Introduction
Located along the eastern Adriatic coast, the Dinaric Alps are one of the most extensive mountainous areas of Europe. This area is known for its extremely high biodiversity and is one of the most floristically diversified regions, on both a European and a global scale (Mutke et al. 2010). In regards to vegetation, the dominant forest community in this region is Omphalodo- Fagetum (Tregubov 1957 corr. Puncer 1980) Marinček et al. 1993, with silver fir ( Abies alba Mill.) and European beech ( Fagus sylvatica L.) as the dominant tree species, with admixtures of other ecologically and economically important species like sycamore ( Acer pseudoplatanus L.), wych elm ( Ulmus glabra Huds.), small-leaved lime ( Tilia cordata Mill.), and European ash ( Fraxinus excelsior L.) (Boncina 2011). In the context of progressing climate change and intensive dieback caused by the pathogen fungus Hymenoscyphus fraxineus (T. Kowalski) Baral, Queloz et Hosoya, the European ash stood out as a species to which attention should be paid in order to conserve its natural populations across Europe and maintain sustainable forest management.
European or common ash is a deciduous, trioecious, and anemophilous tree species, belonging to the Oleaceae family (Pliûra and Heuertz 2003). It is a fast-growing species, reaching a mature height of up to 40 m. Its specific epithet, excelsior (lat. excelsus = noble, tall), refers to the tall growth of the European ash (Šugar 1990), likely in comparison to a related European species, the manna ash ( Fraxinus ornus L.). The European ash is a highly economically significant tree species in forestry, due to its high-quality timber (Herman 1971). As such, it has been used for centuries, particularly in carpentry and wheelwrighting, as well as in the production of looms, handles, agricultural tools, sporting goods, boats, airplanes, train carriages etc.
The natural range of the European ash covers most of Europe, with the exception of the most northern and southern parts of the continent (Herman 1971, Pliûra and Heuertz 2003). The northern limits stretch across southern Norway, Sweden and Finland, whereas in southern Europe, the species can be found in mountainous regions of the Pyrenees, the Apennines, the Alps and the mountains of the Balkan Peninsula. Further east, the species stretches across the northern parts of Asia Minor and the Caucasus, where it encompasses the mountainous southern edge of the Caspian Sea.
The European ash requires moist and rich soils and tolerates soil pH-values as low as 4.5, but prefers pH values above 5.5 (Beck et al. 2016). The species exhibits intermediate properties between a pioneer species and a permanent forest component. It is a species of mixed deciduous forests, in which it occupies moist soils, in plant communities well-adapted to long periods of snow cover or those found in conditions of high air humidity (Herman 1971, Vukelić 2012, Beck et al. 2016). As a young seedling and sapling, it can tolerate shade, but will require full sunlight as a mature tree to fully develop (Pliûra and Heuertz 2003). In northern and western parts of its range, the European ash grows in lowland forests, on cool and moist soils of valleys and plains, where it demonstrates great tolerance to seasonal water-logging. It is not, however, tolerant of prolonged flooding, thus not growing on highly compacted soils of flood-plain forests. The European ash is somewhat opportunistic, and will form near to pure stands in favourable conditions, especially after major disturbances. The species tolerates various altitudes, temperatures and moisture availability, which appear to be the limiting factors to its distribution. In southern part of its range, it grows predominantly in higher altitudes; up to 2200 m a.s.l. (Herman 1971, Beck et al. 2016).
As an economically and ecologically significant species, the European ash has been the subject of much scientific research. The majority of investigations, however, focused on its genetic diversity across the species' range (Heuertz et al. 2004a,b, Sutherland et al. 2010, Tollefsrud et al. 2016, Belton et al. 2022), as well as on the aftermath of the ash dieback disease (Pautasso et al. 2013, Coker et al. 2018). On the other hand, the morphological diversity of the species is largely overlooked. This lack of research into the morphological differences between the numerous populations across Europe is abated only slightly by research into the interspecies hybrids (Fernández-Manjarrés et al. 2006, Gerard et al. 2006) between the European ash and the narrow-leaved ash ( Fraxinus angustifolia Vahl), which are morphologically ambiguous. As a result, the morphological traits, and potential differences among the populations of European ash morphology need to be further investigated, which could benefit the distinction of hybrids as well.
In this research, therefore, we analysed the morphological traits and diversity of seven populations of European ash in the northern Dinaric Alps. This region is thought to have served as a refugium during the last glaciation for many species, including F. excelsior (Heuertz et al. 2004a). Refugium populations in general may accumulate higher genetic, and therefore morphological, diversity, due to their persistence and stability over glacial cycles (Hewitt 1996), which prompted us to investigate the morphological diversity of F. excelsior in one of its refugial areas. In the Dinaric Alps, the European ash is predominantly found in humid ditches and dales, where organic matter accumulates and thus creates unfavourable conditions for the dominant species, which is the beech (Vukelić 2012). It is, however, also found on skeletal, shallow soils on limestone, where the overall conditions are warmer and drier (Herman 1971). Furthermore, the pioneering character of the species comes to the foreground in these habitats, with ash trees occupying and quickly overshadowing other species in small openings and forest edges, following any disturbance. Due to these varying conditions within each population, the selected populations represent and interesting sample of the broader Dinaric range of the European ash, where expected morphological differences between the populations are likely under the influence of the environment.
The aims of this study were: (1) to describe variation of leaf dimension and shape parameters for European ash populations in the northern Dinaric Alps; and (2) to verify whether leaf dimensions and shape of these populations change with geographical or environmental distances among populations.
Plant material
Samples for the morphometric analyses were collected in seven European ash populations of the northern Dinaric Alps (Fig. 1, On-line Suppl. Tab. 1): P1-Žumberak, P2-Crni Lug, P3-Delnice, P4-Vrbovsko, P5-Brinje, P6-Prozor, P7-Perušić.
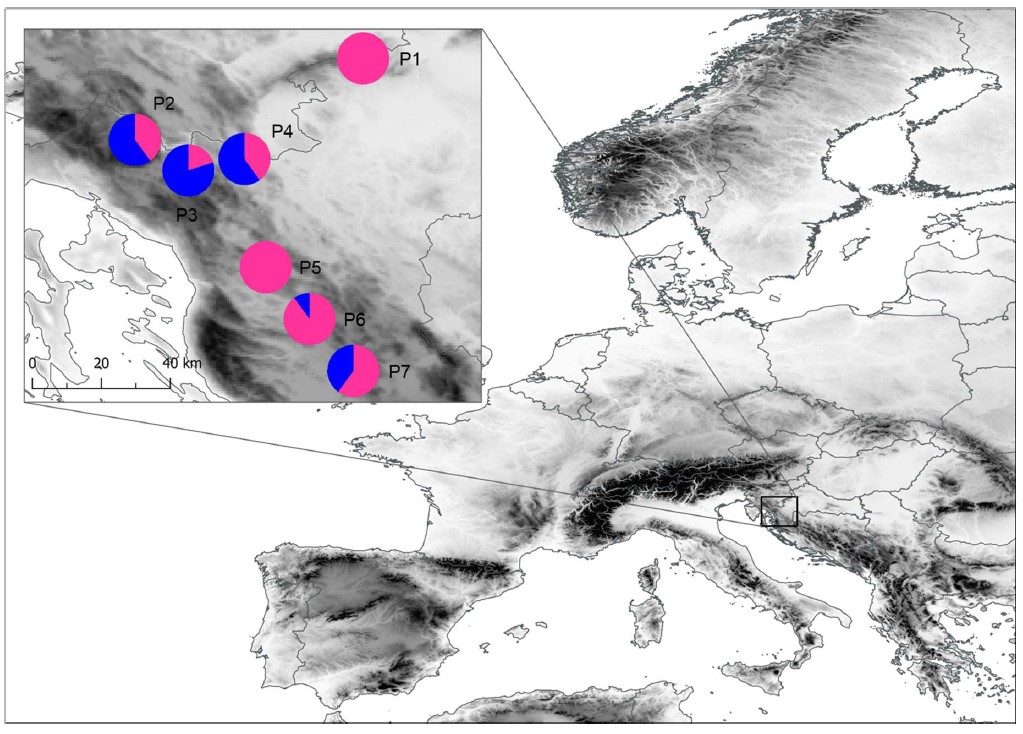
Fig. 1. Results of the K-means clustering method based on 19 leaf phenotypic traits and locations of the seven sampled Fraxinus excelsior populations. The proportions of the membership of each population in each of the defined clusters are colour-coded: cluster A–pink, cluster B–blue). Abbreviations of populations: P1 – Žumberak, P2 – Crni Lug, P3 – Delnice, P4 – Vrbovsko, P5 – Brinje, P6 – Prozor, P7 – Perušić.
A total of 70 trees were sampled, with 20 leaves being collected from every tree. Sampling was conducted in late summer, once the leaves were fully developed. Furthermore, only the optimally developed leaves of short shoots were collected, i.e., those from well-sunlit parts of the crown, with no visible defects or pest and pathogen damage. Once collected, the leaves were immediately placed in cardboard folders and transported back to the Herbarium of the Department for Forest Genetics, Dendrology and Botany, at the Faculty of Forestry and Wood Technology. Finally, leaves were herbarized between sheets of newsprint.
Morphometric analysis
Once fully dried, leaves were scanned, using an A3-format scanner (MICROTEK ScanMaker 9800XL), at a resolution of 600 dpi (.TIF). Image files created in this way were further analysed in the software package WinFolia PRO (Winfolia TM 2001). Using the Interactive Measurement option, the length of rachis (RL) and leaf petiole (PL) were measured on each leaf. Afterwards, on each leaf one top leaflet and one lateral leaflet in the middle part of the leaf was selected and measured using the Leaf Morphology option. In total, eight traits were measured on both the lateral and terminal leaflets, separately, with “LL” de-nominating traits referring to lateral, and “TL” to terminal leaflets: leaflet area (LA); leaflet length (LL); maximum leaflet width (MLW); leaflet length, measured from its base to the point of maximum width (PMLW); leaflet width at 50% of its length (LW1); leaflet width at 90% of its length (LW2); angle enclosed by the main leaflet vein and point on its edge, at 10% of its length (LA1); and angle enclosed by the main leaflet vein and point on its edge, at 25% of its length (LA2). Additionally, petiolule length of the terminal leaflet (TL-PL) was measured.
Population diversity and structure
Arithmetic mean, standard deviation, and coefficient of variation were calculated for the particular trait for each population in order to determine the range of their variation. Pearson correlation coefficients were calculated among all leaf traits including all trees using the CORR procedure in R Version 3.2.2 (R Core Team 2016).
To detect the level of inter- and intrapopulation variability, hierarchical analysis of variance was used. The analysed factors were populations and trees within populations (“tree” factor nested inside the “population” factor). In addition, statistically significant differences in all pairs of populations were identified using Fisher’s LSD multiple comparison test, at P ≤ 0.05. Hierarchical analysis of variance was carried out using the STATISTICA software package version 13 (Statistica, ver. 13 2018).
To analyse structure of the studied populations, two multivariate statistical methods were performed. The K-means method was applied to detect phenotypic structure and define the number of K-groups that best explained the morphological variation of European ash populations. If the proportion of a specific population was equal to or higher than 0.7, that population was assumed to belong to one cluster, and if it was lower than 0.7, that population was considered to be of mixed origin (Poljak et al. 2018). Furthermore, discriminant analysis was performed to evaluate the utility and importance of the measured leaf traits by determining which were most useful in maximally discriminating the populations. In addition, a canonical discriminant analysis was performed based on the leaf traits that best discriminated the studied populations, as determined by stepwise discriminant analysis. Finally, the squared Mahalanobis distances between class means, canonical variables, and eigenvalues were calculated, and the first two canonical variables were plotted. The above multivariate statistical analyses were conducted using the “MorphoTools” R scripts in R Version 3.2.2 (R Core Team 2016) following the manual of Koutecký (2015).
Environmental variability and relationship between morphological variability, environment and geography
To test correlations among morphometric, geographic and environmental data three different matrices were calculated. Data of the bioclimatic conditions for the period from 1970 to 2000, in the area of the studied populations, were obtained from the WorldClim 2 database with a spatial resolution close to a square kilometre (Fick and Hijmans 2017). Firstly, using Spearman´s rank correlation coefficients, highly correlated bioclimatic variables were discarded. In total, six bioclimatic variables were selected and included in the analysis: BIO1 (annual mean temperature), BIO3 (isothermality), BIO11 (mean temperature of coldest quarter), BIO12 (annual precipitation), BIO18 (precipitation of warmest quarter), BIO19 (precipitation of coldest quarter). In addition, altitude values retrieved from GPS data recorded during fieldwork were used to calculate the environmental distance matrix, as well. Finally, environmental differences were calculated as the Euclidian distance between the population means for the first three principal components of the principal component (PC) analysis. Squared Mahalanobis distances among the populations were computed to obtain a matrix of morphometric distances among the studied populations. Geographic distances were calculated from the latitude and longitude of the site of sample collection. Finally, to assess isolation by distance (IBD) and isolation by environment (IBE), response matrix (morphological differences) was compared to the two predictor matrices (environmental differences and geographic distance) using simple Mantel tests (Mantel 1967). The significance level was assessed after 10,000 permutations, as implemented in NTSYS-pc Version 2.21L (Rohlf 2009).
Descriptive statistics
The results of the descriptive statistical analysis are shown in Tab. 1, on the individual population level (N = 200), as well as for the overall sample (N = 1400). Rachis length (RL) had a mean value of 13.99 cm and was relatively variable, with coefficient of variation on the overall sample of 27.15%, with the range between 18.24% (population P2) to 36.15% (population P5). Petiole length (PL) demonstrated a slightly less variable character, with mean length of 6.81 cm and overall CV value of 19.85%. Furthermore, it was also defined by a similarly broad range of variability among the populations, from 11.75% (population P6) to 27.60% (population P7).
The average area of the lateral leaflet (LL-LA) for all seven observed populations was 16.61 cm², and its length (LL-LL) was 8.88 cm. Furthermore, the average maximum lateral leaflet width (LL-MLW) was 2.83 cm. Coefficients of variations for the lateral leaflet area (LL-LA) were within the range from 30.39% (population P5) to 41.71% (population P7). As the most variable traits, width of the lateral leaflet at 90% of leaflet length (LL-LW2) and leaflet area (LL-LA) stood out, with the respective coefficients of variation of 44.92% and 37.02%. On the other hand, the least variable trait was the angle enclosed by the main vein and the line defined by the leaflet basis and point on the edge of the leaflet located at 25% of the total leaflet's length (LL-LA2) (CV = 15.56%).
Tab. 1. Descriptive statistical parameters for studied leaf morphometric traits. Descriptive parameters: M–arithmetic mean; SD–standard deviation CV–coefficient of variation (%). Acronyms of populations: P1 – Žumberak, P2 – Crni Lug, P3 – Delnice, P4 – Vrbovsko, P5 – Brinje, P6 – Prozor, P7 – Perušić. TL – terminal leaflet, LL – lateral leaflet, LA – leaflet area, LL – leaflet length, MLW – maximum leaflet width, PMLW – leaflet length, measured from its base to the point of maximum width, LW1 – leaflet width at 50% of its length, LW2 – leaflet width at 90% of its length, LA1 – angle enclosed by the main leaflet vein and point on its edge, at 10% of its length, LA2 – angle enclosed by the main leaflet vein and point on its edge, at 25% of its length, PL – petiole length, RL – rachis length.
Population P4 was defined by, on average, the largest lateral leaflet area (LL-LA), highest value of the maximum lateral leaflet width (LL-MLW), widest lateral leaflet at 50% (LL-LW1) and 90% (LL-LW2) of the leaflet's width. The longest and narrowest lateral leaflets, with largest distance between the leaflet basis and the point of maximum lateral leaflet width (LL-PMLW), was observed in population P5. The lateral leaflets in population P6 had the lowest mean value of LL-LA and LL-LL, as well as the smallest LL-PMLW value. The largest mean angle value of LL-LA1, was found in population P2, whereas the lowest value of LL-LA1 was noted for population P1. The most variable traits overall, LL-LW2 and LL-LA, demonstrated highest CV values in populations P4 and P7, respectively, whereas the lowest variability for those two traits was noted in populations P1 (CV = 38.58%) and P5 (CV = 30.39%), respectively. On the other hand, the least variable traits were LL-LA2 and LL-LA1, with the lowest CV values noted for population P2, 11.05% and 11.56%, respectively.
Overall, terminal leaflets had somewhat smaller leaflet areas, as well as shorter lengths, than lateral leaflets. The average terminal leaflet area for the seven observed populations was 13.89 cm2, length 8.40 cm, and width 2.82 cm. The angles of LA1 and LA2 demonstrated similar mean values, 21.40º and 22.04º respectively, but differed in the coefficients of variability, with 25.61% noted for LA1 and 18.43% for LA2. The terminal petiolule length was 1.22 cm, which also had the second highest CV value (CV = 43.59%), after trait LW2 (CV=55.12%).
Population P4 stood out for having the highest values for six of the nine measured traits: TL-LA, TL-MLW, TL-LW1, TL-LA1, TL-LA2, TL-PL. Additionally, the remaining maximum values were noted in populations P5 (TL-LL, TL-PMLW) and P7 (TL-LW2). On the other hand, the minimum values were found predominantly in population P6 (TL-LA, TL-LL, TL-MLW, TL-PMLW, TL-LW1), as well as in populations P1 (TL-LA1, TL-PL) and P5 (TL-LW2, TL-LA2). Populations P2, P3 and P7 were of predominantly intermediate values. When the variability of measured traits is considered, the most variable traits were TL-LW2 and TL-PL, with coefficient of variability values of 65.50% (population P7) and 51.32% (population P1), respectively. The least variable traits were TL-LA1 (CV = 13.64%) and TL-LA2 (CV = 13.34%), in populations P3 and P1, respectively. Generally speaking, population P1 stood out as the population with the highest CV values for only two traits (TL-LL, TL-PL), but the lowest values for four traits (TL-LA, TL-MLW, TL-LW1, TL-LA2).
Correlations
A total of 119 statistically significant correlations were noted between the tested pairs of morphological traits (On-line Suppl. Tab. 2). Out of the total 119 correlations, only seven were negative. Furthermore, 30 positive correlations had r-values greater than 0.7, whereas none of the negative correlations demonstrated r-values greater than –0.7.
When lateral leaflet traits are considered, the majority of the trait pairs correlated significantly among themselves, with the exception of the following pairs: LW2 and LL; LA1 and LA, LA1 and LW2; LA1 and LW2 and LA2 and LA. Furthermore, the rachis length (RL) correlated only with the following lateral leaflet traits: LA, MLW, LW1, LW2 and LA1, whereas the petiole length (PL) correlated exclusively with LL of lateral leaflet. This is somewhat expected, as longer petiole automatically means longer leaf, i.e., larger leaflets.
When terminal leaflet traits are considered, the majority too demonstrated significant correlations among themselves. There was, however, no significant correlation of the terminal leaflet traits and traits of the petiole (PL) and rachis (RL). In addition, no significant correlations were noted for the following pairs: LL and LW2, LA1 and LA2; PWLW and LA1 and LA2; and LW2 and LA1.
The correlations between traits of the terminal and the lateral leaflets were also analysed and revealed a large number of significant correlations, with MLW and LW1 standing out by correlating to all other traits. On the other hand, LL did not correlate with LW2, LA1, LA2 or PL. Overall, lateral leaflets demonstrated a far greater number of significant correlations than the terminal leaflets.
Analysis of variance
Statistically significant differences between the observed populations of the European ash were confirmed for 12 out of the 19 analysed traits (Tab. 2). Generally speaking, populations can be best distinguished using the morphometric traits of the terminal leaflets. For the total of seven out of nine traits measured on the terminal leaflets, as well as for four out of eight traits of lateral leaflets, the analysed populations were statistically different. Statistically significant differences were also found for RL, whilst trait PL did not differentiate any of the populations. Furthermore, results of the analysis of variance demonstrated that trees within populations were significantly different, when all measured traits are considered.
Tab. 2. Results of the hierarchical analysis of variance. TL – terminal leaflet, LL – lateral leaflet. Morphometric traits: LA – leaflet area, LL – leaflet length, MLW–maximum leaflet width, PMLW – leaflet length, measured from its base to the point of maximum width, LW1 – leaflet width at 50% of its length, LW2 – leaflet width at 90% of its length, LA1 – angle enclosed by the main leaflet vein and point on its edge, at 10% of its length, LA2 – angle enclosed by the main leaflet vein and point on its edge, at 25% of its length, PL – petiole length, RL – rachis length.
In most of the cases, intrapopulation variability was greater than the interpopulation variability. As an exception, traits TT-LA1, LL-LA1 and TL-PL were defined by having greater interpopulation variability. Furthermore, most of the results indicated that leaf variability within each tree was equal to or slightly lower than the variability of individual trees within a population. In just six instances, leaf variability within each tree (the error component) had a higher share of the total variability, when compared to the variability of trees within populations.
The results of Fisher's LSD test are shown in On-line Suppl. Tab. 3. The number of differing traits among populations varies from one to 10. The most similar populations were P1 and P6, and P3 and P4, which differed only in one trait, LL-LA10 and TL-PL, respectively. On the other hand, pairs of populations with the most differences were P3 and P5, and P4 and P5, each counting 10 differing traits, followed by nine differing traits in pairs of P6 and P2, P3 and P4.
K-means and discriminant analysis
K-means clustering method (Fig. 1) inferred the population structure of the seven populations based on eight morphological leaf traits. Two clusters were clearly visible, mainly corresponding to geographical regions. The first cluster, cluster A, encompassed four populations, marked pink. The three populations were marked blue and formed the second, well-defined cluster, cluster B. Cluster A (pink) was defined by populations P1 (proportion of membership: 1.0), P5 (proportion of membership: 1.0), and P6 (proportion of membership: 0.9). In addition, the population P7 demonstrated a mixed but overwhelmingly cluster A origin. Population P3 demonstrated origin assigned to cluster A (proportion of membership: 0.8), whereas populations P2 and P4 showed a partial cluster affiliation belonging to cluster A (proportion of membership: 0.4) and cluster B (proportion of membership: 0.6).
Five out of eight selected traits have shown to be significant in discriminating the observed European ash populations. The greatest discriminating power was noted for three terminal leaflet traits TL-LA1, TL-LA2 and TL-PL, with partial Wilks’ lambda values of 0.56 (On-line Suppl. Tab. 4). Other significant traits, in descending order of significance, were LL-LA1 and RL. For eight variables and seven groups defined in the canonical analysis, six canonical variates were defined. The first canonical variate (CV1) demonstrated eigenvalue greater than 1 and explained 73.5% of total variability (Fig. 2).
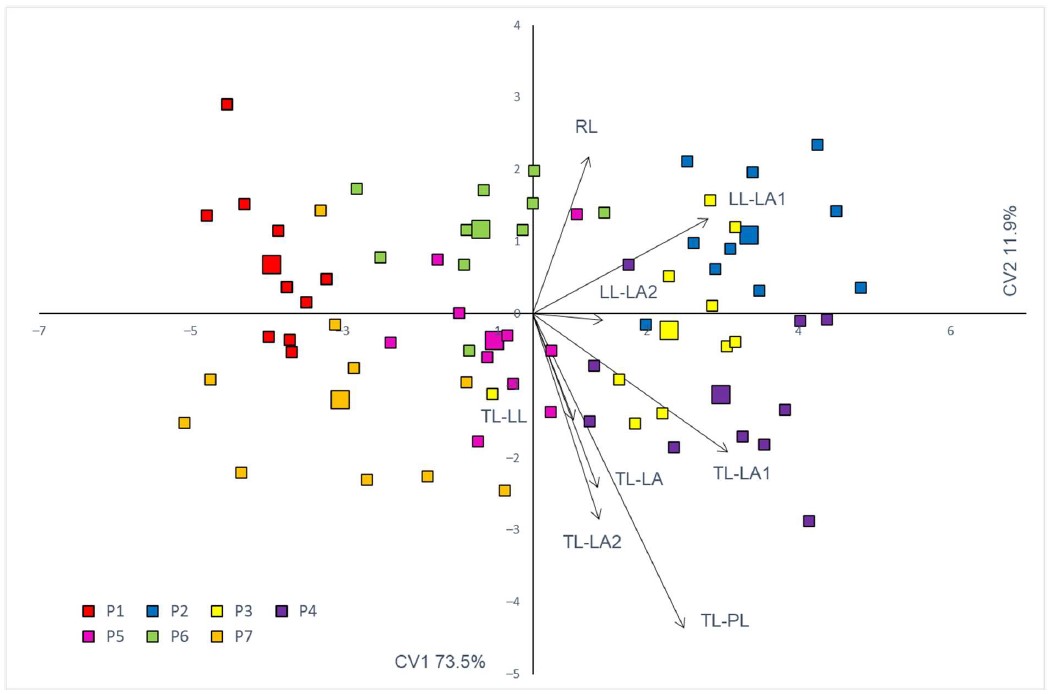
Fig. 2. The first two canonical variates of the canonical discriminant analysis (CV1 and CV2) of seven Fraxinus excelsior populations based on eight morphological traits. Each individual tree is indicated by a small sign, while the population barycenters are represented by larger ones. Leaf morphometric traits: Acronyms of populations: P1 – Žumberak, P2 – Crni Lug, P3 – Delnice, P4 – Vrbovsko, P5 – Brinje, P6 – Prozor, P7 – Perušić. TL – terminal leaflet, LL – lateral leaflet. Morphometric traits: LA – leaflet area, LL – leaflet length, LA1 – angle enclosed by the main leaflet vein and point on its edge, at 10% of its length, LA2 – angle enclosed by the main leaflet vein and point on its edge, at 25% of its length, PL – petiole length, RL – rachis length.
Along the same CV, when the studied individuals and populations were considered in two-dimensional morphospace, although with some overlap, a clear separation of individuals from the central part (P2, P3 and P4) and the northern (P1) and southern (P5, P6 and P7) parts of the studied area is visible. The classification accuracy for all of the populations was 77.1%. The highest classification accuracy, i.e., 100% of correctly classified individuals, was noted for the population P1, whereas the accuracy for the individuals of the populations P3 and P4 amounted to only 50%.
Environmental variability and relationship between morphological variability, environment and geography
Principal component (PC) analysis, based on the environmental data, showed that the first two principal components had eigenvalues greater than 1 and together explained 83.4% of the total variability (On-line Suppl. Tab. 5). The first principal component explained 54.09% of the total variability. The variables displaying the highest negative correlations with the first principal component (–0.7) were altitude and BIO18, while the variables displaying the highest positive correlations (0.7) with the same principal component were BIO1 and BIO11. The second principal component explained 29.28% of the total variability. The variables that showed the highest correlations with the second PC axis were BIO19 and BIO3. The biplot of the principal component analysis based on seven environmental variables is shown in On-line Suppl. Fig. 1. A clear separation of populations along the first PC axis according to the geographical principle was observed, with populations P1-P3 grouped on the left, and populations P4-P7 on the right side of the diagram. Populations on the left side of the biplot are characterized by higher altitudes, higher annual precipitation (BIO12) and precipitation during the warmest quarter (BIO18). On the other hand, populations on the right side are characterized with lower altitudes, with higher annual mean temperature (BIO1) and the mean temperature of the coldest quarter (BIO11). Along the second PC axis separation of P1 of other studied populations is clearly visible.
In order to determine whether the observed morphological variability was caused by geographical (IBD) or environmental distances (IBE) between the studied populations, the Mantel test was performed. Our results showed that population-level pairwise morphological distances were not related to the geographic distances between populations (r = 0.487, P = 0.066), nor to environmental distances (r = 0.326, P = 0.149).
Discussion
The results of the leaf morphometric analysis revealed that both the average terminal and lateral leaflet lengths, of 8.4 cm and 8.9 cm respectively were within the previously reported intervals of 3-12 cm (Herman 1971, Beck et al. 2016). Furthermore, average widths of the lateral (2.8 cm) and terminal (2.8 cm) leaflets were also within the reported interval of 0.8-3 cm (Beck et al. 2016) but were somewhat shorter than values reported by Idžojtić (2009). Petiole length (6.8 cm), on the other hand, demonstrated value on the shorter side of the interval reported by Idžojtić (2009), 5-10 cm. When the whole leaf is observed, the measured mean length (29.2 cm) was within the reported intervals of 20-40 cm (Herman 1971, Idžojtić 2009, Beck et al. 2016). The two most variable traits for the leaflets were those pertaining to leaflet size (LA and LW2). Significant variability of leaflet area in compound leaves has been previously reported (Wang et al. 2022) and could be explained by the general plasticity of leaves as the main photosynthetic organs of the plant (Henry et al. 2020).
Our research showed larger and longer lateral than terminal leaflets, while their width was almost identical, which indicates that leaflet position within the leaf can influence its morphology. According to Chitwood et al. (2012), variability of leaflet shape and size can be influenced by multiple factors, including the heteroblasty, the proximal-distal and left-right axes of leaves, sun and shade conditions and developmental stage. In case of Fraxinus excelsior, the proximal-distal axis of each leaf influences the leaflet shape and developmental stage. Namely, F. excelsior leaves develop basipetally, that is, the terminal leaflet is developmentally older than the proximal pairs of lateral leaflets (Moline and Bostrack 1972, Chitwood et al. 2012), causing the differences in leaflets’ shape and symmetry. Generally, terminal, i.e., older leaflets show greater variability, previously reported for Sorbus domestica L. (Poljak et al. 2015) and various Solanum L. section Lycopersicon species (Chitwood et al. 2012).
The results confirmed significant correlations between the vast majorities of the studied morphological traits. Although both terminal and lateral leaflets showed a moderate to high degree of correlation within and between each other, only the lateral leaflet showed correlation with the length of the rachis and petiole. Interdependence of compound leaf morphological traits has been previously recorded for a number of Pistacia L. species (Kafkas et al. 2002, Karimi et al. 2009), as well as Juglans regia L. (Kabiri et al. 2018) and Robinia pseudoacacia L. (Guo et al. 2022). Although the mentioned studies did not include lateral leaflet, and generally covered fewer leaflet traits, a positive correlation between the length and width of the terminal leaflet was detected. However, in contrast to Kafkas et al. (2002), we did not discover a correlation between petiole length and terminal leaflet length.
Conducted research revealed variability that was high within, and somewhat lower among populations. On the interpopulation level, significant differences were observed for 12 of the studied morphological traits, while seven traits did not show significant differentiation on the intrapopulation level, and have accounted for a very small percentage of total variability. Significantly lower intrapopulation variability could be attributed to lower genetic diversity of F. excelsior among populations in central Europe, confirmed in the study by Heuertz et al. (2004b) and Ballian et al. (2008). According to Hamrick and Godt (1996), such a pattern of genetic diversity is typical for outcrossing, woody plants, as they tend to be more genetically diverse and have less genetic differentiation among their populations. A similar pattern of morphological variability has also been described for narrow-leaved ash ( Fraxinus angustifolia Vahl) populations in Slovenia (Jarni et al. 2011).
For several traits in this research, significant differentiation between the European ash populations was observed, particularly for the traits relating to leaflet base shape, for both the lateral and terminal leaflets. In addition, terminal leaflet petiole length showed great differentiating power as well. These differences can most likely be ascribed to phenotypic plasticity, which is the result of conditions in the microhabitats the observed populations are found in. Namely, multivariate analyses have shown that the ash populations could be separated into two groups, depending on the micro-habitat conditions they grow in. The first group encompasses individuals from the central portion of the researched area, the populations P2-P4, whereas the second group included the northernmost population P1 and populations P5-P7, found in the southern part of the researched area. The first group of populations grows on nutrient-rich and less dry habitats, whereas the second group inhabits skeletal and, generally speaking, drier terrains. In the conditions of extensive gene flow, phenotypic plasticity enables individuals to express different and locally adapted phenotypes formed under the influence of contrasting habitat and environmental conditions (Stotz et al. 2021). In the case of the drier habitats, populations were characterized by more acute and somewhat narrower leaflets, when compared to the more humid habitats, in which leaflets demonstrated rounder base and larger area generally. Leaf and leaflet growth were proved to be significantly affected by water stress in R. pseudoacacia as well (Zhang et al. 2012). This difference in shape between the populations of contrasting habitats, indicates that leaf shape is under the influence of microhabitat conditions, with smaller leaf area likely being the result of lower water availability. This has previously been shown to be the case for numerous plant species, on the global level (Peppe et al. 2011). On the other hand, the influence of altitude and bioclimatic variables on leaflet morphology can be almost completely excluded, as no statistically significant correlation between the ecological and morphological distances between the populations was detected.
Furthermore, no IBD pattern for the European ash populations of the Dinaric Alps was established. IBD, or isolation by distance, is a model of genetic differentiation between populations, in which genetic differences increase with geographic scale (Morente-López et al. 2018). The lack of IBD in this research is visible in the grouping of the P1 from the northernmost part of the researched area with southern populations of P5-P7. This was further confirmed by the Fisher LSD tests. Namely, geographically close populations, e.g., P4 and P5, differed by 10 analysed traits, unlike the two most distant populations, P1 and P7, which only had two traits that differed. Nevertheless, IBD is a well-documented pattern of differentiation, noted in both plant (Twyford et al. 2020) and animal species (Bayne 2017), as well as on the level of whole landscapes (van Strien et al. 2014).
Conclusions
Significant differences between studied European ash populations were found for 12 out of 19 traits. In general, the traits related to shape of the leaflet base have shown to be more significant in describing differences between populations and also had the lowest coefficient of variation. On the other hand, the traits related to the size of the leaflets had a high degree of variability and, using them, the populations did not, in most cases, statistically differ. The high between-tree variation within the populations, and relatively low among-population variation, found in our research can be explained by effective gene flow. Nevertheless, when the traits with the highest discriminatory power between the populations are considered, the results of the multivariate statistical methods revealed two groups of populations defined by the common microsite conditions the researched populations are found in. These differences can most likely be ascribed to phenotypic plasticity, which caused leaflets on the drier and skeletal habitats to be smaller and acute, whereas those individuals from mesophilous and nutrient-rich habitats had somewhat larger and rounder leaflets. Furthermore, no statistically significant influence of the altitude and bioclimate variables on leaf variability was established. Overall, the results of this investigation provide additional insight into the variability of the species, which can be useful in creating guidelines for conservation, breeding, and afforestation programs, which is needed for sustainable forest management in the area.
