Introduction
The genus Centaurea L., comprising more than 300 species, is one of the largest genera in the Asteraceae and is widespread across the world (Bremer 1994). Most species of the genus are distributed in the Balkan Peninsula and Türkiye (Siljak-Yakovlev et al. 2005). Türkiye is one of the main diversity centers of Centaurea, where it is represented by 204 species, 59% of which are endemic (Sirin et al. 2020). Centaurea kilaea Boiss. is a plant endemic to Türkiye that spreads only in the provinces of Kirklareli, Istanbul, Sakarya, and Bolu (Wagenitz 1975). The species is classified as endangered (EN) according to the Red Data Book of Turkish Plants (Ekim et al. 2000). The chromosome number of the species has been reported as 2n = 4x = 36, and the amount of 2C nuclear DNA as 3.68 pg (Meric et al. 2010).
Embryological studies on members of the Asteraceae family date back to earlier years (Desole 1954, Davis 1962, 1964, Renzoni 1970). Recently, the embryology of some species belonging to the Asteraceae family has been studied; some examples are Centaurea achtarovii, Arnica montana, Pilosella brzovecensis, Ageratum conyzoides, and Ageratum fastigiatum (Franca et al. 2015, Yankova-Tsvetkova et al. 2016, 2018, Bonifacio et al. 2018, Janas et al. 2021). Members of the Asteraceae family have an inferior, bicarpellate, and unilocular ovary. The ovary contains an anatropous, unitegmic, and tenuinusellate ovule with basal placentation (Davis 1966). Although most species contain Polygonum-type embryo sacs, some also include Allium-type, Drusa-type, Adoxa-type, and Fritallaria-type embryo sacs (Davis 1966, Musial et al. 2012). In members of the Asteraceae family, the anthers are tetrasporangiate. Although there is generally a plasmodial type of tapetum, the existence of a secretory tapetum has been proven in some species (Bonifacio et al. 2018, Franca et al. 2015). Following the simultaneous type of meiosis, tetrahedral, decussate, or isobilateral type tetrads are seen. Pollen is thrown from the anther as three-celled (Davis 1966).
Scientific data on C. kilaea are also very limited. The chromosome number and nuclear DNA content of the species were determined by Meric et al. (2010). In addition, anti-proliferative compounds of the species were isolated and tested against human tumor cell lines (Sen et al. 2015). A literature review shows that there are no studies on the embryology of this species. The aim of this study is to reveal the reproductive biology of C. kilaea, which is distributed in a limited area and included in the endangered category according to the Red Data Book of Turkish Plants (Ekim et al. 2000).
Materials
Capitula containing the buds, blooms, and fruits of C. kilaea were collected from the coast of Igneada (Kirklareli, Türkiye; 41°52′53″ N, 27°59′32″ E) in July. The samples were fixed in an ethyl alcohol/acetic acid mixture (v/v, 3:1) for 24 h, then transferred to 70% ethanol and kept at 4 °C. Likewise, pollen grains were collected from florets blooming in the same habitat.
Histological method
For histological observations, various sizes of anthers and ovaries were embedded in historesin according to the manufacturer’s instructions (Leica historesin-embedding kit). Longitudinal and transverse serial sections (4 μm thick) of the embedded blocks were obtained using a rotary microtome with a tungsten carbide blade. The sections were stained in 0.5% (w/v) toluidine blue O solution (in 0.1 M phosphate buffer at pH 6.8) at 60 °C for 2 min. They were washed in distilled water for 30 seconds and then dried in air (O’Brien et al. 1964, modified). Finally, they were mounted with EntellanTM (Merck) for microscopy.
Histochemical method
Periodic acid–Schiff reaction (PAS) was applied for the determination of insoluble polysaccharides. The 4 µm-thick sections were kept in 1% (w/v) periodic acid solution (in 90% ethyl alcohol) for 30 minutes and rinsed with distilled water at the end of the period. After staining with Schiff’s reagent (Fisher Chemicals) for 30 minutes, the slides were placed for 5 minutes each in 0.5% (w/v) sodium metabisulfite solution. They were washed for 5 minutes with running water and rinsed with distilled water. Then, the slides were dried in the air and mounted with EntellanTM (Merck). For determination of proteins, the slides were stained with 0.025% (w/v) Coomassie Brilliant Blue G-250 (CBB) in distilled water/acetic acid/methanol (v/v/v, 87:10:3) solution for 10 minutes in an oven (60 °C). They were then washed for 2 minutes in distilled water. Then the slides were dried in air and mounted with EntellanTM (Merck) (Heslop-Harrison et al. 1973).
Pollen viability
Acetocarmine dye was used to investigate pollen viability. Ripe pollen grains from newly opened anthers were transferred onto a clean slide, and a few drops of aceto-carmine were added to the slide. After 20 minutes, stained pollen grains were considered fertile (viable), and the unstained pollen grains sterile (non-living). A total of 4000 pollen grains were counted, and the percentage of viability was calculated.
All observations and photography were carried out using an Olympus CX21 light microscope and the KAMERAM software program (Argenit, Türkiye).
Anther wall and male gametophyte development
In C. kilaea, the anthers are tetrasporangiate, and the anther wall development is of the dicotyledonous type. The anther wall is thin and consists of four layers, each in a single layer of cells from the outside to the inside, as follows: the epidermis, endothecium, middle layer, and tapetum. In the early stages, the anther consists of meristematic tissue surrounded by the epidermis. The archesporial cells differentiate beneath the epidermis of the microsporangium and divide periclinally, forming the primary parietal cells and the sporogenous cells (Fig. 1A). The primary parietal layer undergoes a periclinal division, giving rise to two secondary parietal layers (Fig. 1B). The cells of the outer secondary parietal layer undergo periclinal divisions, forming the endothecial layer (outer) and middle layer (inner). The cells of the inner secondary parietal layer differentiate directly into the tapetum (Fig. 1C). At the microspore mother cell stage, the volume of tapetum cells increases, and they begin to divide before the microspore mother cells (Fig. 1D). At the tetrad stage, the middle layer is flattened, and dark-colored materials appear in the cytoplasm of tapetal cells. However, the transverse inner and side walls of the tapetal cells are still intact (Fig. 1E). At the young microspore stage and the vacuolated microspore stage, the inner tangential and radial walls of the tapetum cells are broken down. The tapetal protoplasmic arms extend into the interior of the locule (Fig. 1F and Fig. 1G, arrowheads). At those stages, the middle layer is flattened or not observed. At the pollen mitosis stage, the tapetal protoplasts move into the locule and fuse to form the multinucleate tapetal periplasmodium. This mass surrounds the pollen grains (Fig. 1H). At the mature pollen stage, the endothecial cells radially elongate and develop fibrous thickenings, which arise from their inner tangential walls. The tapetal periplasmodium disappears. The mature anther wall consists of the epidermis and endothecium with fibrous thickenings (Fig. 1I).
The sporogenous cells develop directly into the microspore mother cells in C. kilaea. They are observed with large nuclei and obvious nucleoli (Fig. 1D). Microsporocytes undergo simultaneous meiotic division, generating tetrahedral tetrads (Fig. 1E).
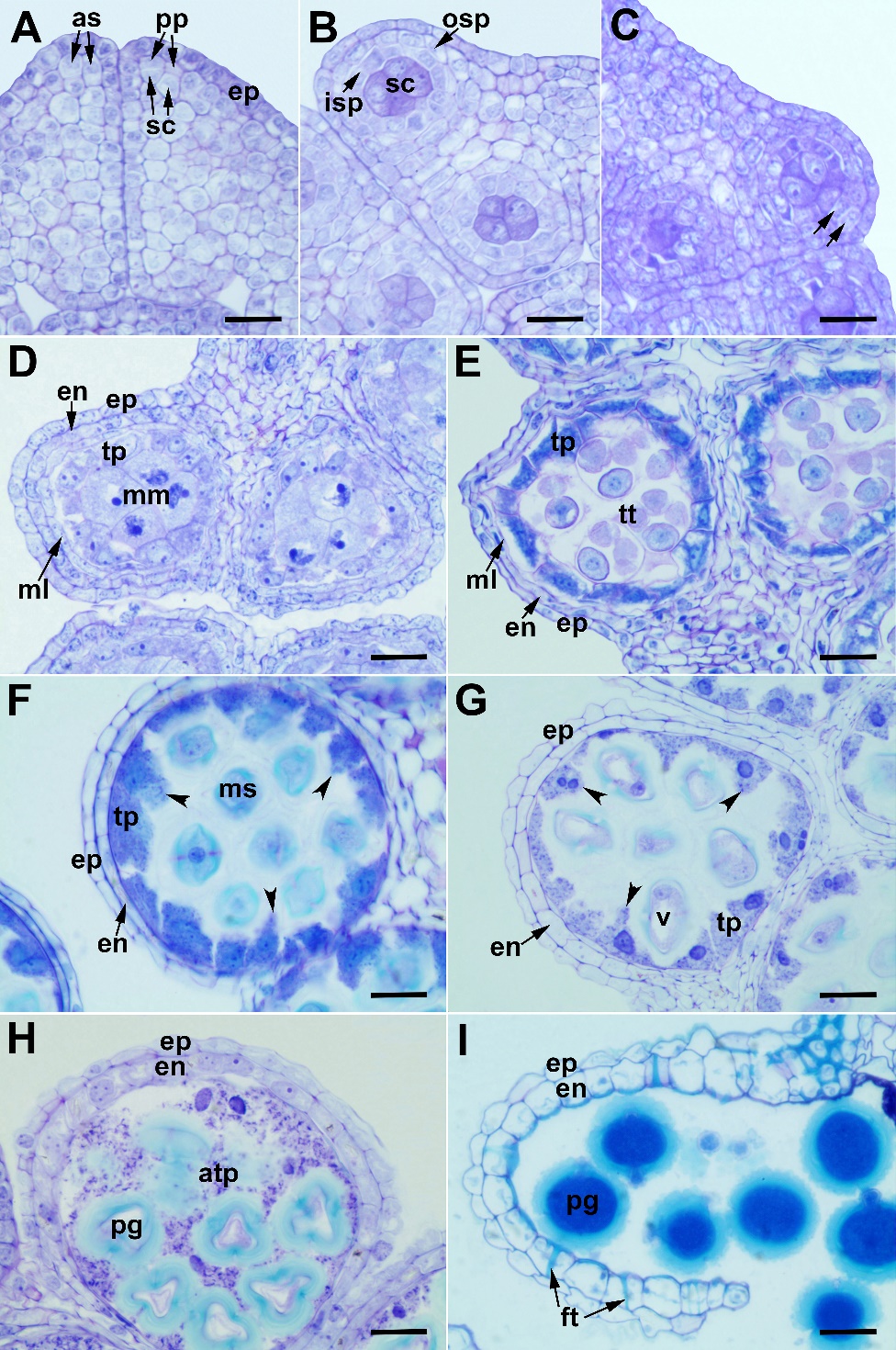
Fig. 1. Anther wall development in Centaurea kilaea: A) archespore cells and their divisions, B) outer and inner secondary parietal layers, C) division of the outer secondary parietal layer (arrows), D) microspore mother cell stage, E) tetrad stage, F) free microspore stage, G) vacuolated microspore stage (arrowheads indicate tapetal extensions), H) pollen mitosis stage, I) anthesis stage. as – archespore cell, atp – amoeboid tapetum, en – endothecium, ep – epidermis, ft – fibrous thickenings, isp – inner secondary parietal layer, ml – middle layer, mm – microspore mother cell, ms – microspore, osp – outer secondary parietal layer, pg – pollen grain, pp – primary parietal cells, sc – sporogenous cells, tp – tapetum, tt – tetrad, v – vacuole. Scale bars = 20 μm.
Microspores released from the tetrad have a prominent nucleus in the middle of the cell (Fig. 2A). Then, the nucleus is pushed to the side due to the vacuole formed inside the cell (Fig. 2B), and it undergoes the first pollen mitosis, resulting in the formation of two unequal cells, a large vegetative cell and a small generative cell (Fig. 2C and Fig. 2D). After the first mitotic division, the vacuole becomes invisible, and the generative cell divides to form two sperm cells (the second pollen mitosis) (Fig. 2E). Sperm cells are initially round in shape, then take the form of threads (Fig. 2F). Mature pollen grains are yellow, prolate-spheroidal, tricolporate and echinate, characteristic of the Asteraceae family.
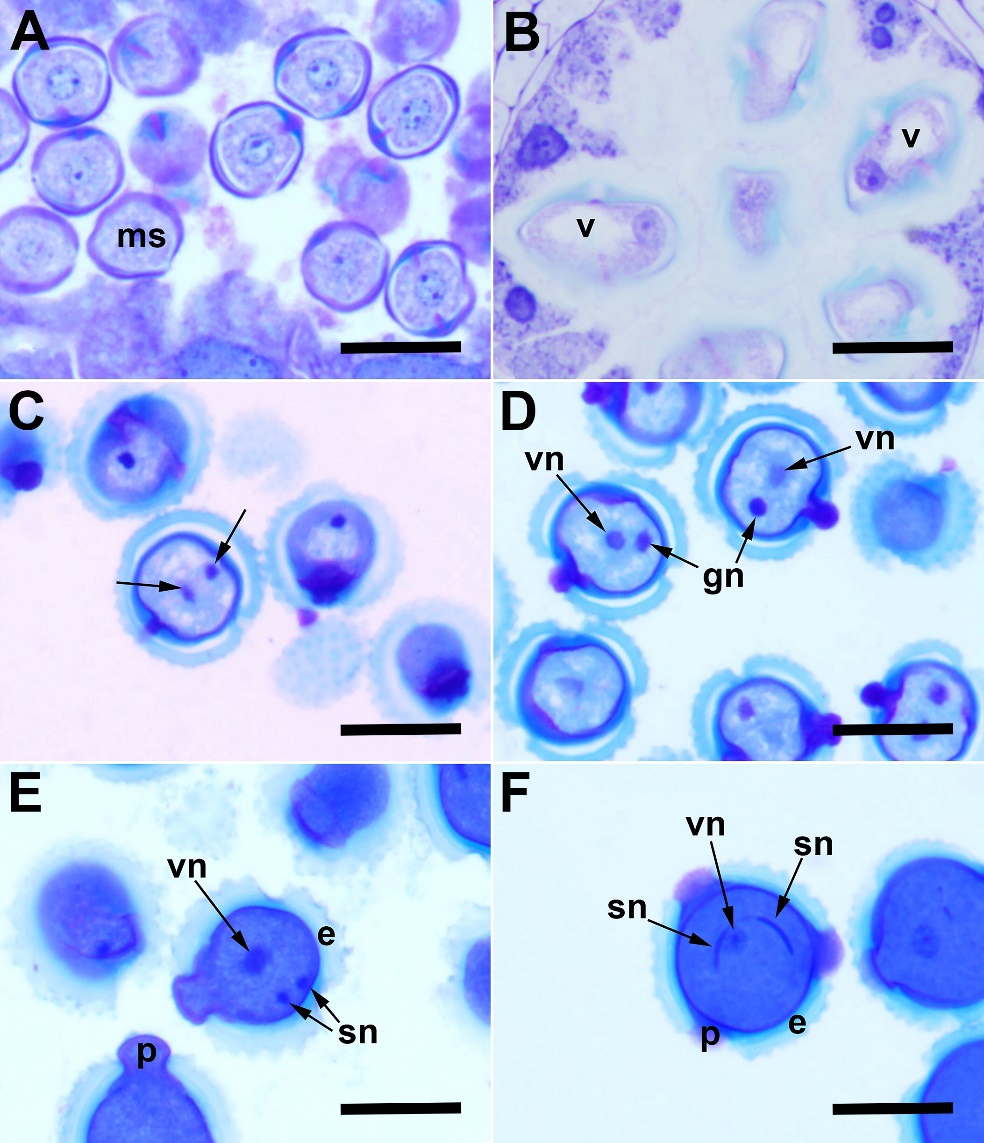
Fig. 2. Male gametophyte development in Centaurea kilaea: A) free-microspore stage, B) vacuolated microspore stage, C) telophase stage at the first pollen mitosis (arrows indicate chromosome groups at poles), D) vegetative and generative nuclei, E) second pollen mitosis, F) mature three-celled pollen grain. e – exine, gn – generative nucleus, ms – microspore, p – pore, sn – sperm nucleus, v – vacuole, vn – vegetative nucleus. Scale bars = 20 μm.
In the PAS reaction employed for staining insoluble polysaccharides in the anther wall layers and pollen, all the anther wall layers and sporogenous cells give a negative reaction in terms of polysaccharide content at the beginning of development (Fig. 3A). In the tetrad stage, tapetum cells react weakly to PAS. At this stage, the epidermis and endothecium cells contain insoluble polysaccharides. Callose gives a positive PAS reaction at this stage (Fig. 3C). Insoluble polysaccharides are seen in the cytoplasm of the mature pollen grain (Fig. 3E). In protein staining with CBB, there is no considerable difference between the protein contents of the anther wall layers and sporogenous cells at the beginning of development (Fig. 3B). However, contrary to the other wall layers, the cytoplasm of tapetum cells becomes rich in protein content starting from the tetrad stage (Fig. 3D). The tapetum layer, which gives a strong positive reaction in the young pollen stage, begins to break down and transfers the protein-structured substances to the maturing pollen grains. Other anther wall layers are poor in protein content at the mature pollen stage (Fig. 3F).
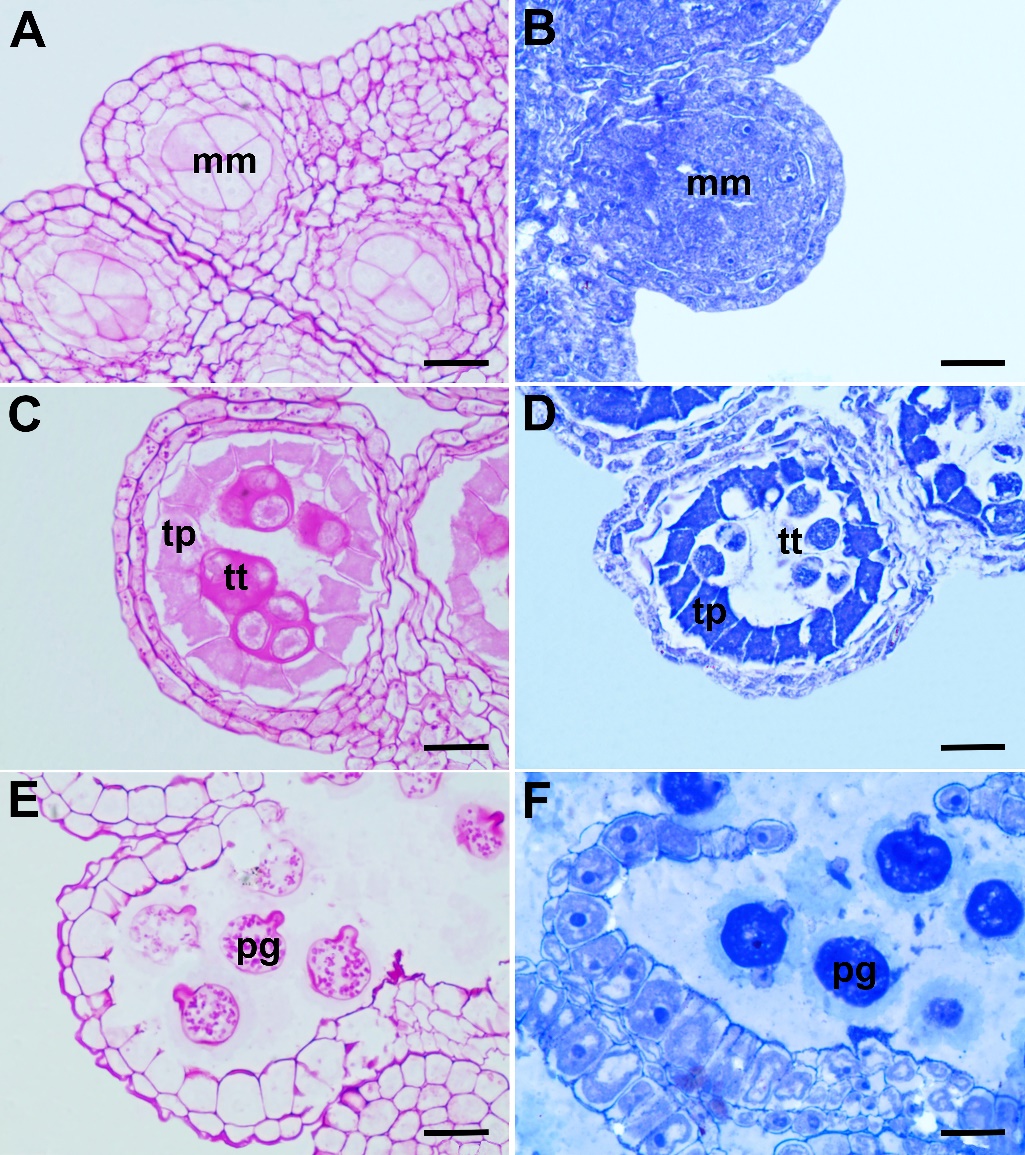
Fig. 3. Histochemical aspects of the anther wall and pollen development in Centaurea kilaea: A) insoluble polysaccharide content in the microspore mother cell stage, B) protein content in the microspore mother cell stage, C) insoluble polysaccharide content in the tetrad stage, D) protein content in the tetrad stage, E) insoluble polysaccharide content in the mature pollen grains, F) protein content in the mature pollen grains. mm – microspore mother cells, pg – pollen grain, tp – tapetum, tt – tetrad. Scale bars = 20 μm.
Pollen viability
After the acetocarmine staining, the pollen viability rate was calculated as 87%, the dyed, large, and properly shaped mature pollen grains being considered fertile, and the unstained pollen sterile.
Ovule and female gametophyte development
C. kilaea has an inferior ovary, characteristic of the Asteraceae family. The ovary is syncarpous, unilocular, and carries a single ovule with basal placentation. The ovule is anatropous, tenuinucellate, and unitegmic (Fig. 4A). A single archespore cell develops under the epidermis, and this archespore cell differentiates directly into a megaspore mother cell (Fig. 4B). The megaspore mother cell undergoes meiosis to produce a linear megaspore tetrad. The megaspore at the chalaza side is the functional megaspore; the other three megaspores rapidly degenerate (Fig. 4C). The functional megaspore undergoes three successive mitotic divisions to produce a two-, four-, and then eight-nucleate megagametophyte (Figs. 4D–H).
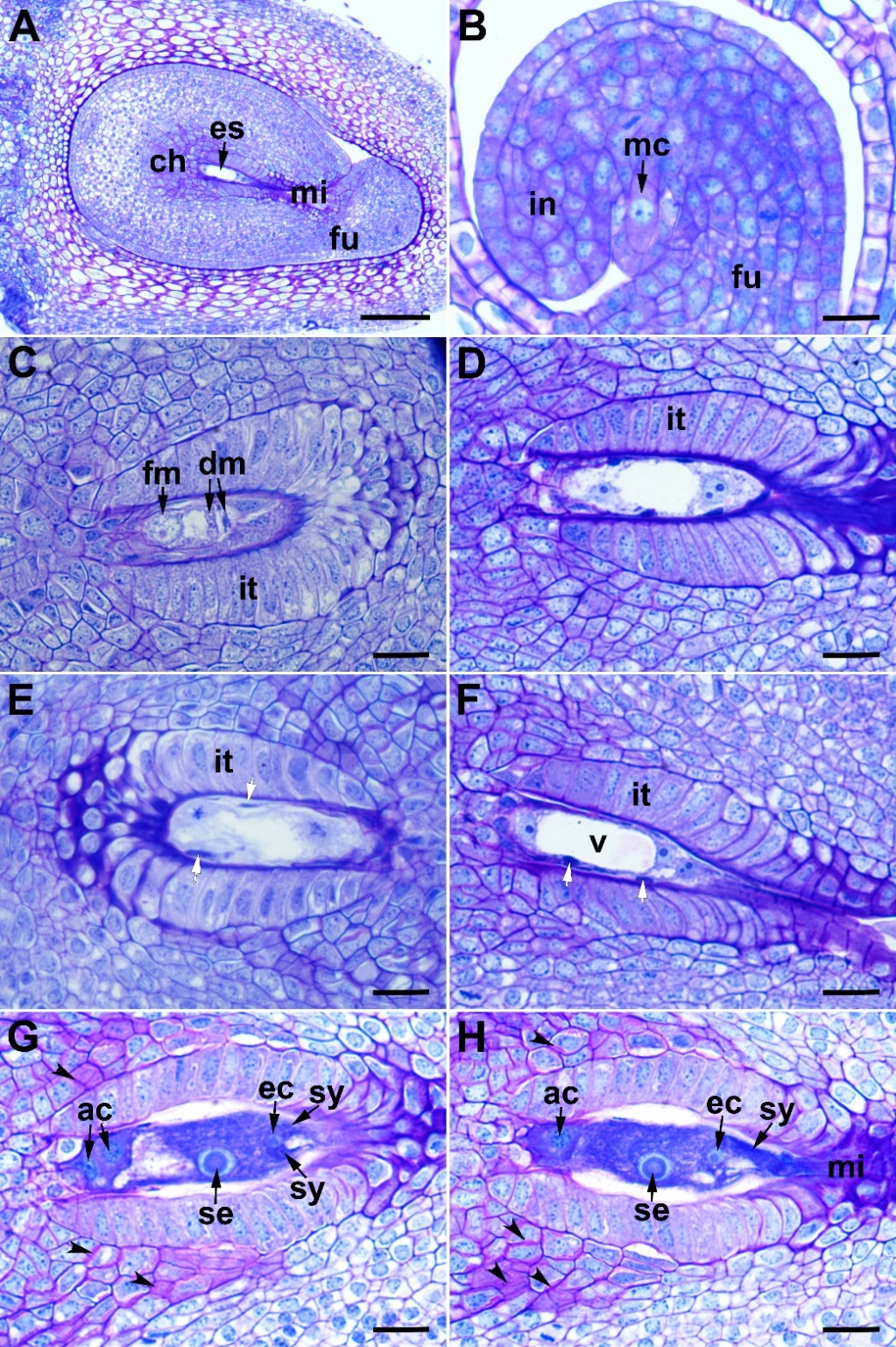
Fig. 4. Ovule and female gametophyte development in Centaurea kilaea: A) ovule, B) megaspore mother cell, C) functional megaspore and degenerated microspores, D) two-nucleate stage of megagametogenesis, E) metaphase stage of the second mitotic division, F) four-nucleate stage of megagametogenesis (white arrows indicate nucellar cell remnants), G) and H) mature embryo sac (the photos are taken from two consecutive sections of the same embryo sac; arrowheads indicate mucilage accumulation). ac – antipodal cell, ch – chalaza, dm – degenerated megaspores, ec – egg cell, es – embryo sac, fm – functional megaspore, fu – funiculus, in – integument, it – integumentary tapetum, mc – megaspore mother cell, mi – micropyle, sy – synergid cell, se – secondary nucleus, v – vacuole. Scale bars = A, 100 μm; B–H, 20 μm.
The embryo sac development is of the Polygonum type. The mature embryo sac consists of a three-celled egg apparatus, (an egg cell and two synergids) at the micropyle side, three antipodal cells at the chalazal end, and two polar nuclei in the central part of the sac. Before fertilization, the polar nuclei fuse to form the secondary nucleus (Fig. 4G and Fig. 4H). The synergids degenerate after fertilization. The antipodal cells persist until the first division of the zygote. Obturator cells originating from the funiculus are long, tubular shaped, and show prominent nuclei. They are elongated toward the synergids. The micropylar canal is completely filled with the obturator cells and the secretion produced by these cells (Fig. 5E and Fig. 5F).
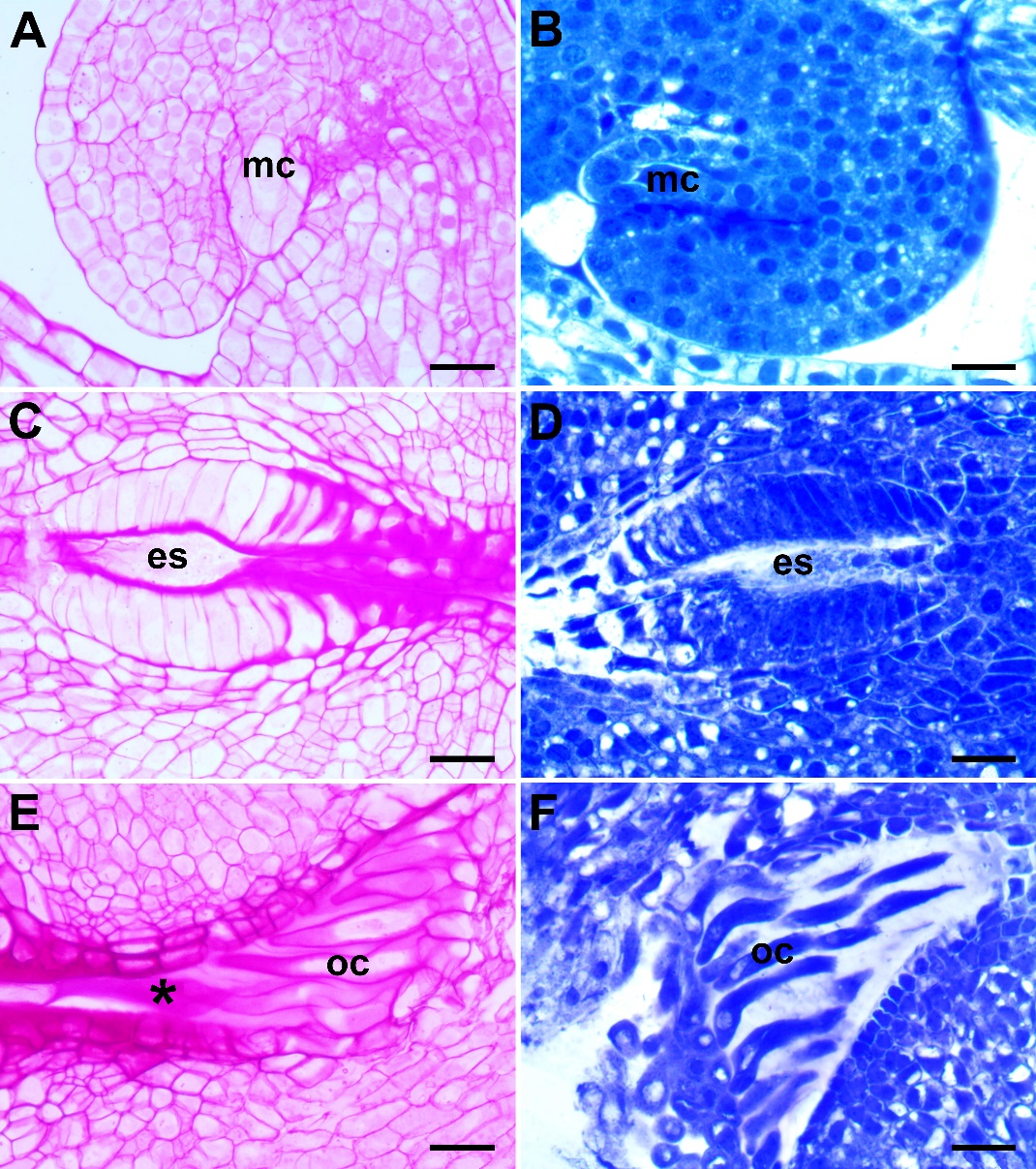
Fig. 5. Histochemical aspects of ovule and embryo sac development in Centaurea kilaea: A) insoluble polysaccharide content at the megaspore mother cell (mc) stage, B) protein content at mc stage, C) insoluble polysaccharide content in the mature embryo sac (es) stage, D) protein content at the mature es stage, E) insoluble polysaccharide content of the obturator cells (oc) and secretion (asterisk), F) protein content of the obturator cells. Scale bars = 20 μm.
In the ovules of C. kilaea, the megaspore mother cell, nucellus, and integument cells react poorly, in terms of insoluble polysaccharides, with the PAS reaction at the beginning of development (Fig. 5A). In the mature embryo sac stage, the part of the integument tapetum facing the embryo sac and the integument cells forming the micropylar opening show an intense PAS-positive reaction (Fig. 5C). In addition, at this stage, the obturator cells and their secretion give an intense PAS-positive reaction (Fig. 5E). In the ovules of C. kilaea, the megaspore mother cell, nucellus, and integument cells react moderately in terms of protein content with CBB at the beginning of development (Fig. 5B). In the mature embryo sac stage, the integumentary tapetum cells give an intense positive reaction to CBB (Fig. 5D). At this stage, it is observed that the obturator cells also give a positive reaction, but the protein is not secreted (Fig. 5F).
Endosperm and embryo development
During fertilization, one of the male gametes fuses with the egg cell, while the other male gamete fuses with the secondary nucleus and forms the primary endosperm nucleus. The primary endosperm nucleus begins to divide before the zygote (Fig. 6A). The endosperm initially develops as free nuclei. The antipodal cells are still intact in this stage (Fig. 6B), and break down after the first zygote division. In the globular embryo stage, wall formation is observed between the endosperm nuclei, starting from the micropylar side (Fig. 6C). The mature seed does not contain endosperm (Fig. 7F).
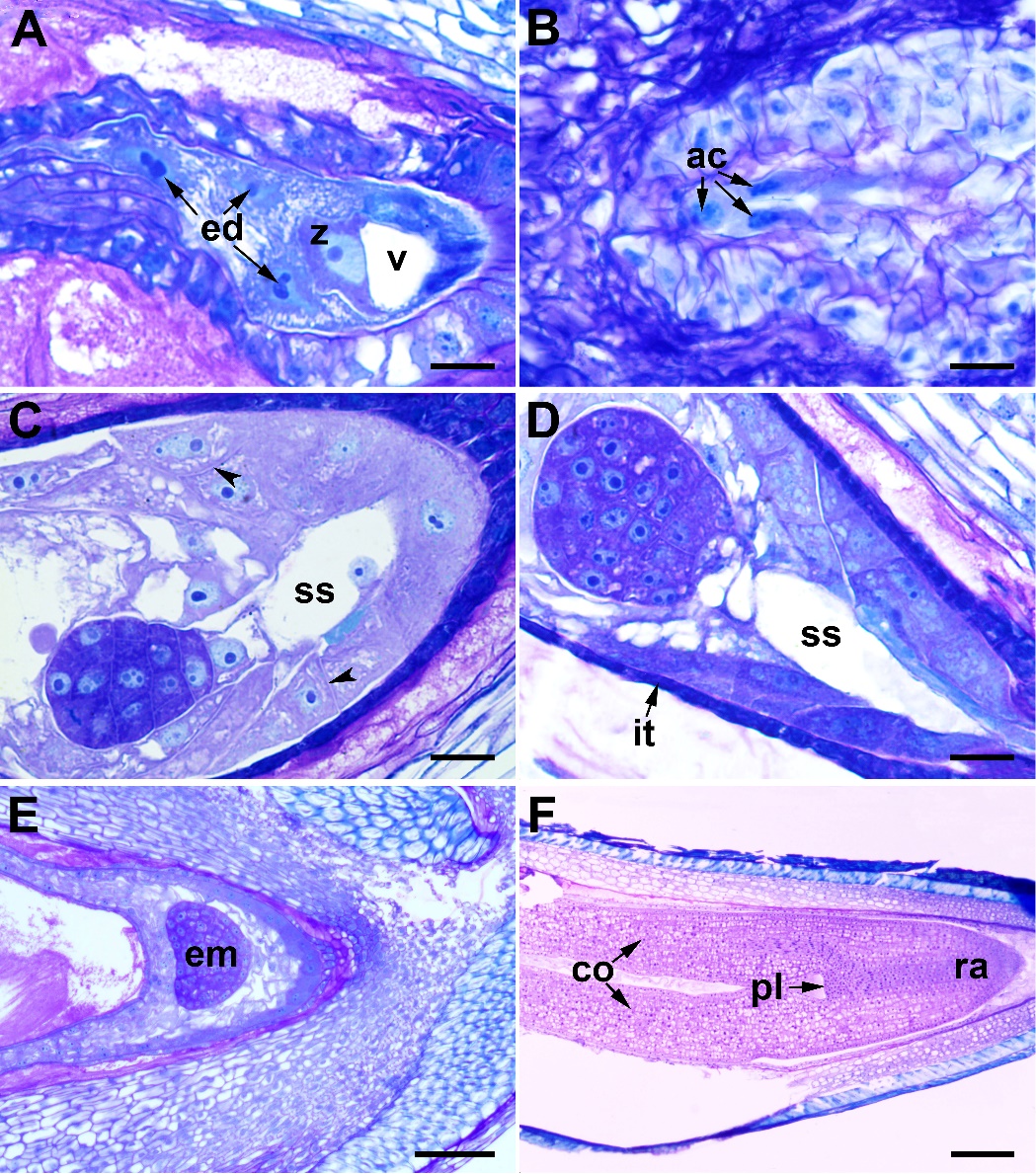
Fig. 6. Endosperm and embryo development in Centaurea kilaea: A) zygote and nuclear endosperm, B) persistent antipodal cells, C) and D) globular embryo with suspensor (arrows indicate the walls of endosperm cells), E) heart-shaped embryo, F) mature embryo. ac – antipodal cell, co – cotyledon, ed – endosperm, em – embryo, it – integumentary tapetum, pl – plumule, ra – radicle, ss – suspensor, v – vacuole, z – zygote. Scale bars = A–D, 20 μm; E, 100 μm; F, 200 μm.
The zygote has a pronounced polarity; the cytoplasm and nucleus are located at the apical end; there is a large vacuole in the basal portion (Fig. 6A). The zygote develops into an embryo by mitotic divisions. The first division is transverse and produces a basal cell toward the micropyle side and an apical cell toward the chalazal side. Embryo development is of the asterad type. The basal cell undergoes transverse divisions to give the suspensor, comprising 4–6 cells (Figs. 6C–E). The globular embryo is formed by the transverse and longitudinal divisions of cells in the proembryo (Fig. 6D). With further development, cells rapidly divide and the globular embryo becomes heart-shaped (Fig. 6E). After successive divisions, a mature dicotyledonous embryo forms with an obvious plumule (Fig. 6F).
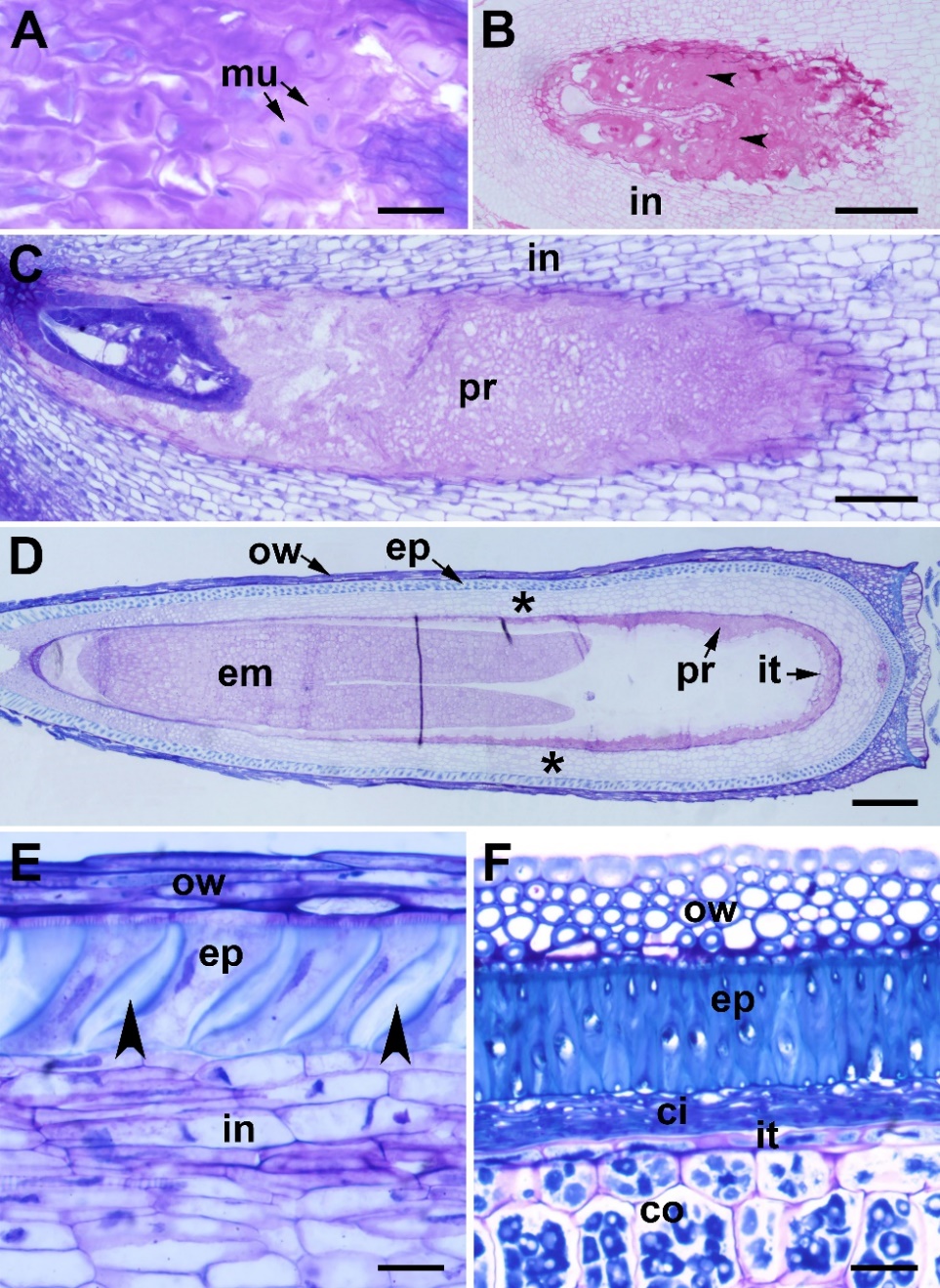
Fig. 7. Integument differentiation in Centaurea kilaea: A) deposition of mucilage (mu) in the integument (in) cells, B) degenerated mucilage cells stained with PAS (arrows), C) peri-endothelial region filled with mucilage, D) peri-endothelial region (pr) and integumentary tapetum (it) in the torpedo-shaped embryo (em) stage (asterisks indicate integumentary cells between the peri-endothelial zone and the outer epidermis), E) outer epidermis cells in the zygote resting stage (arrows indicate wall thickening), F) outer epidermis cells in the heart-shaped embryo stage. cl – cell lumen, co – cotyledon, ci – crushed integument cells, ep – epidermis, ow – ovary wall. Scale bars = A, E, F, 20 μm; B, C, 100 μm; D, 200 μm.
Integument differentiation
In C. kilaea, the integument is initially homogeneous in structure (Fig. 4B). In the zygotene stage of meiotic prophase I, the innermost layer cells of the integument begin to elongate radially to differentiate into the endothelium (integumentary tapetum). The nucellar cells are still intact in this stage. At the end of megasporogenesis, the nucellar cells begin to deteriorate (Fig. 4C). During mitotic divisions (megagametogenesis), the nucellar cells break down and the remnants occur around the embryo sac (Figs. 4D-F). The mature embryo sac is surrounded by single-layered and radially elongated integumentary tapetum (Fig. 4G and Fig. 4H). In this stage, mucilage begins to accumulate rapidly on the walls of the integumentary cells close to the endothelium (Fig. 4G and Fig. 4H, arrowheads). After fertilization, the deposition of mucilage material continues between the primary wall and the plasma membrane on all sides of the cells, and the mucilage pushes the protoplast to the center of the cell (Fig. 7A). Afterwards, the protoplast of the mucilage cells degenerates, and the cell wall breaks down (Fig. 7B). In the globular embryo stage, the peri-endothelial region is completely filled with mucilage (Fig. 7C). Meanwhile, the endothelial cells elongate longitudinally to adapt to the growth of the embryo sac (Fig. 7B and Fig. 7D). In the torpedo-shaped embryo stage, the mucilage material appears homogeneous (Fig. 7D). As the embryo grows, the mucilage material is rapidly consumed, disappearing completely in the mature seed. Endothelium persists in the mature seed (Fig. 7F).
The integument has thin-walled parenchyma cells between the peri-endothelial zone and the outer epidermis (Figs. 7B–D). There is no specific material accumulation in, or in the walls of, these cells. After fertilization, in the resting phase of the zygote, the outer epidermis cells of the integument begin to elongate radially starting from the micropylar pole. In the heart-shaped embryo stage, the outer epidermis cells elongate, and thickening develops on their radial walls (Fig. 7E). In the mature seed, the outer epidermis cells form the seed coat. The testa is represented by a single row of radially elongated, lignified cells (macrosclereids) (Fig. 7F).
Discussion
In this study, the embryology of C. kilaea (subfamily: Carduoideae, tribe: Cardueae), a tetraploid endemic species in Türkiye, was investigated histologically. C. kilaea shows the characteristics of the Asteraceae family in terms of anther structure (tetrasporangiate) and development (dicotyledonous anther wall). The anther wall consists of four layers (epidermis, endothecium, middle layer, tapetum). The outer secondary parietal layer develops an endothecium and a middle layer by periclinal division. The tapetum layer differentiates from the inner secondary parietal layer directly. These results agree with the pattern previously known for Asteraceae (Davis 1966, Yankova-Tsvetkova et al. 2016, 2018).
C. kilaea has an amoeboid type tapetum, which is the most representative of Asteraceae. It has also been observed in other tribes such as the Anthemideae (Li et al. 2010), Astereae (Davis 1968), Calenduleae (Ao 2007), Gnaphalieae (Davis 1962), Heliantheae (Gotelli et al. 2008), Inuleae (Pullaiah 1979), and Senecioneae (Pullaiah 1983). Secretory tapetum occurs in Eupatorieae (Franca et al. 2015), Stifftieae and Wunderlichieae (Bonifacio et al. 2018). Yankova-Tsvetkova et al. (2016, 2018) reported that initially, the tapetum of Arnica montana (Madieae) and Centaurea achtarovii (Cardueae) is secretory, and then it transforms into amoeboid. The persistent epidermis, fibrous endothecium, and ephemeral middle layers observed in C. kilaea are common in Asteraceae (Davis 1966).
Microspore mother cells of C. kilaea show simultaneous cytokinesis, resulting in tetrahedral type tetrads. These findings have also been reported in previous studies (Franca et al. 2015, Bonifacio et al. 2018); the cytokinesis of Ambrosia artemisiifolia is of the successive type (Liu et al. 2011). In C. achtarovii (Yankova-Tsvetkova et al. 2018), the tetrads are generally tetrahedral and isobilateral. A. montana has usually tetrahedral, occasionally isobilateral, rarely T-shaped, and linear tetrads (Yankova-Tsvetkova et al. 2016). The pollen grains of C. kilaea are shed with three cells (a vegetative cell and two sperm cells) from the mature anther, as in many Asteraceae members (Davis 1966). In Stifftia chrysantha, Stifftia fruticose, Wunderlichia mirabilis, and Wunderlichia senae, the pollen grains are shed with two cells (a vegetative cell and a generative cell) from the anther (Bonifacio et al. 2018). Mature pollen grains of C. kilaea contain insoluble polysaccharides and proteins. Similar observations have been also reported in Galinsoga quadriradiata (Kolczyk et al. 2015). Although C. kilaea is a tetraploid species (2n = 4x = 36) (Meric et al. 2010), meiosis is quite regular and the pollen fertility percentage is high (87%).
The ovary of C. kilaea, characteristically for the Asteraceae family, is single-chambered with two carpels and contains a single ovule with basal placentation. This feature has been reported for many Asteraceae family members (Davis 1966, Ao 2007, Chen et al. 2014, Franca et al. 2015, Bonifacio et al. 2018). The single archespore cell forms just below the nucellus epidermis and differentiates directly into the megaspore mother cell. The megaspore mother cell produces a linear type megaspore tetrad as a result of meiosis. The megaspore on the chalazal side is the functional megaspore, and the other three megaspores degenerate rapidly. This is characteristic of many Asteraceae family members (Davis 1966). Embryo sac development in C. kilaea is Polygonum-type, as it is in 70% of angiosperms. Monosporic Polygonum-type embryo sac development is mostly seen in Asteraceae family species, but monosporic Oenothera-, bisporic Allium-, tetrasporic Fritillaria-, Adoxa- and Drusa-type embryo sac developments are also seen (Davis 1966, Li et al. 2009, Musial et al. 2012). Thus, the embryo sac development of plants in the Asteraceae family is highly diverse.
The antipodal cells of C. kilaea persist until the first division of the zygote. They are similarly persistent in Crepis bithynica, C. achtarovii, Wunderlichia spp., Ambrosia spp., and Taraxacum udum (Yurukova-Grancharova and Dimitrova 2006, Musial et al. 2013, Chen et al. 2014, Bonifacio et al. 2018, Yankova-Tsvetkova et al. 2018). However, the antipodal cells in Calendula officinalis degenerate prematurely, so they are not found in the mature embryo sac of C. officinalis (Ao 2007). Before fertilization, the polar nuclei fuse to form the secondary nucleus of the embryo sac. Similar observations have been reported in previous studies (Chen et al. 2014, Yankova-Tsvetkova et al. 2016, 2018, Bonifacio et al. 2018).
Obturator cells with prominent nuclei and their secretions completely filled the micropylar canal in C. kilaea. These cells contain insoluble polysaccharides and proteins. The secretion produced by obturator cells gives an intense PAS-positive reaction; however, there is no protein in the secretion. Similarly, in sunflower and Taraxacum spp., the secretion (extracellular matrix) consists of only insoluble polysaccharides (Yan et al. 1991, Plachno et al. 2015). In C. kilaea, the obturator cells and their secretions play a key role in the growth of pollen tubes toward the micropyle, and further, their nutrition. It is similarly reported that the obturator plays roles in both the feeding of pollen tubes and their directing toward the micropyle in the sunflower (Yan et al. 1991).
In C. kilaea, the polar nuclei fuse before fertilization to form the secondary nucleus. This feature is common for Asteraceae (Davis 1966). Endosperm development is initially of nuclear type in C. kilaea. In the globular embryo stage, cytokinesis occurs between the free nuclei, starting from the micropylar side, and the endosperm transforms into a cellular type. Similar observations have been also reported in Hieracium spp. , A. montana, C. bithynica, and C. achtarovii (Yurukova-Grancharova and Dimitrova 2006, Yurukova-Grancharova et al. 2006, Yankova-Tsvetkova et al. 2016, 2018). The cellular endosperm is observed in Ageratum, Ambrosia, Stifftia, and Wunderlichia (Chen et al. 2014, Franca et al. 2015, Bonifacio et al. 2018). The embryogenesis of C. kilaea is of the asterad type, in which basal and apical cells participate in the formation of the embryo, as previously described for Asteraceae (Davis 1966).
The presence of integumentary tapetum (endothelium) has been reported in 65 dicotyledonous families with thin ovules (tenuinucellate and weakly crassinucellate) (Kapil and Tiwari 1978). The integumentary tapetum is a common feature in Asteraceae and forms in different developmental stages in the different representatives of the family (Davis 1966). The integument cells are initially homogeneously structured in C. kilaea. In the zygotene stage, the innermost layer cells of the integument elongate radially, and the endothelium forms. Endothelium begins to form in C. achtarovii at the one-nucleate stage, in Hieracium pilosella at the end of meiosis, in Taraxacum udum at the diad stage, and in Ambrosia spp. at the leptotene stage (Koltunow et al. 1998, Musial et al. 2013, Chen et al. 2014, Yankova-Tsvetkova et al. 2018).
In the mature female gametophyte stage, mucilage begins to accumulate rapidly on the walls of the integument cells close to the endothelium. The mucilage deposited between the primary wall and the plasma membrane pushes the protoplast toward the center of the cell. This region is called the peri-endothelial region (Pandey et al. 1978, Kolczyk et al. 2016, Plachno et al. 2016, 2017). In Taraxacum, the cells of the peri-endothelial region begin to differentiate at the young ovule stage, while in Hieracium, the wall changes of the peri-endothelial zone cells start during the four-nucleate embryo sac stage (Koltunow et al. 1998, Plachno et al. 2016). After fertilization, during the resting period of the zygote, the protoplast of the mucilage cell degenerates and the cell wall breaks down. Lysigenous cavities filled with mucilage are formed in the peri-endothelial region of C. kilaea. In Hieracium and Pilosella, the formation of lysigenous cavities occurs at the mature female gametophyte stage, while in Taraxacum, the peri-endothelial cells retain their individuality in the mature female gametophyte stage, and the formation of these cavities occurs during embryogenesis (Plachno et al. 2016, 2017, Gawecki et al. 2017). In the globular embryo stage, the peri-endothelial region is completely filled with mucilage. Meanwhile, endothelial cells elongate longitudinally to adapt to the growth of the ovule. In the torpedo-shaped embryo stage, the mucilage material appears homogeneous, and it disappears completely in the mature seed. Endothelium persists in mature seeds in C. kilaea. In A. artemisiifolia, endothelial cells begin to deteriorate during the multicellular proembryo stage. Meanwhile, integument cells adjacent to the endothelium also undergo hydrolysis. In the mature embryo period, the endothelium completely disappears (Chen et al. 2014). It is suggested by the present paper that mucilage might be a carbohydrate source for the developing embryo. Moreover, disruption of peri-endothelial cells creates the necessary space for the growing embryo. In the heart-shaped embryo stage of C. kilaea, the outer epidermis cells of the integument elongate radially, and their radial walls thicken. Like other members of the Asteraceae, the outer epidermis cells form the seed coat in the mature seed (Pandey et al. 1978; Kolczyk et al. 2016).
Conclusion
This study details the embryological characteristics and the fruit development of C. kilaea, a member of Asteraceae, a threatened coastal dune species that is facing a very high risk of extinction in the wild due to human activities. This is the first study on the reproductive biology and chemical composition of the reproductive structures of the species. Тhе revealed features of consisting structures of anthers, ovules, embryo and seeds show the characteristics of the Asteraceae family: tetrasporangiate anthers, dicotyledonous type of anther wall development, amoeboid tapetum, tetrahedral microspore tetrads, three-celled mature pollen grains containing insoluble polysaccharides and proteins, anatropous, unitegmic, and tenuinucellate ovule, Polygonum-type of embryo sac development, antipodal cells persisting until the first divisions of the zygote, obturator cells and their secretions (extracellular matrix) completely filling the micropylar canal, PAS-positive extracellular matrix without protein contains, mature embryo sac surrounded by an integumentary tapetum (endothelium), integument consisting of the integumentary tapetum (endothelium), peri-endothelial region, parenchymatous cells, and outer epidermis, from the inside out, with mucilage accumulation on the walls of the integument cells (peri-endothelial region) close to the endothelium in the mature female gametophyte stage, mature seed without mucilage, Asterad type of embryo development, endosperm of cellular type, mature seed without endosperm and with endothelium. The established normal unfolding of processes in the male and female generative sphere of C. kilaea and the high percent of viable pollen ensure a successful reproduction of populations of the species.
