. 2019). AA also improved chlorophyll fluorescence in C. lanceolate under drought stress by maintaining higher chlorophyll contents due to delay of degradation of pigments or induction of their synthesis (Li et al. 2022). Hawrylak-Nowak et al. (2015) stated that AA had slight effects on carotenoid content in the first leaf of sunflower plant. In the present study, AA decreased chlorophyll and carotenoid contents as well as pheophytin content. A decrease in chlorophyll and carotenoid could be correlated with decreased growth and WC, but we expected accumulation of pheophytin in leaves of AA-treated seedlings because of the acidic environment in which the seedlings had grown. Pheophytin, a breakdown product of chlorophyll is observed mainly during leaf senescence (Lin and Charng 2021) or under oxidative stress (Szafrańska et al. 2017). Acidity replaces magnesium ions in the chlorophylls with hydrogen atoms and chlorophylls are converted to pheophytins (Kusmita et al. 2015). This unexpected result can be explained by the severity of stress, which caused reduced pheophytin content, as it did in duckweed plants exposed to UV-B radiation for 7 days (Farooq et al. 2005).
The growth-restricting effects of AA observed in our study suggests that AA may affect cell viability particularly in roots, which were more susceptible to AA than shoots. We also investigated H2O2 content in shoots, in which AA-induced reductions were less prominent than in roots. H2O2 is a type of ROS characterized by low reactivity. It serves as a signalling molecule due to its long life span and small size (Khan et al. 2018). ROS are produced as byproducts of various metabolic pathways and scavenged by antioxidant defence systems. Under certain (stress or non-stress) conditions, ROS production is elevated in plants (Apel and Hirt 2004). Higher concentrations of H2O2 deplete the ascorbic acid and glutathione pool, cause damage to proteins, nucleic acids and lipids and compromise cell integrity and eventually result in cell death (Khan et al. 2018). Stress conditions increase H2O2 content and decrease cell viability in plants, while compounds that confer stress tolerance usually act inversely (Cikili et al. 2019). Hawrylak-Nowak et al. (2015) reported that AA and particularly malic acid promoted cell viability in roots of Cd-treated plants due to increased high dehydrogenase activity. They also observed that AA decreased the content of H2O2 in leaves and roots of sunflower plants subjected to Cd stress. However, in our study, we observed the accumulation of H2O2 in shoots and decreased cell viability in roots after AA treatment, even after recovery step. Consistently with our findings, Armstrong et al. (1996) reported necrosis of the roots of reed subjected to 1.67 mM AA. These results suggest that AA can induce the oxidative stress in shoots and reduce the root length due to cell death and imply that AA, even at low concentrations, acts as a stressor in barley. In the study by Hawrylak-Nowak et al. (2015) where opposite results were found, 7-day-old sunflower seedlings were treated with 0.25 or 0.5 mM AA in 1.5-times strength Hoagland's II nutrient solution for 14 days. Different plant species and different experimental conditions, especially concerning the pH of the medium may explain the contradictory results.
Plants accumulate phenolic compounds (PCs) when exposed to stress. PCs can be found in several intracellular locations; and stimulate stress tolerance in plants by performing diverse functions such as scavenging of ROS, enhancement of cell division and improvement of photosynthesis (Parvin et al. 2022). The most abundant group of PCs is that of flavonoids, containing anthocyanins as a subgroup (Parvin et al. 2022). Flavonoids, anthocyanins, and other UV-absorbing compounds accumulate under stress conditions, although UV-B exposure was shown to be the most efficient in increasing the levels of UV-absorbing compounds and UV-B marker (Cicek et al. 2012). Depending on the plant species and its genotype, abiotic stresses such as drought affects contents of flavonoids, which act as signalling molecules, antioxidant molecules and UV protectant and regulate hormones (Shin et al. 2021, Parvin et al. 2022, Kumar et al. 2023). Molecules that can enhance stress tolerance of plants can stimulate synthesis of secondary metabolites e.g. flavonoids (Kumar et al. 2023). In the present study, AA treatment increased not only anthocyanin and flavonoid contents but also UV-absorbing compounds (A300) and the UV-B marker (A440). Except for the flavonoids, the increasing trend persisted after recovery step. AA treatment immediately and directly impacted UV-absorbing pigments probably as a result of increased H2O2 which induced synthesis of various phenolic compounds to act as ROS scavengers.
Because AA is a precursor of acetyl-CoA, an essential molecule of carbohydrate metabolism, we decided to investigate how AA treatment affects sugar contents in roots. There are contradictory reports on the effects of acetate on starch synthesis. Starch degradation is usually activated under abiotic stress to redirect carbon for stress responses, but starch can also accumulate when growth is inhibited more than photosynthesis (Ribeiro et al. 2022). Fan et al. (2012) reported acetate-induced starch accumulation in Chlamydomonas reinhardtii. However, Rengel et al. (2018) observed that overexpression of chloroplastic ACSS under nutrient replete conditions enhanced starch content in C. reinhardtii, while acetate treatment did not. Under nitrogen starvation, excess acetyl-CoA was stored as triacylglycerol. Interestingly, Arabidopsis plants with reduced activity of ATP-citrate lyase, which is responsible for conversion of mitochondria-derived citrate to acetyl-coA, have higher amounts of anthocyanin and starch (Fatland et al. 2005). Huang et al. (2017) found that microalgae Chlorella sorokiniana GXNN01 favours AA as a carbon source over glucose under normal and high light conditions. Use of AA as carbon source resulted in higher biomass with high lipid and low starch contents. In this study, soluble carbohydrate contents decreased in AA-treated roots, while insoluble carbohydrate contents increased, which is consistent with the findings of Fan et al. (2012). Moreover, changes in the content of soluble carbohydrates were more prominent than those of insoluble carbohydrates. Soluble sugars in seeds serve as fast-use reserves for energy production but they can also be efficient in protecting membrane integrity during stress conditions (Ferreira et al. 2009).
In conclusion, even at low concentrations, AA treatment can induce dramatic and persistent changes in non-stressed plants that endure even after AA is removed. Previous studies focused on the amelioration of stress, particularly drought, by AA treatment that was usually applied to plants growing in a culture medium or soil. In this study, AA was applied to young (2-day-old) seedlings growing on a filter paper and exhibited its toxic effects i.e. restriction of growth, decrease in WC, photosynthetic pigments and viability in root cells and the accumulation of UV-absorbing pigments and H2O2 in shoots and insoluble carbohydrates in roots.
