Introduction
Different cheese varieties, as sources of valuable proteins, easily digestible fats and some minerals are from the nutritional point of view almost irreplaceable part of human diet. Cheese in general and goat's cheese in particular are also increasingly being considered as health-promoting foods.
Due to the presence of several bioactive compounds (such as bioactive peptides, fatty acids) goat cheese is considered to be potentially useful in cardiovascular disease, metabolic disorders, neurological degeneration, or in promoting intestinal health (Lima et al, 2018). In addition, these compounds showed chemo preventive activity against cancer (Yasuda et al., 2012).
It is well known that nutritive characteristics and functionality of cheese depends on numerous factors including type and quality of milk and cheese making process, which in turn depends on cheese variety. For the most of cheese varieties, the key phase of production is ripening. During this phase specific sensory and nutritional characteristics of each cheese variety are formed. It is well known that the ripening is complex dynamic phase, which includes numerous chemical, microbiological, and biochemical changes of the major cheese compounds (Albenzio and Santillo, 2011; Hatipoğlu et al., 2023; Maslov Bandić et al., 2023). As a result of proteolysis, lipolysis, glycolysis, and further degradation of free amino acids or free fatty acids, appearance, texture, microstructure, and flavour of the final ripened cheese is formed (Hayaloglu et al., 2002; Tudor Kalit et al, 2020). Also, according to numerous studies (Dimitrov et al., 2015; Gupta et al., 2010; Pritchard et al. 2010; Barać et al., 2017; 2019; Fialho et al., 2018) the majority of so-called bioactive peptides are released during proteolysis. These peptides are associated with immunomodulatory, antihypertensive, antimicrobial, antioxidant and opioid activity (Barać and Sarić, 2023). They are mainly derived from αs-, β- and κ- caseins and are result of the action of rennet enzymes, proteinases and peptidases from starter bacteria, secondary microflora, indigenous milk enzymes and exogenous proteolytic enzymes, if used. According to current knowledge, the major source of bioactive peptides is αs- and to lesser extent β- and κ- caseins (Barać et al., 2017). It is well known that the presence of certain caseins depends on the origin of milk and that β-casein is the major casein of goat`s milk. Thus, it can be assumed that besides ripening conditions, the level of bioactive peptides is determined by the origin of milk.
Lider cheese is a commercial full-fat semi-hard cheese which is produced from pasteurized goat`s milk. It is produced in the mountain area of Nikšić (Montenegro) at an altitude above 700 meters, with unique conditions for livestock breeding and the production of milk and dairy products. Since this is a small cheese (the weight of ripened cheese is about 500-550g) it can be assumed that its ripening is specific. Thus, this work aimed to characterize the change of protein and fatty acid profiles, mineral content as well as the change of antioxidant potential during one month of Lider cheese ripening.
Material and methods
Cheese making
The Lider cheese was produced in the small family cheese dairy "Miljanić" near Nikšić from pasteurized (63-65 °C for 30 min) full-fat goat's milk using a freeze-dried starter culture (DELVO TEC, DX-33C DSL DSM Food Specialities) composed of mesophilic lactic acid bacteria Lactococcus lactis ssp. lactis, Lactococcus lactis ssp. cremoris, Lactococcus lactis ssp. lactis biovar. diacetylactis, Leuconostoc spp. Milk was coagulated at 32 °C using commercial rennet Maxiren (DSM Food Specialities, 1g/100 L milk). The obtained curd was cut crossways and tempered at 36-38 °C for 60 minutes. The curd was pressed for 12 hours and salted during 24 hours in brine prepared from the whey, which contained 16 % NaCl. The weight of cheese after pressing was around 720-820g. The cheese was moved to the ripening room at the Faculty of Agriculture and Food Sciences, Sarajevo at a temperature of 11-12 °C and a relative humidity of 85 % in the ripening room for up to 30 days.
Cheese making procedure was carried out on three consecutive days in three replicates. The cheese was sampled at the 1, 15 and 30 days of ripening.
Chemical analysis
The chemical composition of Lider cheese was determined by using standard analytical methods. Dry matter of the cheeses was determined by the standard drying method at 102±2 °C (IDF, 1982) whereas fat content was determined according to the method of Van-Gulik (IDF, 1986). The fat content was expressed as fat in dry matter (FDM). Moisture on a fat-free basis (MFFB) was calculated according to the CODEX ALIMENTARIUS (1978). The nitrogen content was determined by the Kjeldahl method (Ardö and Polychroniadou, 1999), and the protein content was calculated as the nitrogen content multiplied by 6.38 and expressed as percentage of dry matter (%TP/DM). The salt content was estimated by the potentiometric method using MK II chloride Analyzer 926 (Sherwood Scientific, UK) and expressed as salt content and salt in moisture content (S/M) as suggested by Guinee and Fox (2004). The pH of cheeses was measured by using a pH meter (Metrohm 632, Methrom, Switzerland) with WTW - SenTix Sp (Xylem Analytics, UK) electrode.
Assessment of proteolysis
The change of nitrogen compounds of Lider cheese during ripening was followed by using water-soluble nitrogen (WSN) and nitrogen soluble in 12 % trichloracetic acid (TCA-SN) as suggested by Kuchroo and Fox (1982). Briefly, 10 g of previously homogenized cheese was extracted in 50 mL of deionized water for 60 min at 40 °C. After that, the extract was cooled at the room temperature, filtered through Whatman No1 and centrifuged at 4.000 g for 15 min. A quantity of 20 mL of clear supernatant was used to determine the content of water soluble nitrogen (WSN) by the Kjeldahl method (Ardö and Polychroniadou, 1999). A quantity of 10 mL of supernatant was treated with 10 mL of 24 % TCA, filtered and its nitrogen content was determined by the same method. Both parameters are calculated as a percentage of total protein content. Besides these parameters, proteolysis was also monitored by electrophoresis of Tris-HCl extracts of cheese proteins and water-soluble fractions under reducing conditions (SDS-PAGE) using the method of Fling and Gregerson (1986). Electrophoresis was performed on 5 % (w/v) stacking and 12.5 % (w/v) resolving gel (Gel electrophoresis apparatus, LKB-2001- 100, LKB, Uppsala, Sweden). Tris-HCl extract of cheese was prepared as suggested by Barać et al. (2021). Water-soluble fraction which was prepared by the method of Kuchroo and Fox (1982) was diluted with Tris-HCl buffer (0.055 M Tris–HCl, pH 6.8, 2 % SDS, 5 % β-mercaptoethanol (v/v), 7 % glycerol, 0.0025 % bromophenol blue) and filtered. Low molecular mass calibration kit (Pharmacia, Uppsala, Sweeden) was used to estimate molecular masses of the identified polypeptides and proteins. The scanned gels were analysed by SigmaGel software version 1.1 (Jandel Scientific, San Rafalel, CA). Caseins and polypeptides were quantitatively determined by integration of peak volumes. Intensity of casein bands was quantified from the gel on which 25 μL of the samples were applied. Each pattern was analysed in triplicate. Residual content of identified caseins was expressed as a percentage of their initial contents of 1-day-old cheese.
Fatty acid profiles
The fatty acid content of Lider cheese was determined as suggested by Barać et al. (2019). A quantity of 0.5 g of cheese was extracted in 10 mL of heptane in an ultrasonic water bath for 1 hour and then for 24 hours at the room temperature. The extract was filtered through Whatman No. 1 filter paper and evaporated under a stream of nitrogen. Fatty acids were dissolved in 1 mL of hexane and transformed into methyl esters (FAME`s) using 1 mL of 14 % Borontrifluoride-Methanol. The mixture was heated at 100 °C for 1 hour, cooled to the room temperature and metal esters were separated in the hexane phase after the addition of 1 mL of deionized water.
FAMEs were separated using capillary gas chromatography with flame ionization detector (GC/FID) The GC/FID (Agilent Technologies 6890, Santa Clara, CA, USA) was equipped with split/splitless injector and SP-2560 (length 100 m, i.d. 0.25 mm, film thickness 0.20 μm, Supelco, Bellefonte, USA). The obtained chromatographic peaks were identified by using Supelco 37 Component FAME mix standard (Supelco, Bellefonte, USA). Fatty acid content was calculated in mg/g of lipids and expressed in relative quantities as the mass percent of total fatty acids.
Indexes of lipid quality
Based on the fatty acid profile analysis, the unsaturated/saturated fatty acids (SFA/UFA) ratios, desirable fatty acids (DFA), hypercholesterolemic fatty acids (OFA), the atherogenicity index (AI) and thrombogenicity indices (TI) of cheeses were calculated. DFA was calculated according to following equation:
DFA= ΣMUFA +ΣPUFA + C18:0
The OFA was calculated as:
OFA = C12:0 + C14:0 + C16:0
The AI and TI indices were calculated as proposed by Ulbricht and Southgate (1991) through the equations:
AI = [(4 × C14:0) + C16:0]/ΣMUFA +ΣPUFA (1)
TI=(C14:0+C16:0+C18:0)/(0.5MUFA+0.5PUFA-n6+3PUFAn3+PUFA-n3/PUFA-n6) (2)
AI indicates the relationship between the sum of the main saturated FAs (considered as pro-atherogenic) and the main classes of unsaturated FAs (considered as anti-atherogenic). TI reflects the tendency to form clots in the blood vessels and represents the relationship between the pro-thrombogenetic (saturated) and the anti-thrombogenetic fatty acids.
Mineral profiles
Prior to determination of the total content of elements, the homogenized sample (0.5 g) was placed in PTFE vessel, followed by addition of 7 mL 65 % HNO3 and 2 mL of 30 % H2O2. The mixture was digested in microwave oven (CEM Mars 6, US) at the temperature of 210 °C and pressure of 6 atmospheres. The sample was cooled to room temperature and made up to 25 mL with deionized water and conserved at 4 °C until its analysis with ICP.
The content of the macroelements Na, K, Mg, Ca, S and P was determined by the method of Inductively coupled plasma - optical emission spectrometry (ICP-OES), model ICAP 6500 Duo (Thermo Scientific, United Kingdom). The calibration curves were constructed by dilution of the solution obtained by mixing standard PE-CAL4-ASL-1 for Na, K, Mg and Ca, ICP-41W-1 standard for S and ICP standard ICP-41W-1 for P.
The content of 13 micro and trace elements, including Fe, Zn, Cu, Mn, Mo, Co, Cr, Se, B, Al, Ni, Pb, Hg, Cd and As was determined by Inductively coupled plasma mass spectrometry (ICP-MS) (ICAP Q, Thermo Scientific, UK). The system was controlled with Qtegra Instrument Control software. For calibration ICP multi-element standard solution XXI for MS (MES-21-5) and Hg ICP standard suppl. to multi-element standard XXI for MS (MS MES-21-HG-1) were used.
Antioxidant properties
The effect of ripening on antioxidant properties of Lider cheese is determined by using two parameters, total antioxidant capacity (TAC) and ferric reducing power (FRAP). TAC was assayed using so called QUENCHER (Quick, Easy, New, Cheap and Reproducible) according to Serpen et al. (2008) which is based on the direct measurement of the antioxidant activity of solid samples. As the stock solution 7 mM aqueous solution of ABTS (2,2-azino-bis / 3-ethyl-benothiazoline-6-sulfonic acid) with 2.45 mM potassium persulfate was used. ABTS • + working solution was prepared by diluting the stock solution with water/ethanol (50:50, v/v). A quantity of 10 mg of grated cheese was mixed with 1 mL of working ABTS solution (absorbance was 0.7-0.8). The mixture was vigorously vortexed for 7 min and then centrifuged at 17.000 g for 2 min. The absorbance of supernatant was measured at 734 nm. The ability of the cheese to neutralize free radicals was calculated using the following equation:
% of ABTS inhibition = ((Ac-As)/Ac) x 100 (3)
where:
Ac - absorbance of control
As - absorbance of control
Total antioxidant capacity was also calculated as the Trolox equivalent antioxidant capacity (TEAC) in mg of Trolox per kg of sample.
Ferric reducing power was measured according to Meira et al. (2012) slightly modified by Barac et al. (2019). Grated cheese was dissolved in potassium phosphate buffer (15 mg/mL) and stirred vigorously on a mechanical shaker for 2 h. Then, the extract was centrifuged at 3.000 x g for 10 min and the obtained supernatant was used for FRAP analysis. Briefly, 2.5 mL of the supernatant was mixed with 2.5 mL of potassium ferricyanide and incubated at 50 °C for 20 minutes. After that, 2.5 mL of TCA was added and the precipitate was separated by centrifugation at 3000 g for 10 min. The supernatant (2.5 mL) was mixed with 2.5 ml of deionized water and 0.5 mL of iron chloride. The mixture was vigorously vortexed and the absorbance was measured at 700 nm. An increase of the absorbance indicates better FRAP activity of the samples.
Results and discussion
Chemical composition of Lider cheese
The chemical properties of Lider cheese at different stage of ripening are shown in Table 1.
Table 1. Chemical composition and relative content of the major caseins in Lider goat's cheese at different stages of ripening
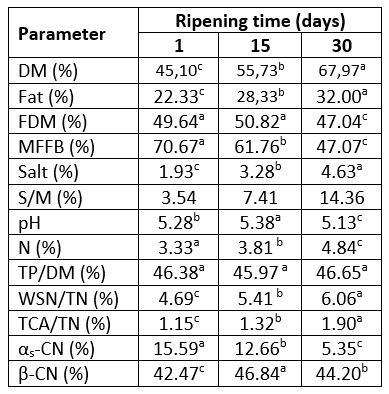
*values represent means; values with the same letter within the same row are not statistically significant at p<0.05; relative content based on densitometric analysis of SDS-electrophoregrams; DM - dry matter, FDM - fat in dry matter, MFFB - moisture on a fat-free basis, S/M - salt in moisture, TP/DM - total protein in dry matter, WSN/TN - water soluble nitrogen per total nitrogen, TCA/TN - nitrogen soluble in 12 % trichloracetic acid per total nitrogen.
According to the results presented in Table 1, ripening under conditions used in this study significantly (p˂0.05) affects chemical composition of Lider cheese. The only exception was the TP/DM content, since no significant influence of ripening on the TP/DM content was observed. Due to the continuous loss of moisture throughout 30 days of ripening the average DM content of Lider cheese increased from 45.10 % to 67.97 %. This is followed by the reduction of FDM and MNFS up to 47.04 % and 47.07 %. Furthermore, due to the reduction of moisture and increase of the NaCl content, the salt in moisture increased to 14.36 %. Besides ripening conditions, such decrease of moisture content as well as MNFS values can be attributed to the size of cheese. Lider cheese is prepared as a small one (720-820g after pressing) that loses moisture easily.
Ripening during 30 days affects pH of Lider cheese. In fact, after 15 days of ripening pH slightly increased from 5.28 to 5.38 and then decreased up to 5.13.
According to the content of FDM, goat's "Lider" cheeses belong to the category of full-fat cheeses. As the moisture on fat-free basis (MFFB) of 30-days-old cheese was less than 51 % (47.07 %) it can be characterized as extra-hard cheese (CODEX ALIMENTARIUS, 1978). Furthermore, based on the results of the moisture content and MNFS values it can be estimated that under conditions used in this study ripening for 15-20 days provides characteristics of semi-hard full-fat cheese. The average parameters of 15-days-old Lider cheeses were in accordance with parameters for semi-hard full-fat goat`s cheese ripened for 45 days reported by LópezRuiz et al. (2023) whereas chemical parameters of 30-days old- cheese were similar to those reported by Alvarez and Fresno (2021) for Palmero goat cheese of the same age.
Assessment of proteolysis
The effect of 30 days of ripening of Lider cheese on nitrogen compounds is characterized by the change of water-soluble (WSN) and trichloracetic acid soluble nitrogen fraction (TCA/SN); both fractions are expressed as percent of total nitrogen (Table 1). The content of WSN/TN reflects the level of proteolysis in general, whereas TCA-SN/TN as a part of WSN fraction indicates the level of low molecular weight products formed during the secondary proteolysis (Kuchroo and Fox, 1982). Also, proteolysis was followed by the SDS-PAGE of total proteins and of water-soluble fractions (Figure 1). Based on the results of densitometry analysis of the obtained SDS-profiles the residual content of the major casein fractions was determined (Figure 2).
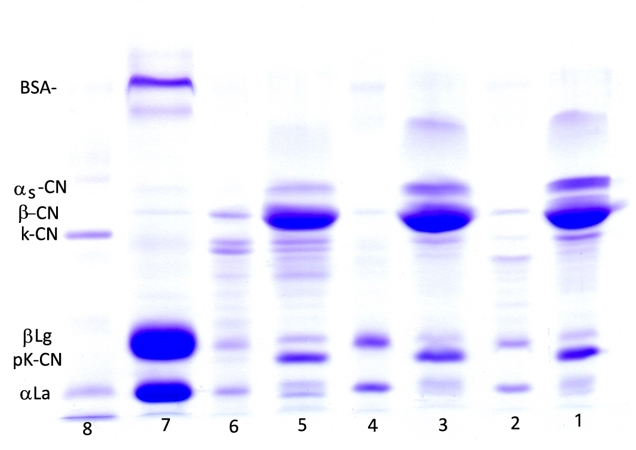
1, 3, 5 - Tris-HCl extracts of cheese ripened for 1, 15 and 30 days, respectively;2, 4, 6 - WSN of cheese ripened during 1, 15 and 30 days, respectively; 7. Goat`s whey; 8. Molecular weight standards
Figure 1. The change of SDS-profiles of Tris-HCl extracts and WSN fractions of Lider cheese
According to the results presented in Table 1, nitrogen compounds underwent noticeable but slow changes during 30 days of ripening. The one-day-old cheese had the low average value of the WSN/TN content. The average WSN/TN content of the one-day-old cheese was 4.69 %. This indicates a slow proteolysis that occurred during cheese making process and the first day of ripening. Since the TCA-SN/TN content was only 1.15 %, the WSN fraction was mostly composed of high molecular weight peptides that were products of primary proteolysis. This was in accordance with the SDS-profile of WSN fraction of one-day-old cheese which is composed of several high molecular weight products (Figure 1, Line 2). It is well known that primary proteolysis occurs due to the activity of chymosin, plasmin and other indigenous milk enzymes mainly on αs-casein and β-casein. Ripening of Lider cheese during 30 days induces continual but slow increase of both, WSN/TN and TCA-SN/TN contents; after this period of ripening the value of these parameters increased to 6.06 % and 1.90 %, respectively. In other words, a slight increase in WSN/TN and TCA-SN/TN clearly indicates a slow primary and secondary proteolysis which could be attributed to the loss of a significant amount of moisture and the increase of salt and salt in moisture content reported in Table 1. It is known that the loss of moisture and the increase of salt content create unfavourable conditions for the activity of both, exogenous and endogenous proteolytic enzymes, as well as bacteria primarily responsible for secondary proteolysis. Similar level of WSN/TN and TCA-SN/TN in goat`s cheese after 30 days of ripening was reported by Burgos et al.(2016). The observed trend of WSN/TN and TCA-SN/TN are in good accordance with the SDS-patterns of water-soluble fractions (Figure 1, Lines 2, 4 and 6). These patterns were mainly composed of peptides with m.w. range of 16-20 kDa. It can be assumed that they were products of primary proteolysis.
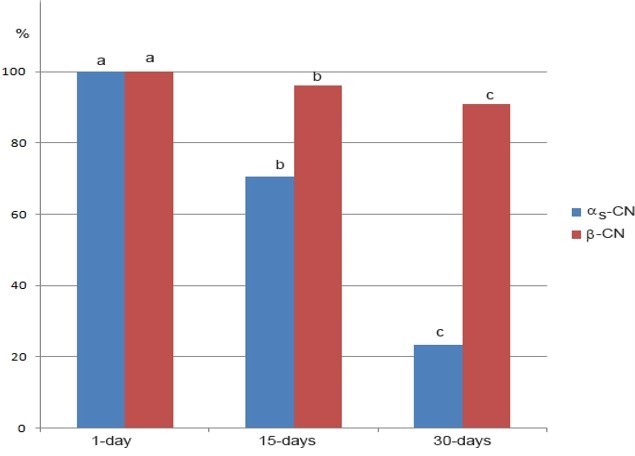
*data marked with different letter within the same casein are significantly different at p<0.05
Figure 2. Changes of the residual content (%) of αs-CN and β-CN during 30 days of ripening of Lider cheese
The 30-days of ripening affected the distribution of the major caseins, αs-CN and β-CN of Lider cheese (Figure 1, Table 1). Ripening reduced the content of both caseins, but to the different extent. In addition, the level of residual αs-CN and β-CN in Lider cheese depends on the time of ripening (Figure 2). Different susceptibility of αs-CN and β-CN to proteolysis in the case of different cheese varieties is well documented. Numerous authors (Hayaloglu et al., 2002; Sarantinopoulos et al., 2002, Barać et al., 2016) showed higher resistance of β-CN to proteolysis compared to αs-CN. Similarly, under ripening conditions used in this study, β-CN expressed higher resistance to proteolysis compared to αs-CN. After 30 days of ripening, the relative ratio of αs-CN decreased from the initial 15.59 % in one-day-old cheese extracts to 5.35 % (Table 1). Consequently, the ratio of β-CN slightly increased from 42.47 % (one-day-old cheese) to 44.20 %. However, during the 30 days of ripening the residual content of both caseins decreases; after that period initial content of αs-CN and β-CN was reduced to 23.25 % and 90.92 % of initial quantity (Figure 2). In addition, it is evident that more intensive proteolysis, especially of αs-CN after 15 days occurred.
Fatty acid profiles and health lipid indices
The effect of 30 days of ripening on fatty acid composition and health lipid indices of Lider cheese is shown in Table 2 and Table 3. According to the results presented in Table 2, in fresh (one-day-old) Lider cheese 15 fatty acids were detected; ten of them were saturated (SFA) whereas five were unsaturated (monounsaturated, MUFA and polyunsaturated, PUFA) fatty acids. As expected, SFAs were dominant and represented 66.81 % of all identified fatty acids of 1-day old cheese; three of them, miristic acid (C14:0), palmitic acid (C16:0) and stearic acid (C18:0) were found to be the major and represented 15.46 %, 13.10 % and 26.0 % of all detected fatty acids, respectively (Table 2). The major unsaturated fatty acids (USF) of one-day-old cheese were oleic (C18:1n9c) and linoleic (C18:2n6c). Fatty acid profiles of investigated cheeses qualitatively were quite similar. The absence or presence of behenic (C22:0) and miristoleic acid (C14:1) was the only difference between fresh and ripened cheeses samples. However, ripening induced the change of fatty acid contents and consequently the ratio of SFAs and UFAs contents. During 30 days of ripening the content of SFAs decreased up to 60.40 %, mainly due to the reduction of long-chain saturated fatty acids (LCSFAs, C16:0-C24:0) content, especially stearic acid (Table 3). This can be attributed to the ability of stearic acid (C18:0) as the most abundant SFA of goat`s cheese to rapidly convert into healthier C18:1 (oleic acid) fatty acid (Jakobsen et al., 2009). This was supported with the increased level of oleic acid detected in 15- and 30-days-ripened samples.
The observed content of SFAs of a 30-days-old cheese was in good agreement with data for commercial hard goat cheese reported by Paszczyk and Łuczynska (2020). It is interesting to note that under conditions used in this study in all examined cheeses short-chain fatty acids (C4-C6, SCFA) were not detected. Furthermore, lauric acid (C12:0) and capric acid (C10:0) were not detected whereas myristic acid (C14:0) was the major medium-chain fatty acid (C8:0-C14:0). The absence of these fatty acids in the case of hard Kupres cheeses also was reported by Sarić et al. (2022).
Table 2. Changes in the fatty acid profile during 30 days of ripening of Lider cheese
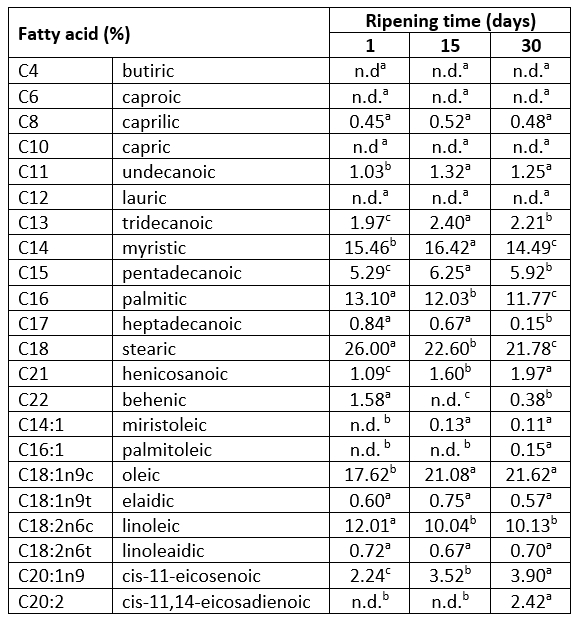
*values represent means; values with the same letter within the same row are not statistically significant at p<0.05
For a long time SFAs in cheese were associated with negative effects on blood cholesterol levels and with the development of numerous diseases, including cardiovascular disease, type II diabetes mellitus, obesity, and cancer. Today it is known that there is no clear evidence that cheese lipids contribute to any disease. Also, it is known that the total plasma blood cholesterol raising effect depends on fatty acid chain length and that LCSFAs, as the major group of SFAs in Lider cheese (36.05-42.61 %, Table 3) are less potent than MSCFAs. Furthermore, lauric acid which is not detected in Lider cheese increased blood cholesterol levels to the higher extent than miristic or palmitic acid (Parodi, 2009).
The fat extracted from Lider cheese also contained pentadecanoic acid (C15:0) and heptadecanoicacid (C17:0) which originated from milk and are the result of microbial fermentation in rumen. Their content depending on time of ripening varied from 0.84-0.15 % and 5.29-5.92 %, respectively (Table 2). These fatty acids are generally accepted as biomarkers of milk fat intake (Yakoob et al., 2014). Today, it is believed that these fatty acids have a positive effect on insulin sensitivity and thereby reduce the risk of diabetes type II development (Forouhi et al., 2014). The presence of pentadecanoic acid and heptadecanoic acid in high percentages is associated with organic production of milk (Kusche et al., 2015).
According to the Table 3, thirty days of ripening significantly reduced the content of SFAs. As a result, the level of UFAs increased, mainly due to the increase of MUFAs content. During this period of ripening, the content of UFAs increased from 33.19 % to 39.60 % whereas the level of PUFAs was almost unchanged and represented 13.25 % of identified fatty acids (Table 3). A similar trend of the UFAs content in the case of Kupres hard cheese was observed by Sarić et al (2022). These authors showed that more intensive bioconversion of a part of MUFAs into PUFAs of Kupres cheese started after 30 days of ripening. Since the most abundant MUFA in 30-days-old Lider cheese was oleic acid (C18:1n9c) which is known as a precursor for linoleic acid biosynthesis (Chamberlin et al., 2014), it could be expected that longer-ripened cheeses would have higher content of linoleic acid and other PUFAs.
The analysed cheeses are characterized with high content of UFAs detected even in one-day-old cheese. This can be attributed to grazing of goats on mountain pastures. It is known that grazing on mountain pastures affects milk fat composition. Romanzin et al. (2013) established that grazingon mountains increased the level of unsaturated acids, especially PUFAs whereas Moneeb et al. (2019) suggested that an intensive silvopastoral system of grazing caused high content of lauric, myristic and palmitic as well as high content of stearic, linoleic and γ-linolenic acid in milk and in fresh Domiati cheese.
Table 3. Changes in health indices of Lider cheese during 30 days of ripening*
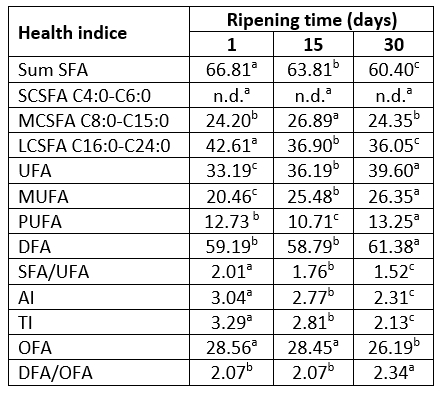
*data were presented as average values; values with the same letter within the same row are not statistically significant at p<0.05; SFA - saturated fatty acids; SCSFA - short chain saturated fatty acids, MCSFA - medium chain saturated fatty acids, LCSFA - long chain saturated fatty acids, UFA - unsaturated fatty acids, MUFA - monosaturated fatty acids, PUFA - polyunsaturated fatty acids, DFA - desirable fatty acids, AI - atherogenicity index; TI - thrombogenicity index; OFA - hypercholesterolemic fatty acids.
According to the results in Table 3, due to the observed changes of fatty acid profiles, ripening improved fatty acid health indices of Lider cheese fat. The 30-days-ripened cheeses are characterized with significantly (p<0.05) lower values of SFA/UFA, AI and TI compared to one-day-old samples. The observed values of AI and TI parameters of 30-days-old Lider cheese (2.31, 2.13) were lower than those reported by Bodnár et al (2021). These authors showed that AI and TI of goat`s cheeses prepared from milk obtained from extensively grazed goats were 2.47 and 2.68, respectively. This can be attributed to the higher content of PUFAs, especially linoleic acid. It is known that AI and TI reflected atherogenic and thrombogenic potential of cheese. Thus, lower values of these parameters indicate that the consumption of Lider cheese can contribute to the reduction of the risk of coronary heart disease.
Mineral profile of Lider cheese
Mineral content of Lider cheese expressed on dry matter basis is shown in Table 4. Under experimental conditions in Lider cheeses,6macroelements (calcium, phosphorus, magnesium, potassium, sodium and sulphur) and 6 microelements including iron, zinc, copper, manganese, cobalt and boron were detected. In addition, potentially harmful trace elements such as Al, Pb, Cd and Hg that are usually associated with environmental or cheese making process contamination were not detected. According to Table 4, mineral profiles of investigated cheeses were quite similar. In fact no significant differences (at p<0.05) between the content of detected macro- and microelement contents were observed during cheese ripening. The only exception was the low content of Cr (0.216 μg/100 g) detected only in 30-days-old samples. The absence of significant differences between the mineral profiles can be attributed to the ripening method, but also to the fact that the content of macro and microelements is expressed on dry matter.
Table 4. The change of mineral contents of Lider cheese during 30 days of ripening
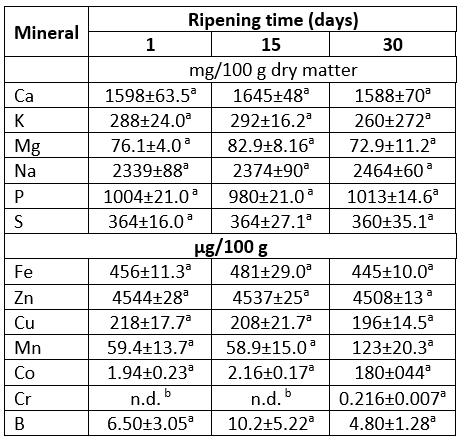
*values with the same letter within the same row are not statistically significant at p<0.05
The major macroelements of Lider cheeses were Ca, Na and P. Their contents were in the range of 1588-1645 mg/100 g, 2339-2464 mg/100 g and 980-1013 mg/100 g, respectively (Table 4). It is known that numerous factors including type and quality of milk (genetic characteristics, the stage of lactation, environmental conditions, the type of pasture, soil contamination etc.) and cheese processing, particularly ripening, effected mineral content of cheese. Thus, data related to mineral content of various types of goat cheeses reported in literature greatly varied. However, in general mineral content of Lider cheese contents of the major macroelements are in accordance with data for different goat's cheeses presented by da Paixão Teixeira et al (2022) and Gebreyowhans et al (2020). Consequently, the ratio of Ca/P in Lider cheese (1.57-1.68) was similar to those found in several Brazilian goat cheeses investigated by da Paixão Teixeira et al (2022). Besides macroelements, Lider cheese is a good source of Fe, Mn and especially Zn, which is the major microelement detected in investigated samples. Its content was 4508-4544 μg/100 g. Therefore, in light of the results of this investigation (which is supported with current scientific literature), consumption of Lider cheese could be recommended for the daily intake of macroelements such as Ca, P, Mg and K, and also for an additional supply of Zn.
Antioxidant properties of Lider cheese
The change of in vitro antioxidant properties of Lider cheese during 30 days of ripening is shown in Table 5. Antioxidant properties of investigated cheeses are determined as Total antioxidant capacity (TAC) and as ferric reducing power (FRAP). The total antioxidant capacity is presented as the ability of whole cheese samples to scavenging free ABTS radicals are and expressed as % of their inhibition and as the Trolox equivalent antioxidant capacity per kg of wet sample (TEAC).
Table 5. The effect of ripening on antioxidant properties of Lider cheese
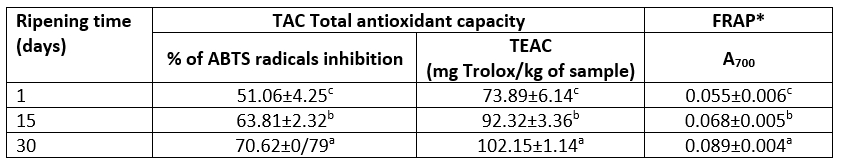
*FRAP - ferric reducing power; TEAC- Trolox equivalent antioxidant capacity; data marked with different letter are significantly different at p<0.05
According to the results presented in Table 5, it is evident that the ripening process greatly influences the level of the ability to scavenging free ABTS radicals and to a lesser extent FRAP. However, all detected values of both characteristics (TAC and FRAP) of Lider cheeses were significantly different (at p<0.05). The one-day old Lider cheese had a relatively high ability to scavenging ABTS radicals and low reducing power; the average percent of ABTS inhibition was 51.05 % and the average TEAC value was 73.89 mg Trolox/kg whereas FRAP was 0.055. These properties are mainly associated with free amino acids, low- and high molecular peptides and proteins of cheese. It is known that their function as natural antioxidants is complex and is related to the several properties including the ability to scavenging or quenching of reactive oxygen species and/or free radicals, to chelate the transition of metal ions, to reduce ferric ions and their ability to inhibit oxidation of lipids, proteins and DNA. These properties in turn are dependent on amino acid sequences of peptides and proteins, correct position of individual amino acid residues, molecular weight and physical structure, the content of amino acid residues that contain sulfhydryl groups, the level of aromatic amino acid residues and histidine (Žilić et al., 2012; Esfandi et al., 2019).
As previously mentioned, antioxidant properties of whole cheeses were determined in this work. Due to the lack of data related to the total antioxidant capacity of whole cheeses, as well as the difference in the methods used for their determination, the comparison of the obtained values with the data present in current literature is difficult. Namely, the largest number of reported data refers to the antioxidant capacity of water extracts of cheeses but not to whole cheeses. Although the water-soluble fraction exhibits a significant ability to capture free radicals, Barać et al. (2019) showed that the water-insoluble fraction, which makes up about 80 % of whole cheese proteins, had a significant ability to capture free radicals. However, the established values of TAC and reducing power are consistent with those for hard Kupres cow`s cheese reported by Saric et al. (2022).
Ripening during 30 days increased both investigated antioxidant properties; after that period the average values of TEAC and FRAP raised up to 70.62 % and 0.089, respectively (Table 5). In other words, after 30 days of ripening TEAC increased by 38.25 % and FRAP by 61.82 %. Such trends of these parameters agreed well with data reported for different cheese varieties. Namely, based on the work of numerous authors (Gupta et al., 2007, Meira et al., 2012; Barać et al., 2019, 2021, Vučić et al.,2020), it is known that the most intensive increase of antioxidant capacity of cheese occurs during initial three month of ripening. The improvement of the total TAC and FRAP of Lider cheese can be associated with the increase of the content of both, WSN- and TCA-soluble fractions. Furthermore, according to SDS-electrophoretic analysis, the increased level of peptides with molecular weight higher than 10 kDa may be responsible for the improved antioxidant properties of 30-days ripened Lider cheese. Pritchard et al (2010) suggested that these fractions possess medium antioxidant ability due to increased electron donating properties and increased ability to react with free radicals. The results related to the change of residual contents of the major caseins (Table 1) showed that αs-CN was to the much higher extent degraded than β-CN. Thus, it can be assumed that the most of products responsible for improved antioxidant properties originate from this casein.
Conclusion
From the results reported in this study it is evident that ripening even within 30 days greatly influences nutritive and functional characteristics of Lider cheese. Ripening of Lider cheese during one month is characterised by a relatively slow proteolysis greatly due to the intense loss of moisture. In addition, the improvement of the SFA/UFA ratio and other fatty acid health indices occurred. Proteolysis mainly occurred on αs-CN; its residual level after 30 days was 23.25 %. Simultaneously, the content of β-CN was 90.92 %. One-month-old cheese had improved indexes of lipid quality (such as AI, TI and DFA/OFA ratio) as well as reducing power and ability to scavenge free radicals. Ripening had no significant (at p<0.05) influence on the content of macro- and microelements in DM of Lider cheese.
References
da Paixão Teixeira, J.L., Lima Pallone, J.A., Andrade, C.D., Mesías, M., Seiquer, I. (2022): Bioavailability evaluation of calcium, magnesium and zinc in Brazilian cheese through a combined model of in vitro digestion and Caco-2 cells. Journal of Food Composition and Analysis 107, 104365.https://doi.org/10.1016/j.jfca.2021.104365
