Introduction
Due to the bioactive properties and health-promoting benefits of polyphenols from medicinal plants and food, pharmaceutical and cosmetic industries are increasingly turning to natural resources and their usage in the production of functional food, drugs, and cosmetics (Sharma and Singh, 2010). However, the effectiveness of polyphenols in the prevention of diseases depends on the preservation of their bioavailability, since only a small proportion remains available following oral administration. Also, polyphenols are sensitive to various environmental conditions, making them prone to degradation during different processing conditions (Bell, 2001).With the aim of their successful incorporation into various products, during the last several decades encapsulation was highlighted as an effective technique for the protection of polyphenolic compounds, since it allows their efficient immobilization into various delivery carriers. Among various materials used for the delivery of active compounds, sodium alginate has found widespread applications in food and pharmaceutical industries due to its favourable properties, like biocompatibility, non-toxicity, and low price (Lee and Yuk, 2007). However, due to the pronounced porosity and poor retention of the active ingredients of low molecular weight in the alginate gel network, it is often reinforced with natural biopolymers, like proteins, which, thanks to their functional features, may enhance retention of the entrapped substances. Food proteins are a versatile group of biopolymers possessing many functional properties, including emulsification, gelation, and foaming, and their application in terms of delivery vehicles for the immobilization of numerous bioactive compounds is considerable (Chen et al., 2006). Zein is the prolamin of maize (Zea mays L.) with existing and potential applications in the food, agricultural, pharmaceutical, and biotechnology industries, where it is used mainly in terms of coatings, films, and tabletting (Hurtado-López and Murdan, 2008). Zein-based micro- and nanoparticles have been used for encapsulation, stabilization, and the controlled release of polyphenols, essential oils, food grade antimicrobials, bioactive lipids, functional micronutrients, and some food colouring agents (Wan et al., 2015). Polyphenols like curcumin (Patel et al., 2010), quercetin (Patel et al., 2012), tangeretin (Chen et al., 2014), and cranberry procyanidins (Zou et al., 2012) were encapsulated in zein nanoparticles using the liquid-liquid dispersion method. Also, zein/tannic acid complex was produced using the antisolvent approach (Zou et al., 2015).Neo and co-workers (2013) incorporated gallic acid into zein ultra-fine fibres in order to develop an encapsulating technology for functional ingredient delivery using electrospinning, while green tea polyphenol (-)-epigallocatechin gallate was encapsulated in zein fibres using the same method (Li et al., 2009).
Ganoderma lucidum is a medicinal mushroom mostly used in Asian traditional medicine, but due to numerous pharmacological effects it is often used worldwide (Boh et al., 2007). Ganoderma contains a variety of active compounds, such as proteins, amino acids, alkaloids, fatty acids, enzymes, polyphenols, vitamins, and lignin, however, two major chemical groups are polysaccharides ß-glucans and triterpenes (Leung et al., 2002; Yuen and Gohel, 2005). Even though the phytochemical composition of Ganoderma is well documented, there is a lack of investigations dealing with its polyphenolic profile, especially with regard to the encapsulation of Ganoderma polyphenolics. According to our knowledge, the polyphenolic extract of Ganoderma was encapsulated using electrostatic extrusion and ionic cross-linking of alginate and pectin, and carrageenan and chitosan reinforcement in a study byBelščak-Cvitanović et al. (2016a).
In this study, the bioactive profile of Ganoderma lucidum was evaluated and ionic gelation was applied to immobilize the aqueous polyphenolic extract of Ganoderma in different alginate-based delivery systems. In that order, alginate (4%, w/v) was combined with whey protein isolates and zein. The obtained hydrogel beads were characterized in terms of their physico-chemical (particle size, texture, water content) and morphological properties (scanning electron microscopy), and encapsulation efficiency (polyphenols and retained antioxidant capacity). The release profile of polyphenols and the antioxidant activity from alginate-based hydrogels were accompanied in simulated gastrointestinal fluids.
Materials and methods
Preparation of the Ganoderma lucidum extract
Firstly the dried Ganoderma mushroom was ground to appropriate particle size using a domestic grinder. Afterwards extracts were prepared by pouring 250 ml of distilled water (T = 80 °C) over 15 g of dried plant and stirred with a glass rod for 30 minutes, while maintaining the water temperature during the whole extraction time. The obtained extract was filtered through a tea strainer and cooled to room temperature.
Determination of the bioactive profile of the Ganoderma extract
Total polyphenols (TP) content was analysed using the Folin-Ciocalteu reagent, according to a modified method ofLachman et al. (1998). Formaldehyde precipitation of flavonoids was used for the determination of total flavonoids (TF) content, and flavonoids were calculated as the difference between total polyphenol and non-flavonoid contents. Results were expressed as mg gallic acid equivalents (GAE)/g of dry weight (dw) of plant. The method for the determination of hydroxycinnamic acids - HCA (mg caffeic acid (CaffA)/g dw) was adapted fromMatkowski et al. (2008), while the content of flavan-3-ols (Fla-3-ols) was determined using the vanillin assay (VA) as described byDi Stefano et al. (1989), and expressed as mg (+)-catechin/g dw. Trolox equivalent antioxidant capacity (TEAC) was estimated by the ABTS radical cation decolourization assay (Re et al., 1999), while antioxidant capacity evaluated using the DPPH radical was described byBrand-Williams et al. (1995). The results of the antioxidant capacity were expressed as mmol Trolox equivalents/g dw. All measurements were performed in triplicate, and the results were expressed as means ± SD.
Preparation of alginate-based hydrogel beads
Three alginate-based delivery systems were formulated in this study: plain sodium alginate (A), alginate combined with whey protein isolates (A-WPI), and alginate combined with zein (A-Z). Alginate solution (4%, w/v) was prepared by dissolving sodium alginate in the previously prepared Ganoderma extract and stirring overnight, as well as WPI and Z solutions (10%, w/v). Afterwards, the plain alginate solution was mixed with WPI and Z solutions in mass proportion of 80:20 (w/w). Solutions were well homogenised and drawn into a syringe to which a blunt tip and an appropriate metal needle (18-23 gauge) were attached. The prepared encapsulate mixtures were dripped into a receiving beaker containing the cross-linking solution, which consisted of 3% (w/v) calcium chloride previously dissolved in Ganoderma extract. After the ion exchange, hydrogel beads were allowed to stir gently in the collection solution, they were washed several times with extract, and stored that way in the dark at 4 °C, while a proportion of the hydrogel beads was subjected to freeze drying. Also, blank samples containing distilled water instead of the polyphenol extract were prepared in the same way as described.
Physico-chemical and morphological properties of alginate-based beads
The particle size of the prepared hydrogels was determined by recording the particles with a digital camera on the Dino-Lite calibration plate, and then reading the particle size. The texture analysis was carried out using a TA.HDPlus texture analyzer equipped with a cylindrical steel probe P/2 with a flat bottom (Stable Micro Systems, Godalming, Great Britain), at the penetration rate of 0.5 mm/s. The hardness of the samples was expressed through the maximum force (N) needed for compression. Elasticity (mm) of the samples was measured as the travel distance of the probe to the breaking point of each sample, and expressed on 50% of the readings for the hardness. The water content of the obtained hydrogel beads was determined according to the modified standard AOAC 966.02. method (AOAC, 1990). Scanning electron microscopy (SEM) analysis was performed using a TESCAN Mira3 microscope (Czech Republic). Freeze-dried beads were attached to stubs using two-sided adhesive tape, coated with a layer of gold (50 nm), and examined using acceleration voltage of 4-5 kV.
Encapsulation efficiency of polyphenols and retained antioxidant capacity
The total polyphenols content and antioxidant capacity entrapped in the obtained beads were estimated by dissolving a known amount of hydrogel beads in 2% sodium citrate (w/v) at ambient temperature, until total dissolution occurred. The total polyphenols content was determined using a Folin-Ciocalteu reagent, while antioxidant capacity was evaluated by ABTS and DPPH radical scavenging assays (as previously described). The possible interactions of the Folin-Ciocalteu reagent with the polysaccharides and proteins were neutralized by readings and subtractions of blank samples (not containing Ganoderma extract). The percentage of loading efficiency was calculated as the ratio of total polyphenols and retained antioxidant capacity in the citrate solution of dissolved beads and their content in the initial delivery solution.
Release profile of polyphenols and antioxidant activity
The release profile of total polyphenols and antioxidant capacity of the hydrogel beads were determined in simulated gastric (pH=1.2) and intestinal (pH=7.4) fluids (without pepsin and pancreatin), according to the modified method ofBelščak-Cvitanović et al. (2016).
The release profile was determined by evaluating the total polyphenol content using the Folin-Ciocalteu assay (mg GAE/g of beads), and the retained antioxidant capacity by the ABTS and DPPH assays (mmol Trolox/g of beads) (as previously described).
Statistical analysis
The results were analysed statistically using the Statistica 7.0 software to determine the average value and standard error. Variance analysis, with a significance level of α=0.05%, was performed to determine the influence of delivery system formulations on the encapsulation attributes of obtained beads.
Results and discussion
Bioactive profile of Ganoderma lucidum
Total polyphenols (TP) content exhibited 1.45 mg GAE/g, among which total flavonoids amounted up to 45% of TP. Among the evaluated polyphenolic groups, hydroxycinnamic acids were assessed in remarkable proportion, with 0.49 mg CaffA/g, while flavan-3-ols, determined by vanillin assay, were present in low content, accounting to 0.05 mg (+)-catechin/g (Table 1).Saltarelli et al. (2009) evaluated higher TP in different mycelium of Ganoderma lucidum from central Italy. Such values could be subscribed to different parameters used in extract preparation, but maybe mostly to the applied solvent, since the authors used an ethanol solution for the extraction of polyphenols, which is often reported as a more efficient extraction solvent for polyphenolics in comparison to water. However, in this study, water was used as the extraction solvent for Ganoderma polyphenols for encapsulation purposes, since it is known that polysaccharides, like alginate, precipitate in ethanol solutions. Furthermore, as presented inTable 1, antioxidant capacity determined by two radical scavenging assays was evaluated to similar extent, with 0.14 mmol Trolox/g (ABTS) and 0.10 mmol Trolox/g (DPPH).
| Polyphenolic compounds | Antioxidant capacity | ||||
| TP mg GAE/g | TF mg GAE/g | HCA mg CaffA/g | Fla-3-ols mg (+)-catechin/g | ABTS mmol Trolox/g | DPPH mmol Trolox/g |
| 1.45±0.30 | 0.66±0.05 | 0.49±0.02 | 0.05±0.00 | 0.14±0.00 | 0.10±0.00 |
TP- total polyphenols; TF- total flavonoids; HCA- hydroxycinnamic acids; Fla-3-ols- flavan-3-ols
Physico-chemical and morphological properties of alginate-based beads
In comparison to the particle size of plain alginate beads (2.18 mm), introduction of WPI to the alginate network reduced the particle size (2.10 mm), while the addition of zein resulted in the formulation of larger hydrogel beads (2.76 mm) (Table 2). However, the influence of the delivery materials on particle size of obtained beads was insignificant (p>0.05). When encapsulating rosemary polyphenols in different alginate-based carriers, the employment of WPI also resulted in the smallest particle size of obtained hydrogel beads (1.7 mm) (Bušić et al., 2016). However, the authors obtained smaller beads when comparing to the ones produced in this study, which could be attributed to the 2-fold lower concentration of alginate (2%), but also to the influence of the different encapsulant (medicinal plant).The beads composed of binary delivery systems exhibited lower values for hardness, indicating softer texture of A-WPI and A-Z beads, where the latter were significantly different (p <0.05) in comparison to plain ones. Also, softer hydrogels were characterized by lower elasticity, thus the hardest plain alginate beads (0.18 N) were the most elastic (0.31 mm). The water content in evaluated alginate-based beads was ranging from 92.12% (A-Z) to 94.26% (A-WPI) (Table 2). By employing an ionic gelation technique, with calcium ions as the cross-linking agents, large quantities of water are retained within the three-dimensional hydrogel network, justifying the approach of using liquid herbal extracts as the solvents for the preparation of encapsulant solutions. Such approach directly implies that a certain amount of bioactive compounds from the extract will remain entrapped in the hydrogel bead (Belščak-Cvitanović et al., 2016). The same authors also reported that up to 95% of water is retained in the alginate beads obtained by external/hydrophilic gelation, which is consistent with the results of this study. No significant impact on elasticity and water content of obtained beads was reported among the employed carriers.
| Delivery system | Particle size | Hardness | Elasticity | Water content |
| mm | N | mm | % | |
| A | 2.18±0.10 | 0.18±0.01a | 0.31±0.01 | 94.21±0.05 |
| A-WPI | 2.10±0.13 | 0.15±0.00 | 0.29±0.00 | 94.26±0.07 |
| A-Z | 2.76±0.09 | 0.12±0.00 a | 0.26±0.01 | 92.12±0.02 |
Values superscripted with the same letters are significantly (p<0.05) different. Delivery systems encapsulating Ganoderma lucidum polyphenols: plain alginate (A); alginate-whey protein isolates (A-WPI); alginate-zein (A-Z).
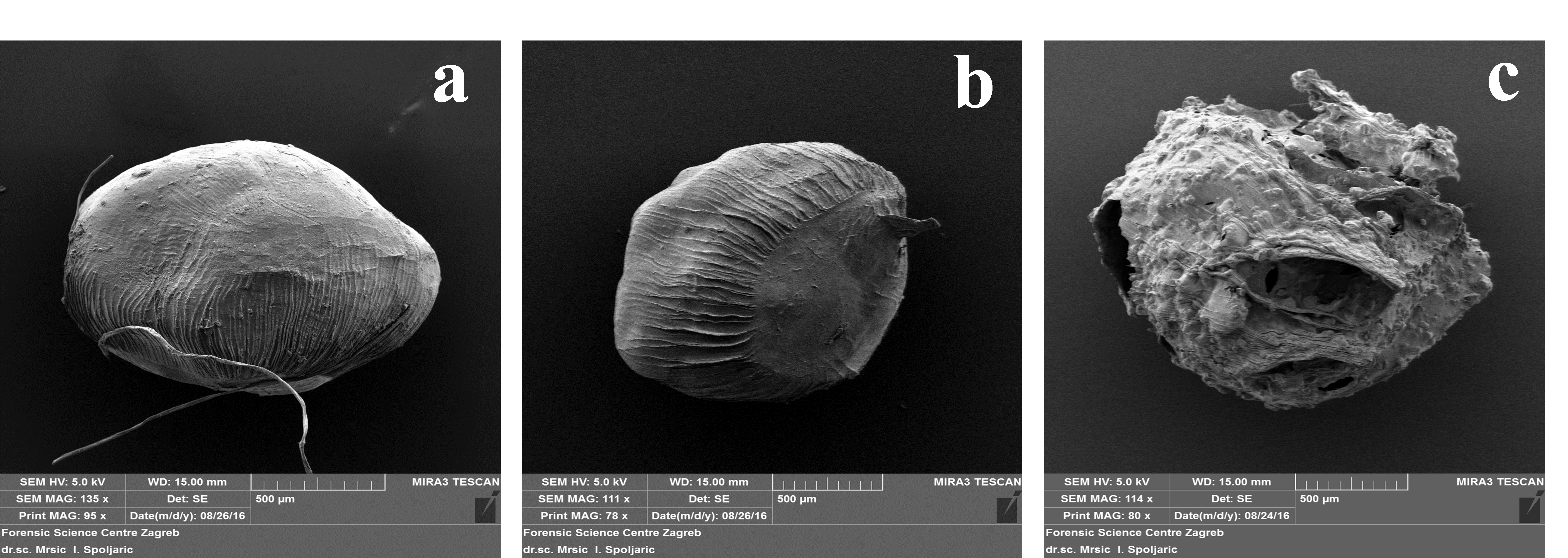
SEM micrographs of freeze-dried beads, describing their shape and surface morphology, are displayed onFig. 1. In comparison to A-Z beads, plain alginate and A-WPI beads exhibited more morphologically acceptable shape and surface, which remained mostly spherical or slightly oval and unchanged after freeze-drying (FD). On the other hand, heterogeneous surface, pronounced collapse of the walls of pores, shrinking, and structure cracking was observed in A-Z beads after FD. Artefacts are formed upon FD of porous Ca-cross linked alginate beads due to water leakage from the particles, which resulted in the weakening of the gel structure of the matrix in the end (López Córdoba et al., 2013), and such changes were also previously reported in many studies. Also, it seems that the particle size of beads influenced the changes in morphological properties of beads after the drying step. A-Z beads which were characterized by the larger particle size were more prone to shape and structure collapse, while smaller beads (A and A-WPI) preserved their spherical shape and regular surface appearance after FD. Longitudinal indentations on the surface of the beads were also observed, which could be ascribed to the usage of the narrow diameter needle used for the preparation of beads. Therefore, in terms of the preservation of the morphological characteristics, obtained results indicated WPI as better filler for an alginate matrix than zein.
Encapsulation efficiency of polyphenols and retained antioxidant capacity
The formulated alginate-based delivery vehicles proved to be very efficient for entrapping polyphenolic compounds from Ganoderma, since all evaluated carriers exhibited polyphenol loading capacity higher than 76% (Fig. 2). The highest encapsulation efficiency of TP was achieved in zein-reinforced alginate beads (85.42%). However, even in a high proportion, the lowest and significantly different (p<0.05) polyphenols entrapment, in comparison to other alginate beads, was observed in A-WPI beads (76.91%). Such results indicate a proportional relationship between particle size and encapsulation efficiency of TP, since the largest beads exhibited the highest encapsulation efficiency. Moreover, proteins are known to interact strongly with polyphenols through hydrogen bonding and hydrophobic interactions (Li et al., 2009). Due to the good binding affinity of polyphenols to proteins and interactions that could occur between proteins and polysaccharides, potential formation of protein-polyphenol and protein-polysaccharide complexes is possible, which could result in the successful chemical entrapment of polyphenols inside such a delivery system. Therefore, the introduction of zein and whey protein isolates as additional fillers justified their implementation to the alginate gel network, since both enabled successful retention of Ganoderma polyphenolics in the alginate-protein matrix. When zein was used as additional coating to chitosan, α-tocopherol was also successfully encapsulated into the zein/CS complex in a range of 76.6-87.7%, depending on fabrication parameters (Luo et al., 2011). Also, microencapsulates containing Ganoderma aqueous extract retained a high proportion of TP (up to 71%) when using different natural biopolymers, like alginate, pectin, carrageenan, and chitosan reinforcement (Belščak-Cvitanović et al., 2016a), which is consistent with the results obtained in this study. Furthermore, the retention of antioxidant capacity (AC) in alginate hydrogels determined by ABTS and DPPH assays was more effective when both proteins were employed to the alginate matrix. In this regard, the highest retention of AC was observed in A-WPI (67.85% - ABTS) and A-Z (58.20% - DPPH) hydrogel beads, while plain alginate beads showed the lowest retention of AC evaluated by both radical scavenging assays (Fig. 2.). The ABTS assay showed better affinity for the retention of AC , which is not surprising considering that the ABTS radical reacts with both hydrophilic and lipophilic antioxidants, while the DPPH radical reacts only with lipophilic ones (Prior et al., 2005).
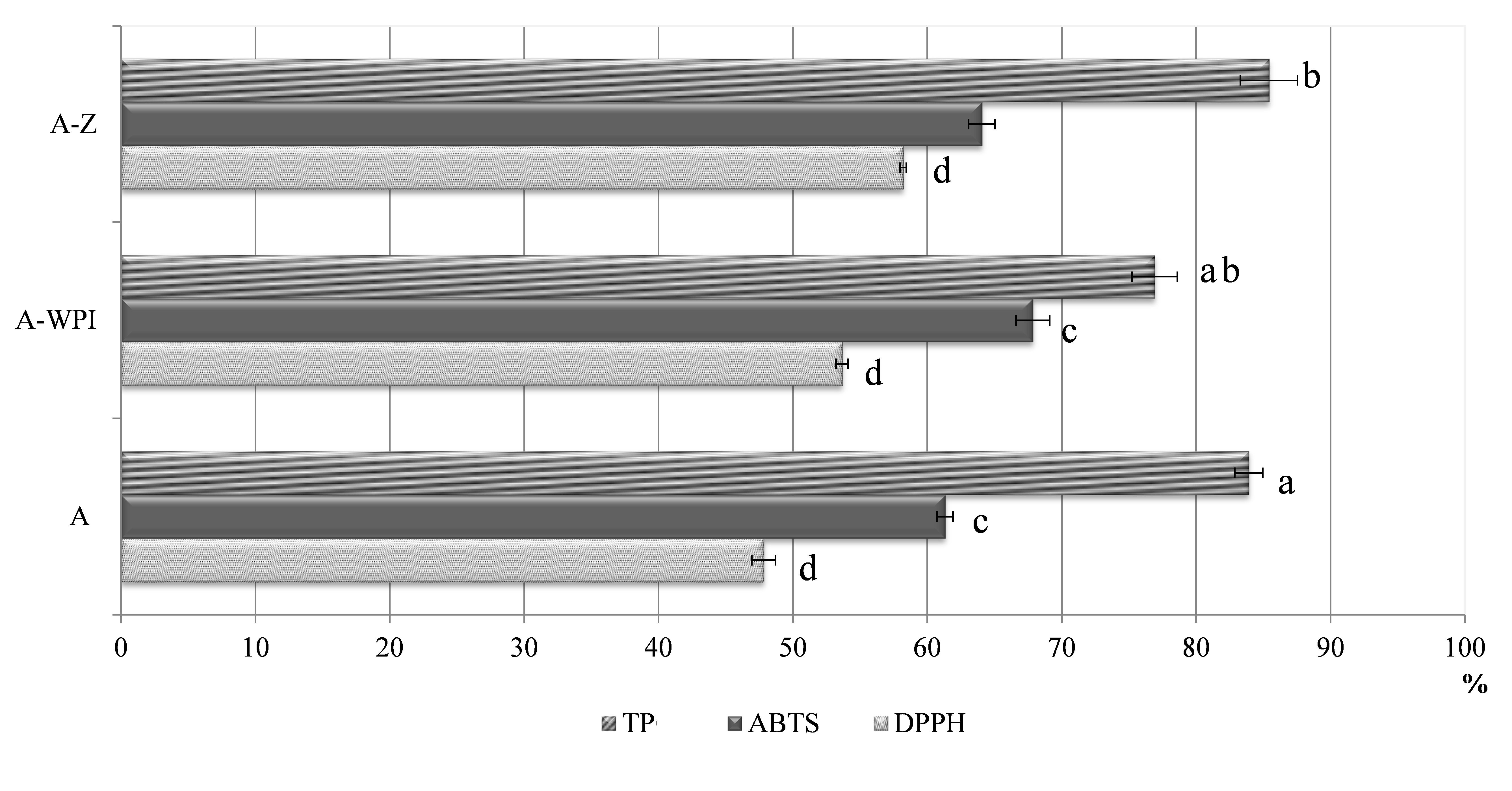
Values superscripted with the same letters are significantly (p<0.05) different. Delivery systems encapsulating Ganoderma lucidum polyphenols: plain alginate (A); alginate-whey protein isolates (A-WPI); alginate-zein (A-Z).
Release profiles of polyphenols and antioxidant capacity
According toFig. 3, the best release profiles of TP and AC, determined by both radical scavenging assays, was observed in the delivery system A-WPI, since the application of this carrier enabled the most prolonged release profile of the evaluated attributes. When observing the release profile of TP, the fastest release was observed in A-Z hydrogels (in the first 10 minutes in SGF, followed by negligible fluctuations), however, the release of TP continued again when hydrogels were transferred to SIF. TP from plain alginate hydrogels were completely released in the first 20 min in SGF, with no additional release in SIF. On the other hand, the release of TP from the A-WPI delivery system was markedly extended in both SGF and SIF. As shown onFig. 3a, release of TP from A-WPI was gradually increasing in SGF and continuing in SIF, even up to 130 min, with 0.67 mg GAE/g of beads. In the case of A-Z, TP were completely released in 122 min (0.48 mg GAE/g beads). The prolonged release of polyphenols in the delivery systems reinforced with proteins indicates the potential ability of proteins to reduce the porosity of the alginate gel network. That way the potential protein-polysaccharide interactions could enable a firmer protein-alginate matrix, aggravating and retarding the liberation of polyphenols to the surrounding media. Such results were also reported in a paper byBelščak-Cvitanović et al. (2016). Also, potential protein-polyphenol chemical interactions could slow down TP release from hydrogel beads. Furthermore, the release profiles of antioxidant capacity for both assays showed much faster release in SGF, with no additional release in SIF. WPI-reinforced beads again showed the slowest release of AC determined by ABTS and DPPH radical assays (up to 30 min in SGF), while the majority of AC from plain alginate and A-Z carriers were rapidly released in the first 5 and 10 minutes (Fig. 3b, 3c).

Delivery systems encapsulating Ganoderma lucidum polyphenols: plain alginate (A); alginate-whey protein isolates (A-WPI); alginate-zein (A-Z).
Conclusions
Selected materials - whey protein isolates and zein, used as fillers in an alginate matrix network, proved to be very successful delivery materials for enhancing the encapsulation efficiency of polyphenols extracted from Ganoderma lucidum, and allowing the extended release profile of polyphenols and antioxidant capacity from formulated alginate-based hydrogels.
