Introduction
Algae are commonly found in seas and fresh or brackish waters. However, they can survive in deserts, moist soils and stones, thermal springs, snow at the poles, and in all conditions with very little moisture. Thermal springs, in particular, are extreme ecosystems for algae. Certain groups of algae are common in thermal springs, such as Cyanobacteria and Bacillariophyta. Examining the species living in thermal springs is important in determining the factors conducive to life in these environments. Thus, the potential economic value of these species is revealed, and their biotechnological use improves.
Because it is located on fault lines, Türkiye has numerous thermal springs, concentrated mainly in the west of the country. The city of Denizli has numerous and varied thermal springs, which are famous worldwide for travertines and historical and cultural features. Travertines are formed by the precipitation of calcium in layers like thick lime deposits (Güner 1970, Pentecost et al. 1997). The most important characteristic of thermal springs in Denizli is that, although they are very close, they show different physio-chemical parameters (Kozak 2020). These thermal springs are in Pamukkale, Karahayıt, Yenicekent, Inalti, Şanlıalp, Umut, and Gölemezli. While Karahayıt, Yenicekent, and Umut have deep circulation and a high temperature, Pamukkale and Gölemezli have a low temperature and shallow water characteristics (Yaman and Özgür 2005). Pamukkale, or “Hierapolis,” is on the UNESCO list of world heritage sites and is perhaps one of the oldest spa centers in Türkiye. The Hellenistic spa town of Hierapolis was a focus of interest for visitors at the end of the second century BC (UNESCO 2022).
The first study in Türkiye about the biology of thermal springs was from Pamukkale (Regel and Skuja 1937), and there are other studies on the algal flora of the Pamukkale thermal spring (Güner 1966, Pentecost et al. 1997, Altunöz et al. 2016, Öztürk Ulcay et al. 2017). Also, Aysel et al. (1992) examined cyanobacteria in the Ilıksu thermal springs (Zonguldak, Türkiye) and reported 33 Cyanobacteria taxa. Öztürk Ulcay et al. (2007) explored the thermal springs in Dikili (İzmir, Türkiye) and identified 19 Cyanobacteria taxa. Öztürk Ulcay and Kurt (2017) identified a total of 27 taxa (21 Cyanobacteria, five Bacillariophyceae and one Conjugatophyceae) in Alangüllü (Aydın, Türkiye). Öztürk (2020) reported 13 Cyanobacteria taxa in the thermal springs in Kütahya (Türkiye). Thermal algae studies have also intensified in the west of Türkiye where many thermal springs are located. Although there are many thermal springs in Denizli, thermal algae studies in this region have only been carried out in the Pamukkale and Karahayıt thermal springs.
The aim of the present study was to provide for the first time a study of the algal flora in the thermal springs of Denizli and their relationship to the ecological conditions of seven thermal springs in Denizli.
Material and methods
Sampling
Denizli is one of the important thermal regions of Türkiye for health tourism and the touristic thermal springs there were chosen as the study area (Fig. 1).
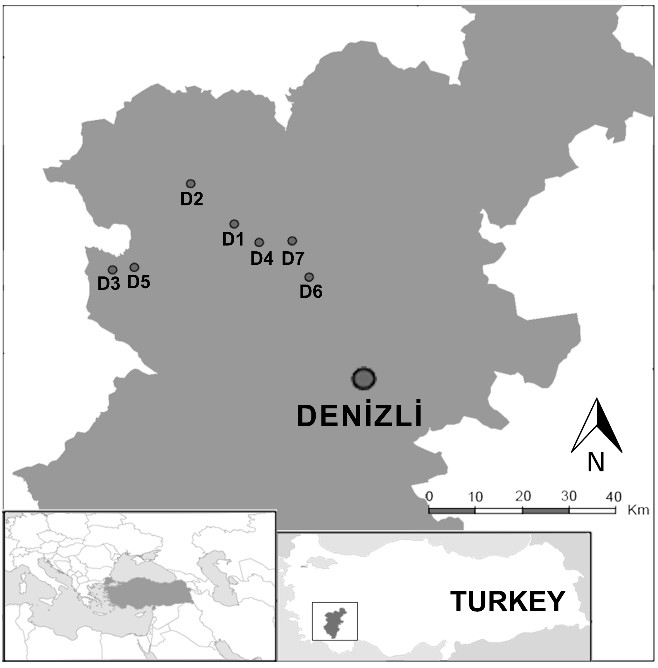
Fig. 1. The map of the study area showing the investigated thermal springs, and their locations in Denizli (Türkiye). D1 − Gölemezli, D2 − Yenicekent, D3 − İnaltı, D4 − Şanlıalp, D5 − Umut, D6 − Pamukkale, D7 − Karahayıt.
Gölemezli (D1; 37°59' N 29°02' E), Yenicekent (D2; 38°02' N 28°57' E), İnaltı (D3; 37°54' N 28°45' E), Şanlıalp (D4; 37°57' N 29°04' E), Umut (D5; 37°55' N 28°49' E), Pamukkale (D6; 37°55' N 29°07' E) (Fig. 2), and Karahayıt (D7; 37°58' N 29°06' E) (Fig. 3).

Fig. 2. The Pamukkale thermal spring (D6). A – general view of travertines, B – the Hellenistic spa town of Hierapolis, C – details of the travertines (photo: Sevilay Öztürk).

Fig. 3. The Karahayıt thermal spring (D7). A – general view of travertines, B – details of the travertines, C – thermal water outlet points (photo: Sevilay Öztürk).
Samples were taken from several points (thermal water outlet points, natural and artificial pools, travertines, and thermal water channels) of these thermal springs. Sample sites were scattered over approximately 207 km2, ranging from an altitude of 148 m to 420 m a.s.l.
Algae sampling was done monthly between May 2013 and June 2014 using forceps, a spatula, and a plankton net with a 30 μm mesh size. Two algae samples were taken separately from each sample site in 50 mL falcon tubes. Then, a 4% formaldehyde solution was added to one of the samples for fixing. The second algae sample was used for identification. The algae samples were labeled and brought to the laboratory in the dark. Algae samples were examined under an Olympus BX 50 (phase-contrast) microscope and photographed using a Sony DSC-TX7 camera in the laboratory. The literature was used to identify the algae taxa: Komárek and Anagnostidis (1999, 2005), and Komárek (2013) for Cyanobacteria taxa and Cantonati et al. (2017) for Bacillariophyta. The nomenclature was checked on the AlgaeBase database (Guiry and Guiry 2023).
Water samples were taken simultaneously in sterile bottles during the algae sampling. The pH and temperature were measured in-situ, with a Hanna HI 9812-5 Portable Meter. Sodium (Na+), potassium (K+), calcium (Ca2+), lithium (Li+), magnesium (Mg2+), ferrous (Fe2+), chloride (Cl-), bicarbonate (HCO3-), and sulphate (SO42-) analyses were made ex-situ The concentrations of Na+, K+, Ca2+, Li+, Mg2+, and Fe2+ ions were measured by atomic absorption spectrometer, the concentrations of Cl- and HCO3- ions were measured by titrimetric analysis, the concentration of SO42- ion was measured by theoretical methods, and the ion concentration results are given as mg L-1. In-situ measurements were made every month (between May 2013 and June 2014), while ex-situ measurements were made seasonally (in July and October 2013 and in January and April 2014). The results of in-situ and ex-situ measurements are given as their means.
Statistical analysis
Canoco 5.0 software for Windows (Microcomputer Power, Ithaca, NY, USA) was used to determine the correlation between the physio-chemical characteristics of the thermal springs and algal flora (Ter Braak and Šmilauer 2012). Firstly, detrended correspondence analysis (DCA) was done to determine the gradient length and whether the studied gradient was suitable for linear or unimodal models. The results of the DCA were ideal for principal component analysis (PCA). The algal flora composition (total of 46 taxa from 7 sample sites on 12 sampling dates) with binary data was used in this analysis. Tests of significance of the first and all canonical axes were performed to statistically assess the relation between algal flora composition and physio-chemical characteristics (Monte Carlo test: 499 permutations under the reduced model).
Additionally, the Monte Carlo permutation test was applied to determine the statistical significance of physio-chemical characteristics in explaining the composition of algal flora. This involved a stepwise "forward selection" of explanatory variables, as available in Canoco. The process was begun by selecting the most effective explanatory variable (the one that best explained the overall variance in the data). Subsequent variables were chosen based on their decreasing importance in explaining the total variance in the dataset, in conjunction with the previously selected variables. The statistical significance of each variable was also assessed. The variation in algal flora composition explained by the physio-chemical characteristics included in the analysis was expressed as a percentage, representing the ratio of the sum of all canonical eigenvalues to the total variance (total inertia).
The Kruskal-Wallis H-test was conducted using SPSS 28.0 software to determine the statistical significance of the difference in values of physio-chemical parameters of thermal springs by sample sites. The Kruskal-Wallis test used the 12-month means of pH and temperature values and the seasonal means of Na+, K+, Ca2+, Li+, Mg2+, Fe2+, Cl-, HCO3-, and SO42- values of water samples from seven thermal springs. None of these data were transformed. The Kruskal-Wallis test is a non-parametric analysis of variance test used to assess the significance of differences among means of three or more groups in cases where the data does not follow a normal distribution.
Physio-chemical characteristics of the thermal springs
Differences were observed among the physio-chemical parameters of seven thermal springs in Denizli (Tab. 1).
Tab. 1. The physio-chemical parameters of the thermal springs (D1-D7) in Denizli (Türkiye) and the results of the Kruskal-Wallis test (with SPSS 28.0) were used to reveal the statistical significance of the similarities and differences of the physio-chemical parameters of the sample sites (P < 0.001). D1 − Gölemezli, D2 − Yenicekent, D3 − İnaltı, D4 − Şanlıalp, D5 − Umut, D6 − Pamukkale, D7 − Karahayıt. T − temperature, Ca − calcium, Mg − magnesium, Fe − ferrous, SO − sulphate, K − potassium, Na − sodium, Cl − chloride, and Li − lithium. The Kruskal-Wallis column contains the test values among the sample sites. Values that show significant differences among sampling sites are shown in bold.
The highest temperature value was measured at D3 as 60 °C, the lowest temperature value was measured at D6 as 34 °C, and the mean temperature value of all sampling sites was 47 °C. In terms of calcium values, D7 had the highest value of 377.10 mg L-1, while D3 had the lowest value of 8.7 mg L-1. With potassium values, D4 had the highest value of 134.0 mg L-1, while D6 had the lowest value of 7.5 mg L-1. A difference in ferrous values was also seen among sample sites, with D7 having the highest value of 2.45 mg L-1 and D3 having the lowest value of 0.034 mg L-1. In terms of bicarbonate values, D3 (689.53 mg L-1) is quite low compared to the other sample sites. It was observed that the lithium values of D6 and D7 were considerably lower than those of the other sample sites. The measured sodium values at D4 (1392.2 mg L-1) were twenty times higher than at D6 (68.6 mg L-1) (Tab. 1). The D6 and D7 thermal springs were clearly distinguished from the others by their high value of Ca2+ concentration and low Cl-, Na+, and K+ concentrations. In this study, it was determined that the most important physio-chemical parameter was temperature, and considering the temperature differences of thermal springs, it was seen that the most significant difference was found for D6, which has the lowest temperature. Another notable point was the differences in the concentration of Fe2+ which was higher in D2 and D7 than in other thermal springs (Tab. 1). A significant difference was found when the Cl−, Fe2+, K+, Mg2+, Na+, Li+, Ca2+, HCO3-, SO42-, pH and temperature values of the thermal springs were compared with the Kruskal Wallis test (P < 0.001) (Tab. 1).
Algal flora
A total of 46 algae taxa (43 Cyanobacteria, 3 Bacillariophyta) were identified according to their morphological and ecological characteristics (Tab. 2). Synechococcales was the dominant order with 18 taxa among other Cyanobacteria orders.
Tab. 2. Taxa, taxa codes and distribution of algal flora determined in thermal springs (D1-D7) in Denizli (Türkiye). D1 − Gölemezli, D2 − Yenicekent, D3 − İnaltı, D4 − Şanlıalp, D5 − Umut, D6 − Pamukkale, D7 − Karahayıt.
The D5 sample site had the most biodiversity with 22 taxa. The most common taxon was Spirulina subsalsa Oersted ex Gomont, collected from the five sample sites, followed by Jaaginema geminatum (Schwabe ex Gomont) Anagnostidis & Komárek, and Pseudanabaena mucicola (Naumann & Huber-Pestalozzi) Schwabe from four sample sites. In the present study, three Bacillariophyta taxa were identified from the sample sites of D5 and D6 that had water temperatures of 32–36 °C.
Statistical analysis
First, the DCA was performed to find a suitable analysis, and then gradient lengths were assessed (Axis 1: 0.7356; Axis 2: 0.4883). According to the gradient lengths obtained from the DCA, the data were found to be suitable for PCA. Among the physio-chemical parameters analyzed, nine were included in the forward selection (temperature, Cl−, SO4−2, Fe2+, K+, Mg2+, Na+, Li+, and Ca2+). In the PCA, the identified 46 taxa, the investigated thermal springs, and the physio-chemical parameters were used (Fig. 4).
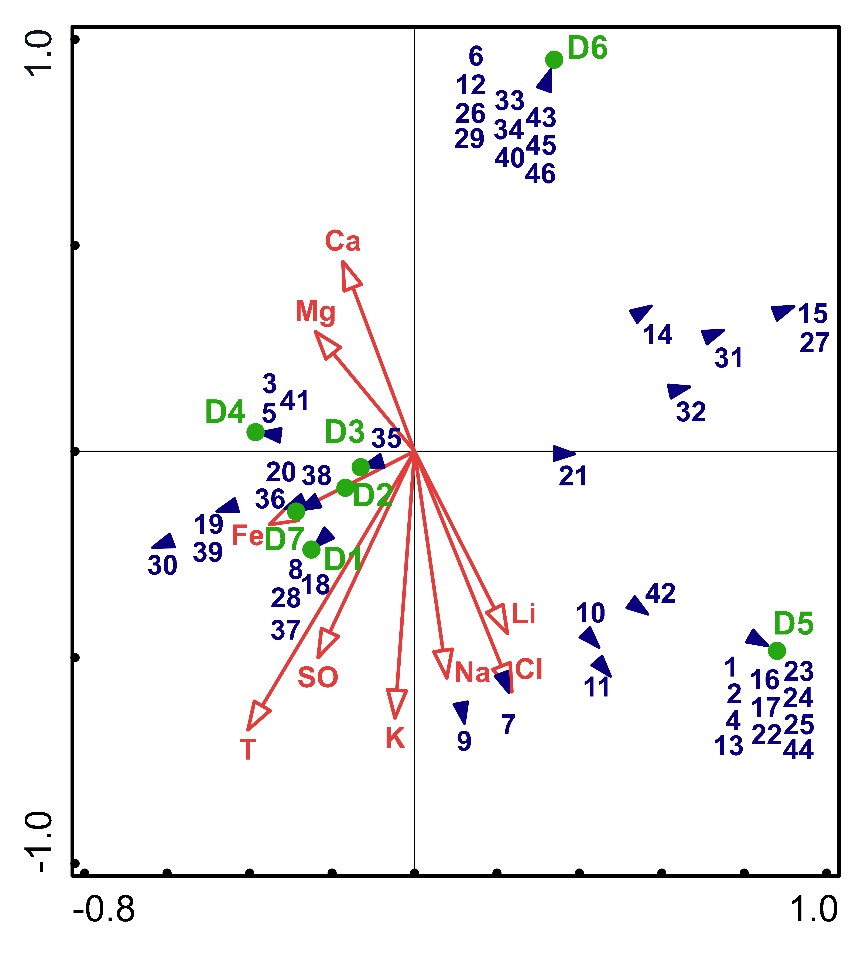
Fig. 4. Principal component analysis (PCA) diagram of the algal flora, the physio-chemical parameters, and the sample sites of thermal springs (D1-D7) in Denizli. The graph shows the algal taxa codes (blue triangles), the sample sites (green circles), and the physio-chemical parameters, temperature, and concentration of different ions (red arrows) with the canonical axes. The algal taxa codes are given in Tab. 2. T − temperature, Ca − calcium, Mg − magnesium, Fe − ferrous, SO − sulphate, K − potassium, Na − sodium, Cl − chloride, and Li − lithium.
The significance effect was supported by a Monte Carlo permutation test (499 permutations, F-ratio < 0.1, P-value = 1). As a result of the PCA, the total variation was 49.71429, and the first two axes explained 58.59% of the variance (Fig. 4). The most determining factor was the temperature (T), followed by K+ and Cl−. Although D5 (with 22 taxa) and D6 (with 16 taxa) had the highest number of taxa, they did not closely correlate with all the parameters (Fig. 4). Interestingly, D6 is partially positively correlated to Ca and Mg and negatively correlated to the rest. Conversely, D5 is negatively correlated to Ca and Mg, while it is partially positively correlated to the rest. Additionally, it can be seen that all the other sample sites are positively correlated to almost all the physio-chemical parameters (Fig. 4).
Discussion
In this taxonomic study, the algal flora and physicochemical parameters of seven thermal springs in Denizli were examined. We observed that these thermal springs were different in terms of their physio-chemical properties. As in similar studies in the literature (Ward and Castenholz 2000, Papke et al. 2003, Sompong et al. 2005), the relationship of species diversity in thermal springs and physio-chemical parameters and substrate structure was observed in this study. Accordingly, the correlation between the algal flora of the thermal springs and their physio-chemical parameters was examined statistically by PCA (Fig. 4).
The critical factor determining the distribution of algal flora in the thermal springs was temperature, with diatoms preferring relatively low temperatures. Pinnularia microstauron (Ehrenberg) Cleve, which was sampled from sites with low temperatures in this study (D5 and D6), has been reported from similarly low-temperature thermal springs (Fazlutdinova et al. 2020, Kaštovský and Komárek 2001). Kaštovský and Komárek (2001) defined the region below 36 °C in thermal waters as the mesothermophilic diatom region and stated that in this region, diatoms and cyanobacterial taxa form clusters together. Similar to Kaštovský and Komárek (2001), especially at low temperatures in D6, P. microstauron and Diploneis interrupta (Kützing) Cleve were observed to form mats with cyanobacteria taxa. The results of the PCA (Fig. 4) showed the negative correlation of these two diatom taxa with temperature, confirming diatom preference for lower temperatures in thermal springs.
Cyanobacteria taxa are common in thermal springs because they can survive at higher temperatures than other algae. Jaaginema angustissimum (West & G.S.West) Anagnostidis & Komárek is one of the common taxa of thermal and sulfate (SO42-) springs (Güner 1970, Komárek and Anagnostidis 2005, Arman et al. 2014, Öztürk Ulcay and Kurt 2017), and it was determined from D5, which is also characterized by a high SO42- value in this study.
Interestingly, although Gloeocapsa gelatinosa Kützing was found to have a low correlation with Cl- in this study (Fig. 4), Roy et al. (2015) reported that the taxon was associated with higher Cl- by canonical correspondence analysis. In this study, G. gelatinosa and Calothrix fusca Bornet & Flahault were collected together from the same sample site which is consistent with the report of Lukavsky et al. (2011) that they formed a mat together.
The most common taxon in this study was Spirulina subsalsa, which did not have a significant correlation with the physio-chemical parameter results of the PCA. Altunöz et al. (2016) identified this taxon in the Pamukkale thermal spring and reported that this taxon did not show a distribution correlated with physio-chemical parameters, which is consistent with the present study. However, Roy et al. (2015) reported that the combined effect of pH, K+, and HCO3- was effective in the distribution of S. subsalsa. As in the study of Kanellopoulos et al. (2016), S. subsalsa and Phormidium terebriforme (C.Agardh ex Gomont) Anagnostidis & Komárek were sampled together from the travertine substrates in this study (D1). The travertines are frequently colonized with Cyanobacteria mats, commonly composed of more than one taxon (Pentecost 2003, Kanellopoulos et al. 2016). Pentecost (2003) reported that travertines are often covered with a mixture of Oscillatoria, Spirulina, and Phormidium taxa. This result was also observed in this study; Jaaginema geminatum, Spirulina major Kützing ex Gomont, S. subsalsa, and P. terebriforme were collected from travertine substrates in D1, D2, D5, and D7. In addition, the D2 and D7 thermal springs had a high value of Fe2+ concentrations. Pierson and Parenteau (2000) reported well-developed cyanobacterial mats in high Fe2+ concentrations. As can be seen from the PCA (Fig. 4), Planktolyngbya contorta (Lemmermann) Anagnostidis & Komárek and Oscillatoria proboscidea Gomont were identified from D7 with the highest Fe2+ concentration. Also, common taxa of D2 and D7, with high Fe2+ concentrations, were Pseudanabaena mucicola and S. subsalsa, and the distribution of these species in this study overlaps with the related data from the literature. However, S. subsalsa does not appear to be associated with Fe2+ concentrations (Fig. 4). The possible reason may be the ecological tolerance of S. subsalsa.
Conclusions
This study enlarged the worldwide knowledge of algal flora inhabiting thermal springs, which helps to improve the ecological data on the physio-chemical parameter preferences of Cyanobacteria and Bacillariophyta taxa and contributes to the understanding of the effects of physio-chemical parameters on the diversity of these ecosystems. Our findings also confirm that algal flora diversity is directly affected by temperature, followed by potassium and chloride ions in the water of thermal springs.
Further ecological, physiological, and biotechnology studies are required for an understanding of the potential usability of these thermophilic species of algae. For all these reasons, determining the species diversity in thermal springs can also underpin new economic and industrial uses.
