Introduction
The diatom genus Cymbella C. Agardh was established almost 200 years ago (Agardh 1830), with C. cymbiformis C. Agardh (1830) chosen later as the type species of the genus (Håkansson and Ross 1984). With advances in observation tools and increased understanding, 19 genera have been separated from Cymbella (Liu et al. 2021, Kulikovskiy et al. 2022). After several taxonomic revisions, Krammer (2002) revised and summarized the characteristics of this genus as follows: the shape of the valve with cymbelloid symmetry, the terminal raphe fissures bent towards the dorsal margin, at each apex the presence of one apical pore field, and one or more stigmata positioned on the ventral side of the valves. Subsequently, a series of papers reported many new species in this genus (Cantonati et al. 2010, Le Cohu et al. 2015, Bahls 2019, Solak et al. 2021). At present, AlgaeBase notes more than three hundred described taxa in the genus (Guiry and Guiry 2023). In China, our research over the last 20 years also found many new species. (Li et al. 2003a, 2005, 2019, Gong and Li 2011, Gong et al. 2013, Hu et al. 2013, Liu et al. 2021, Zhang et al. 2021).
New species of Cymbella have been described worldwide: from Europe, South America, Asia, North America, Africa, Australasia and Antarctica (Krammer 2002, Cantonati et al. 2010, Le Cohu et al. 2015, Wengrat et al. 2015, Bahls 2019, Li et al. 2019, Liu et al. 2021, Solak et al. 2021, Zhang et al. 2021). In China, research has been conducted in Fujian Province, Heilongjiang Province, Sichuan Province, Guizhou Province, Yunnan Province, Liaoning Province, Gansu Province, Qinghai Province, Hubei Province, Inner Mongolia, Xinjiang and Tibet (Li et al. 2003b, 2004, 2005, 2007a,b, 2012, You et al. 2005, Liu et al. 2006, Wu et al. 2008, Liu et al. 2012, Fu et al. 2013, Sun and Zhi 2015). There have been no systematic diatom surveys in the Pearl River basin.
In this paper, we describe a new species of Cymbella from a tributary of the Pearl River: the Modaomen Channel of Guangdong Province, China. We present light and scanning electron microscopy observations on the valve of a new species and provide information on its ecology. Cymbella stomachsis sp. nov. is also compared with six most similar species: C. tumida (Brébisson) Van Heurck (1880: 64), C. stuxbergii (Cleve) Cleve (1894: 173), C. stuxbergioides Kulikovskiy, Metzeltin & Lange-Bertalot (2012: 81), C. pseudostuxbergii Kulikovskiy, Metzeltin & Lange-Bertalot (2012: 80), C. mexicana (Ehrenberg) Cleve (1894: 177) and C. australica (A.W.F. Schmidt) Cleve (1894: 176).
Material and methods
The Modaomen Channel is located in Zhuhai city, Guangdong Province, China. It is one of the tributaries of the Xijiang River, which in turn is a major tributary of the Pearl River (Hu 2010). The region is characteristic by a subtropical monsoon climate with distinct dry and wet seasons (Hu 2010, Han et al. 2022). According to Zhang et al. (2022), the water quality in this area may be considered mesotrophic. In July 2021, the sample containing the species of Cymbella studied here was collected from rock scrapings from the Modaomen Channel (22°24.523′ N, 113°36.976′ E) (Fig. 1).
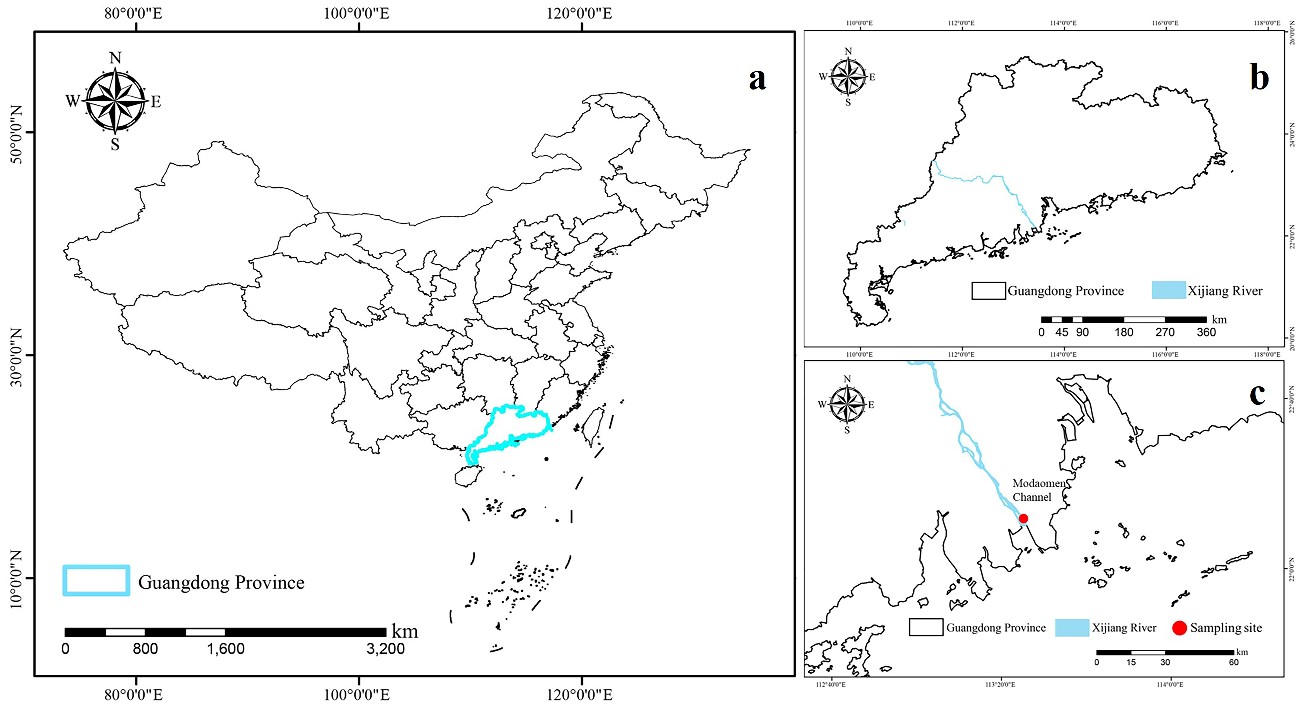
Fig. 1. Study areas and the sampling sites: a – Map of China and Guangdong Province, b – Guangdong Province and Xijiang River, c – Xijiang River and sampling point.
pH and specific conductance of the river water were measured in situ using a YSI 650 multi-parameter display system (650 MDS, YSI Incorporated 1700/1725 Brannum Lane, Yellow Springs, OH 45387 USA) with a 600XL probe. The results are shown in Tab. 1.
Tab. 1. Physical and chemical parameters in Modaomen Channel on 29th July 2021.
| Modaomen Channel | |
|---|---|
| Latitude (°N) | 22°24.523′ |
| Longitude (°E) | 113°36.976′ |
| Altitude (m) | 78 |
| Water temperature (℃) | 29.8 |
| pH | 7.81 |
| Dissolved Oxygen (mg L-1) | 7.46 |
| Conductivity (μs cm-1) | 262 |
Algae was removed from the rocks with a toothbrush. 10% HCl and 30% H2O2 were used to dissolve and remove the calcium carbonate and organic matter, respectively (Battarbee 1986). After being washed several times in distilled water, a part of the cleaned diatom material was air-dried onto cover slips and mounted on glass slides using Naphrax. The sample and slides were deposited in the Herbarium of the Institute for Ecological Research and Pollution Control of Plateau Lakes, Yunnan University, Kunming, P.R. China (YUK). The isotype slides are stored in the Key Laboratory of Biodiversity of Aquatic Organisms, Harbin Normal University.
Morphological features of diatoms were observed under light microscopy (LM) using a ZEISS Axioscope 5 (DIC, ×1000, oil immersion lens) research microscope with a Canon EOS 6D Mark Ⅱ digital camera. At least 500 valves of the associated diatom community were identified and counted. Cleaned material for scanning electron microscopy (SEM) was air-dried onto cover glasses, mounted onto stubs, and coated with 20 nm of Au (EMSCOP SC 500 sputter coater). The resulting stubs were examined and photographed in a LEO 1530 SEM with an acceleration voltage of 5–10 kV. The image analysis and plate arrangement were processed with the program Adobe Photoshop (CS5) (V. 12.1, Adobe Systems, San Jose, CA, USA). Description of the new species follows the terminology provided by Krammer (2002).
Taxonomy
Class Bacillariophyceae Haeckel 1878: 95
Subclass Bacillariophycidae D.G. Mann in Round et al. 1990: 125
Order Cymbellales D.G Mann in Round et al. 1990: 653
Family Cymbellaceae D. Siliva and W. José 2016
Genus Cymbella C. Agardh 1830: 10
Cymbella stomachsis Li, sp. nov. (Figs. 2–4)
LM (Fig. 2): Valves semielliptic, strongly dorsiventral. Dorsal margin strongly convex. Ventral margin slightly convex, straight and with slightly tumid central portion. Valve ends distinctly constricted on both the dorsal and ventral sides, protracted, with truncate and bluntly square apices. Length 40.0 – 52.0 μm, width 14.0 – 17.0 μm. Maximum length/width ratio about 3.0 (N = 30). Axial area narrow, arched. Central area, orbicular-rhomboidal, occupying 2/5 – 2/3 the width of the valve. Raphe lateral, becoming filiform near the distal and proximal ends. External central raphe ends with distinct central pores. External distal raphe fissures hooked towards dorsal margin. Striae radiate more strongly towards the central portion, punctate-lineate, slightly wavy at the valve center dorsally, density 11 – 13/10 μm in the central valve portion becoming 13 – 14/10 μm near the ends. Individual areolae visible, 21 – 25/10 μm. 1 – 2 (mostly one) stigmata present at the ventral side of the central nodule.
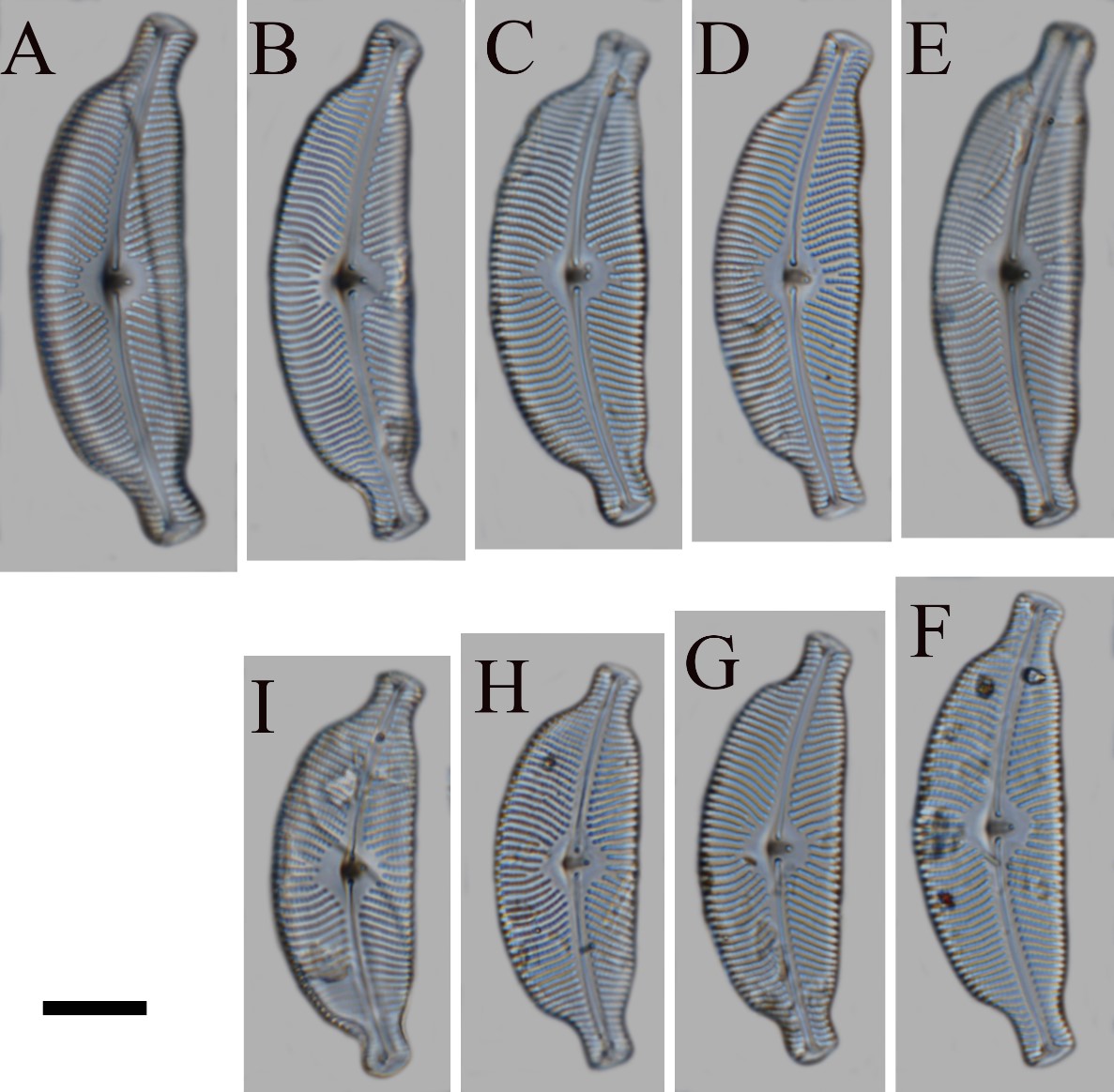
Fig. 2. Cymbella stomachsis sp. nov., LM, DIC. Fig. 2B is of the holotype. A-I – valve views, showing size range and variability of the holotype population. Scale bar = 10 μm.
In SEM external valve view (Fig. 3): Raphe with central ends dilated, drop-shaped pores and curved to the ventral side (Fig. 3A, 3C). The external distal raphe ends hooked, bent towards the dorsal side, and terminating on the mantle (Fig. 3A, 3B). Apical pore fields (APFs) are composed of vertically aligned porelli, not bisected, evidently present at the valve mantle of both apices (Fig. 3A, 3B). One small round stigma located on the ventral side of the central nodule (Fig. 3A, 3C). Transapical striae formed by slit-like areolae with apically oriented foramina, although there are small, rounded partly T-shaped, V-shaped or irregularly shaped areolae adjacent to the axial and central areas (Fig. 3A – 3C). The number of puncta per stria varies between 25 and 30 in 10 µm.
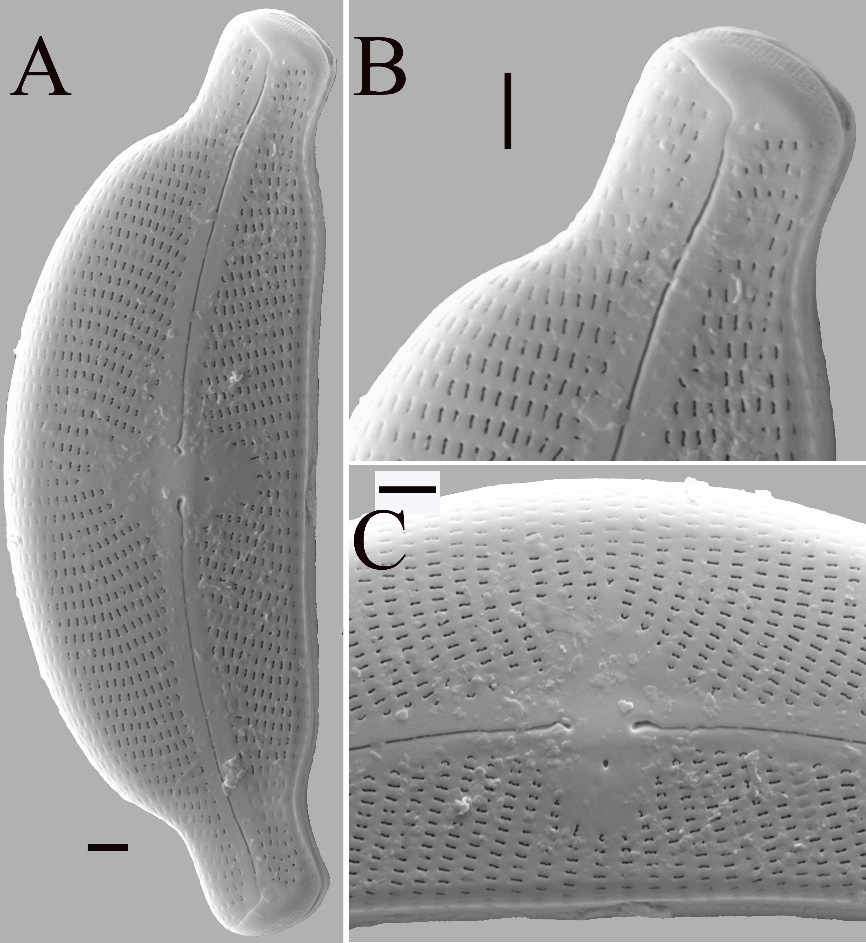
Fig. 3. Cymbella stomachsis sp. nov., SEM, external views. A – External view of an entire valve. B – Valve apices, striae with areolae mostly slit-like, the distal raphe ends dorsally bent and APFs composed of vertically aligned porelli, not bisected. C – External view of valve center, the central ends dilated with drop-shaped pores deflected ventrally and one small round stigma located on the ventral side. Scale bar = 2 μm.
In SEM internal valve view (Fig. 4): Stria opening in depressions between virgae elliptical in shape (Fig. 4A – 4C). The internal central raphe ends undulate, discontinuous, revealed with the absence of a hooded structure over the central nodule (Fig. 4A, 4C). The internal distal proximal raphe ends terminate in helictoglossae which are raised prominently from the internal valve surface (Fig. 4A, 4B). APFs composed of small, parallel arranged porelli (Fig. 4A, 4B). The large, slit-like stigma opening has irregular projections (Fig. 4A, 4C). The proximal ends of 11–13 striae form a semi-circular central area.
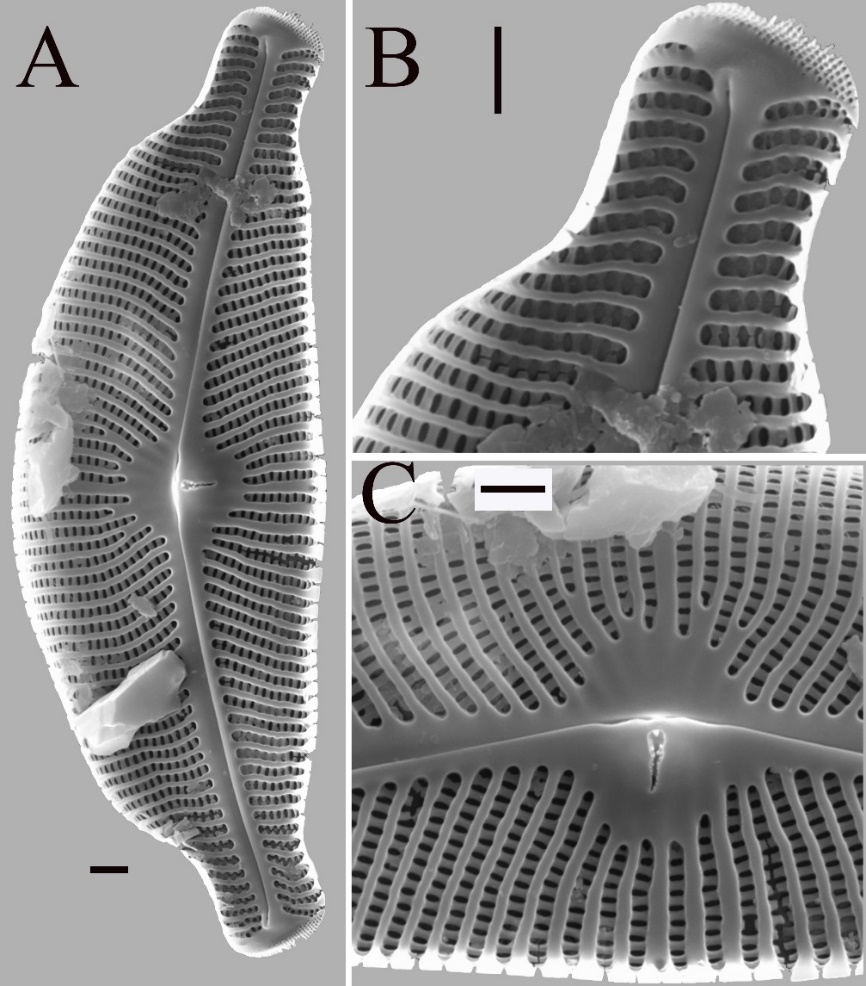
Fig. 4. Cymbella stomachsis sp. nov., SEM, internal views. A – internal view of entire valve. B – internal view of valve apices, the internal distal raphe ends terminate in helictoglossae, APFs composed of small, parallel arranged alveoli. C – showing the valve central area. one large, slit-like stigma opening with irregular structures. In this specimen the hooded structure over the central area is not present, revealing the raphe is not continuous. Scale bar = 2 μm.
Type: China. Guangdong Province: Modaomen waterway, freshwater, 22°24.523′ N, 113°36.976′ E, elevation 78 m a.s.l., 29th July 2021, rock scraping samples collected by Hong-Qu Tang (Holotype MDM202172902 in Coll. Hong-Qu Tang, Jinan University, Guangzhou, China = Fig. 2B; Isotype YUNGL20220320, Harbin Normal University, Harbin, China)
Etymology:stomachsis, referring to the stomach-like shape of new species.
Ecology:Cymbella stomachsis is found in the Modaomen Channel, Zhuhai city, Guangdong Province, on stone surfaces. Environmental measurements from the site at the time of collection include moderately alkaline pH (7.81), dissolved oxygen of 7.46 mg L-1, conductivity of 262 µs cm-1, and water temperature of 29.8 ℃. At the type locality, the related species in decreasing order of abundance were Amphora linearis F. Meister (1935: 97), Seminavis strigosa (Hustedt) Danieledis & Economou-Amilli (2003: 30), Nitzschia clausii Hantzsch (1860: 40), Navicula viridula var. rostellata (Kützing) Cleve (1895: 15), Navicula schroeteri F. Meister (1932: 38), Gomphonema parvulum (Kützing) Kützing (1849: 65), Aulacoseira granulata (Ehrenberg) Simonsen (1979: 58).
Discussion
Cymbella stomachsis sp. nov. is assigned to the genus Cymbella by its cymbelloid valve shape, dorsally deflected external distal raphe fissures and by the presence of APFs and stigmata. C. stomachsis has some unique features, such as valve ends that are distinctly tapered on the dorsal and ventral sides, with truncate and bluntly square apices, with undulate striae near the ends on the dorsal side and with intermissio separating the proximal raphe ends. In particular, the intermissio structure is not typical of other species of Cymbella. However, the intermissio in C. stomachsis and Didymosphenia are nearly identical, and this may prove close phylogenetic relationships between these taxa (Kociolek and Stoermer 1988, Kulikovskiy et al. 2012, Metzeltin and Lange-Bertalot 2014). Comparing the outline of the valve and striae pattern, C. stomachsis is more similar to six species C. tumida, C. stuxbergii, C. stuxbergioides, C. pseudostuxbergii, C. mexicana and C. australica, the morphological characteristics of which are compared in Tab. 2.
Tab. 2. Morphological characteristics of Cymbella stomachsis sp. nov. compared with other similar taxa.
Although there are some similarities among the six similar species, the new species can be easily distinguished from Cymbella tumida, C. stuxbergii, C. stuxbergioides, C. pseudostuxburgii, C. mexicana and C. australica by the shape of the valve end and the central area. According to the characteristics mentioned above, C. stomachsis most closely resembles C. tumida (Fig. 5), but there are also some features that can be used to separate C. stomachsis.
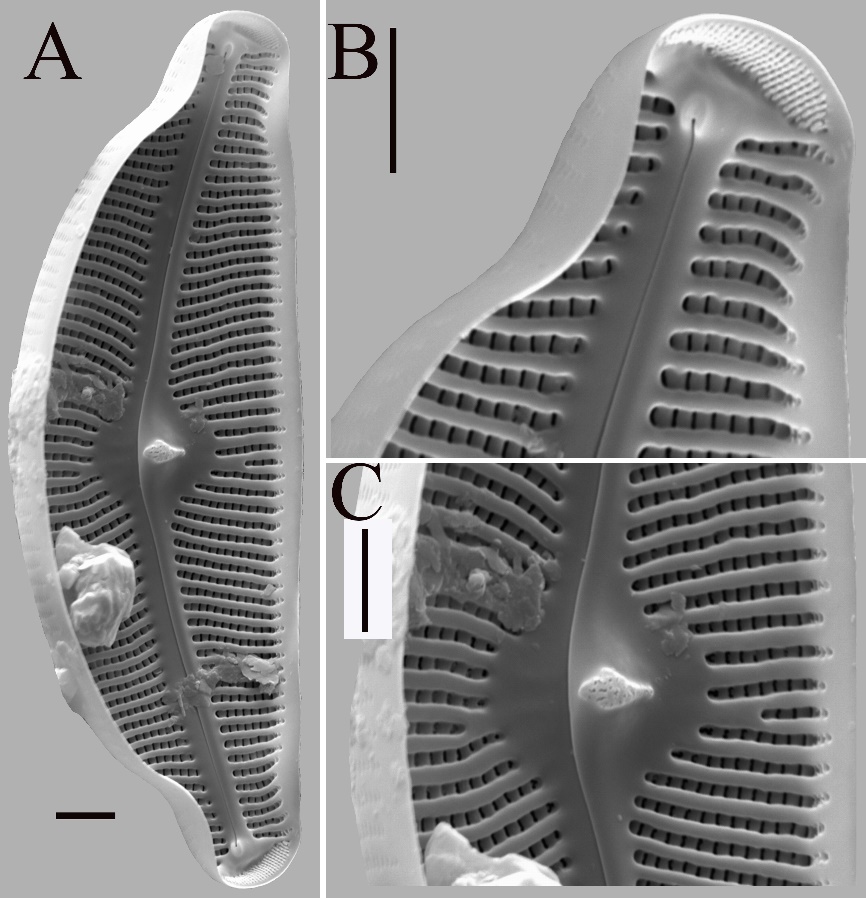
Fig. 5. Cymbella tumida, SEM, internal views. A – Internal view of entire valve. B – Internal view of valve apices, the internal distal raphe ends terminate in helictoglossae. APFs composed of small, parallel arranged alveoli. C – showing the valve central area. One water-drop shape stigma alveoli and filled with silica located on ventral side. Scale bar = 3 μm. C. tumida was collected from North River, in the sample NR 27 (113°30′26″ E, 24°38′11″ N), in July 2021.
For example, when the shape of the end is compared, the new species has more obvious constrictions near the ends. In terms of the internally proximal raphe ends, the proximal raphe ends of the new species are discontinuous with the intermissio while they are continuous in C. tumida. Taking into consideration the areola shape, the areolae of C. tumida are more oblong than those of C. stomachsis. With respect to the internal shape of stigmata, the stigmata of the new species are slit-like, while the stigmata of C. tumida are water-drop shaped. Besides, the size range of C. stomachsis is smaller than that of C. tumida. Finally, the areola density is very high compared to C. tumida. In summary of the above, these differences are sufficient to justify the description of C. stomachsis as an independent species.
Regarding the ecological distribution of Cymbella, the genus Cymbella tends to be distributed in lakes (Gong and Li 2011, Hu et al. 2013, Liu et al. 2021), reservoirs (Çelekli and Arslanargun 2019, Hansika and Yatigammana 2019), and rivers (Li et al. 2019, Long et al. 2022). For example, of the six species similar to our new species, C. stuxbergii is found not only in ancient lakes, but also in rivers (Cleve-Euler 1955, Foged 1993). In addition, some of these taxa are reported from ancient lakes. For example, C. stuxbergioides and C. pesudostuxbergii were reported in Lake Baikal (Kulikovskiy et al. 2012), while C. australica was found in Lake Tanganyika (Krammer 2002). Finally, others may live in lotic environments. For example, C. tumida and C. mexicana were found in river systems (Terao et al. 1993, Gou et al. 2015), which is same environment in which we found C. stomachsis sp. nov.
With global climate change and human activities, freshwater ecosystems are facing multiple crises (Dudgeon et al. 2006). As a member of the Generic Index (the sum of the ratio of the relative abundance of Achnanthes, Cocconeis and Cymbella to the relative abundance of Cyclotella, Aulacoseira and Nitzschia was calculated) (Wu 1999), Cymbella plays an important role in the indicators of water quality. In freshwater, Cymbella species can survive in oligotrophic, mesotrophic and eutrophic environments (Krammer 2002, Hu et al. 2013). For example, for similar species mentioned above, C. stuxbergii lives in freshwater with oligotrophic environmental conditions (Krammer 2002). Cymbella tumida has been found both in epiphyton and phytoplankton, within oligotrophic or mesotrophic water quality conditions (Gou et al. 2015, Cespedes-Vargas et al. 2016). In this study, C. stomachsis sp. nov. was found from rock scraping samples in the Modaomen Channel which is a mesotrophic environment according to Zhang et al. (2022). In short, the new species found in this genus it is helpful in increasing our understanding of Cymbella taxa and their ecological preferences and tolerances, and thus in providing more accurate diatom indices used in water quality evaluation.
