Introduction
Water melon (Citrullus lanatus) is a tropical fruit which can be found in most parts of Africa, Asia, United States and Russia (FAOSTAT, 2016). It is an important horticultural crop, mostly grown in warm regions for its sweet and juicy taste in (et al., 2012). It belongs to the cucumber family (Cucurbitacea). Water constitutes about 92% of the total fruit weight and it is of great importance in Africa due to thirst quenching ability during water shortage (Ensminger et al., 1986). The crop is mainly harvested for juice and juice concentrates being an excellent source of vitamins, such as vitamin A and C (Edwards et al., 2003. However, the seeds are not routinely eaten with the pulp, they are discarded each year either as cheap animal feed or simply thrown away.
The major challenge in food processing is the disposal of waste products generated. These wastes pose ecological problems related to the proliferation of insects and rodents; therefore research towards finding a potential utilisation of this material is required (Hussein et al., 2001. Edible portions of fruits are processed into diverse products such as puree, canned slices, whereas seeds are often disposed as waste since there are no current commercial utilisation purposes (Ajila et al., 2007, but they are a promising source of useful compounds due to their favourable nutritional properties (Schieber et al., 2001. Water melon seeds, are currently regarded as a waste product since they are often disposed of after eaten the pulp; this constitutes about 1 to 4% of total fruit weight (Kiin-Kabari and Akusu, 2014.
Recently, more attention has been focused on the potential utilisation of agricultural by-products in the development of new functional ingredients for food enrichment to provide an alternative for industries and sustainability of the environment (Salgado et al., 2001. Often, agricultural waste products are sources of phytochemicals with functional properties, such as phenolics, alkaloids and saponins which have radical scavenging ability in a biological system. There have been increases to the utilisation of agricultural waste to reduce environmental pollution, taking into account the nutrients and phytochemicals contents (Seidu et al., 2015).
Antioxidants are substances that are capable of counteracting the damaging but normal effects of the physiological process of oxidation in animal tissue. There is a strong evidence that reactive oxygen species (ROS) including free radicals can lead to lipid peroxidation and oxidative stress which damage biological structures such as proteins, lipids and DNA. The results in body include: ageing; non-communicable chronic diseases such as heart disease, stroke, certain cancers, neuro-degenerative disease and lung disorders. In general, human body has its own natural antioxidant system to stand against free radicals using enzymes. It is believed that an intake of antioxidants reinforces the defense of human antioxidant system (Edeoga et al., 2005). Oxidative stress occurs when the production of harmful molecules called free radicals is beyond the protective capability of the antioxidant defenses. While a lot of information abounds in the proximate and oil content of watermelon seeds, there is dearth of information on the phytochemicals and radical scavenging potential of the seeds. Hence, the present study aimed at the phytochemicals and antioxidants activities of water melon seed constituents.
Materials ans methods
Materials
Water melon (Citrullus lanatus) seeds were procured from a local market in Ado-Ekiti, Nigeria.
Chemicals
Ascorbic acid, acetic acid, ethanol, ammonium hydroxide (NH4OH), Folin–Ciocalteu’s reagent, gallic acid, sodium carbonate (Na2CO3), Sodium nitrite (NaNO2), aluminium chloride (AlCl3), sodium hydroxide (NaOH), isobutyl alcohol, magnesium carbonate (MgCO3), iron (III) chloride (FeCl3), hydrochloric acid (HCl), ammonium thiocynate (NH4SCN), Potassium permanganate (KMnO4), Sulfuric acid (H2SO4), potassium persulfate (K2S2O8), 2, 2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid), Trolox, 2, 2-diphenyl-1-picrylhydrazyl, methanol, potassium ferrocyanide (K₄[Fe(CN)₆] · 3H₂O.) and trichloroacetic were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Pascal Scientific LTD (Akure, Nigeria).
Methodology
Production of watermelon seeds flour
The seeds were washed and air-dried (at 50 °C) for 24 h, divided into two parts, one part was shelled manually to remove the shells. The shelled seeds, shells and whole seeds were milled into flour in an electronic blender, kept in an air-tight plastic container at 4 °C prior to analysis.
Determination of alkaloid
The five grams (5 g) of the water melon seed flour were weighed into a beaker and 200 ml of 10% acetic acid in ethanol was added and allowed to stand for 4 min. This was filtered and the extract was concentrated on a water bath to one quarter of the original volume. Ammonium hydroxide solution was added drop wise to the extract until the precipitation was completed. The precipitate was collected, washed with dilute ammonium hydroxide and filtered. The residue was dried and weighed (Harbone, 1973).
Determination of total polyphenols
This was determined using the Folin-Ciocalteu method (Singleton et al., 1999) Distilled water and Folin-Ciocalteu’s reagent were added to a 125 μl water melon seed extract. The mixture was allowed to stand for 6 min before the addition of 7% sodium carbonate solution. The mixture was allowed to stand for 90 min; absorbance was read at 760 nm and the result was expressed as gallic acid equivalents (GAE).
Determination of flavonoid
Approximately 0.25 g of water melon seed flour was dissolved in 1 ml distilled water; 5% NaNO2 solution, 0.150 ml of freshly prepared aluminum chloride (AlCl3) and 1 M NaOH solutions were added. The mixture was allowed to stand for 5 min and absorbance measured at 510 nm. The result was expressed as quercetin equivalents (QE).
Determination of saponin
The saponin content was determined using spectrophotometric method described by (Brunner, 1984). About 2 g of water melon seed flour were weighed into a beaker and isobutyl alcohol (but-2-ol) was added. The mixture was filtered through No 1 Whatman filter paper into a beaker containing 40% magnesium carbonate (MgCO3) solution. Approximately 1 ml of the solution was transferred into volumetric flask; 2 ml iron (III) chloride (FeCl3) solution was added and made up to mark with distilled water. This was allowed to stand for 30 min for the colour development and absorbance was read at 380 nm.
Determination of phytate
The four grams (4 g) of water melon seed flour were soaked in 2% hydrochloric acid for 3 h and filtered through a No 1 Whatman filter paper. About 25 ml of the filtrate was transferred into a conical flask and 5 ml of ammonium thiocynate solution was added as an indicator. After which distilled water was added to give it the proper acidity and this was titrated against standard iron (III) chloride solution until a brownish yellow colour persisted for 5 min (Wheeler and Farrel1971
Determination of oxalate
The 1 g of sample was soaked in plastic bottle followed by the addition of 75 ml 1.5 N sulphuric acid solution. The content was mixed properly and allowed to extract for 1 h with constant agitation using mechanical shaker. The resultant solution was filtered through No 1 Whatman filter paper and the filtrate was titrated against 0.1 M potassium permanganate (KMnO4) solution while hot (80-90 °C) until a purple colour was observed.
Determination of cardiac glycosides
Approximately 10 ml of water melon seed flour dissolved in water was transferred into a conical flask containing chloroform, the mixture was filtered into a conical flask with an addition of 10 ml pyridine and 29% sodium nitroprusside and thoroughly shaken for 10 min. Thereafter, 20% NaOH was added for colour development and absorbance was read at 510 nm (Olaleye, 2007.
ABTS radical scavenging activity
The radical scavenging activity of water melon seed against 2, 2’-azinobis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) was determined by the 2, 2’-azinobis 3-ethylbenzothiazoline-6-sulfonic acid diammonium salt radical cation (ABTS) test (Re et al., 1999. 2, 2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS.+) decolourization test is a spectrophotometric method widely used for the assessment of antioxidant activity of various substances. The absorbance of the ABTS+ solution was equilibrated to 0.70 (± 0.02) by diluting with water at room temperature. The predetermined volume of ABTS+ solution was mixed with 100 µl volume of the test sample. The absorbance was measured at 734 nm after 6 min of starting the reaction.
DPPH radical scavenging activity
Watermelon seeds flour capacity to scavenge lipid-soluble 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical, which resulted in the bleaching of the purple color exhibited by the stable DPPH radical was determined using the procedure of Amarowicz et al. (2008). A known volume of sample extract was added to a methanolic solution of DPPH. The solution was kept in a dark chamber for 30 min before measuring the absorbance at 517 nm. Free radical scavenging ability was calculated as percentage of DPPH radical discolouration.
Ferric reducing power
The reducing property was determined by assessing the ability of the water melon seed flour to reduce iron III chloride solution. An appropriate dilution of the seed extract (0.5 ml) was mixed with sodium phosphate buffer (pH 6.6) and 1% potassium ferrocyanide. The mixture was incubated at 50 °C for 20 min after which trichloroacetic acid was added. The mixture was centrifuged at 650 rpm for 10 min. The upper layer was mixed with equal volume of distilled water; 1% ferric chloride and absorbance was measured at 700 nm (Olaleye, 2007).
Statistical analysis
All analyses were carried out in triplicates and data generated was subjected to statistical analysis using statistical package for social scientist (SPSS) 16 computer package. Level of significance was determined by Analysis of variance (ANOVA) and means were separated by New Duncans’ Multiple Range Test at the level of p ≤ 0.05.
Results and discussion
Phytochemicals composition
The result of phytochemical composition is depicted inTable 1. The most abundant phytochemical in water melon seeds was saponin (11.62-32.48 mg/g) and cardiac glycosides (9.94-14.35 mg/g). Cardiac glycosides were more abundant in the whole seeds, they are known to be useful in the treatment of congestive heart failure (Schneider and Wolfling, 2004). They exert some effects on neural tissue, thus indirectly influence the activity of the heart and modify vascular resistance and capacitance (Olaleye, 2007). Saponin was significantly (p ≤ 0.05) higher in shelled seeds, values in this study are higher than the value reported for some Nigerian medicinal plants (Cleome nutidosperma, Physalis angulata, Richardia bransitensis, Scopania dulcis, Sida acuta etc) (Edeoga et al., 2005Kim et al., 2003). Pharmaceutically, saponins have been patented for the treatment of various conditions such as inflammation, pre-and post-menopausal symptoms (Bombardelli and Gabetta, 2001), cardiovascular and hypertension (Yao et al., 2005). Purified saponins have been used in the manufacture of food and drinks primarily as foaming agents and antioxidants (Takashi et al., 1986.
Values are means of triplicate determinations ± Standard deviation
Means with different letters in the same row are significantly different (p ≤ 0.05)
Alkaloids (47.2-95.8 mg/g) in this study were significantly (p ≤ 0.05) higher than those reported for some tropical legumes such as Vigna uniguiculata (3.7 mg/g) and Arachis hypogea (2.5 mg/g) (Mbagwu et al., 2011). Plant alkaloids and the synthetic derivatives are used as a basic medicinal agent due to analgesic, antispasmodic, anti-cholinergic and anaesthetic properties (Okwu, 2004), antimicrobial activity of alkaloids have also been reported (Olaleye, 2007).
Total phenol (5.63-8.40 mg GAE/g) was higher than (0.3-1.0 mg/g) value recorded for various species of V. unguiculata (Oboh, 2006). Phenolics have been found to inhibit autoxidation of unsaturated lipids, thus preventing the formation of oxidised low-density lipoprotein (LDL), which is known as a causative factor of cardiovascular disease. Flavonoids are a group of secondary metabolites found in foods of plant origin as well as medicinal plants which have been shown to exert potent antioxidant activity against the superoxide radical. Flavonoids (3.51-7.76 mgQE/g) have been used as natural antioxidants in foods and pharmaceutical drugs due to their ability to scavenge reactive oxygen species (Bombardelli and Gabetta, 2001). The result showed phytate content in the range of 16.4 to 66.0 mg/g far above the values reported for some commonly consumed legumes. Phytates have been shown to posses anticancer and antioxidant property (Sudheer et al., 2004), by forming an ion chelate in blocking iron-driven hydroxyl radical generation (McCarty, 2004). Industrially, it has been employed as a preservative for some products, reduction of fermentation time and in taste enrichment.
In-vitro antioxidant activity
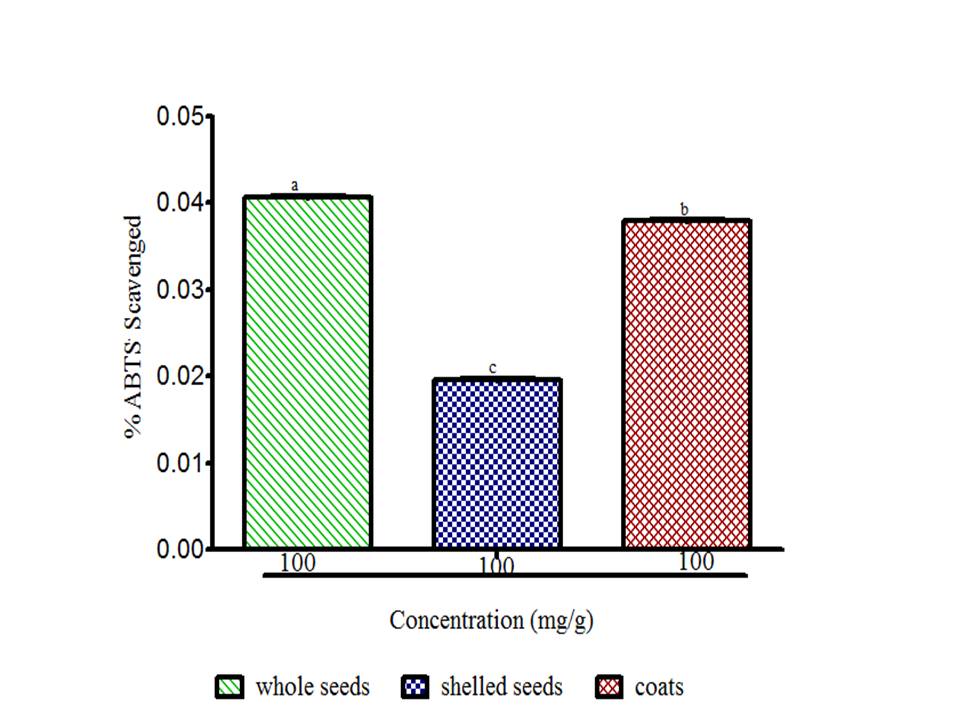
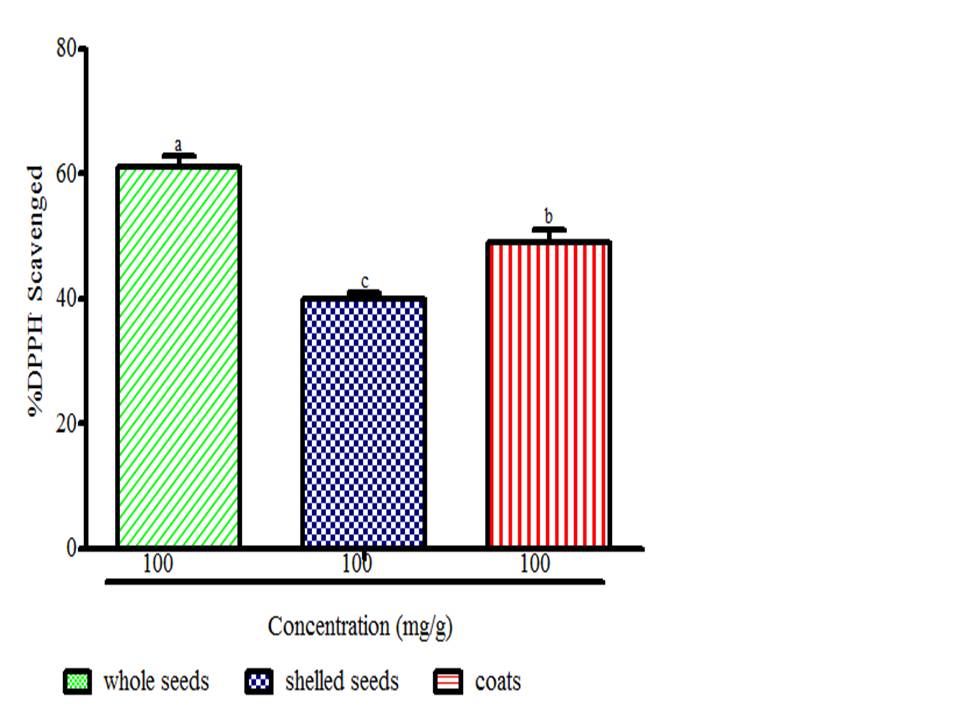
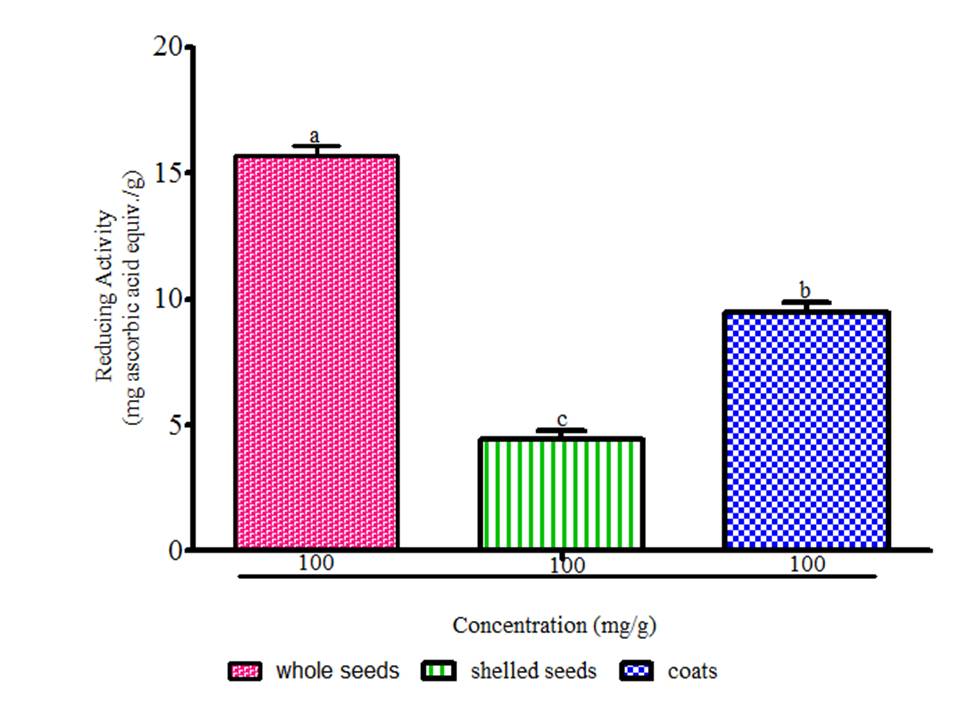
There is a growing awareness that many phytochemicals found in whole plant foods can provide versatile health protection; in particular, these compounds are believed to help preserve vascular health and diminish cancer risk (McCarty, 2004). The results showed radical scavenging ability of the seeds constituents against ABTS in the range of 0.02 to 0.04 mg Trolox equivalent /g seed flour (Fig. 1), DPPH was 39.89 to 61.11 mg ascorbic acid equivalent /g seed flour (Fig. 2) free radicals and reducing power (Fig. 3). The samples reduced the radical to the corresponding hydrazine (Fig. 2) when it reacted with hydrogen donors in the antioxidant principle thus, exhibiting considerable antioxidant activity by the scavenging of the cation radical. The free radical-scavenging activity of the constituents could be attributed to the hydrogen-donating ability. All the constituents exhibit considerable antioxidant activity by the scavenging of the ABTS and DPPH free radicals. Whole meal and shell flours have higher free radicals inhibitory power than shelled flour, in agreement to the observation ofSeidu et al. (2014) on lima bean seed coats. The transformation of Fe3+ to Fe2+ in the presence of water melon seed flour and the reference compound ascorbic acid was used to measure the reductive capability (Fig. 3). The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. Water melon seed constituents possess considerable reducing properties as demonstrated by its ability to reduce Fe3+ to Fe2+. The antioxidant activities suggest that the polyphenolics available in the seeds could act as reductones through electrons donation to free radicals and terminate the free radical mediated chain reactions (Gordon, 1990).
Conclusions
The phytochemical contents coupled with radical scavenging activities of water melon seeds constituents studied underline the potential use of watermelon seeds rather than disposing them as a waste product after consuming the pulp. The seed could be used in the development of new functional foods to promote good health.