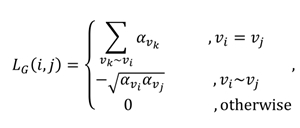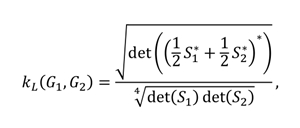Introduction
Thymus cilicicus Boiss. et Balansa, a member of the Lamiaceae family, is an endemic and Eastern Mediterranean element and its natural population spreads on the gravelly ground and open rocky areas of South and Southwest Anatolia (Davis 1982).
Rare and endemic plant species are generally endangered due to different environmental factors such as pollution, climate change, urbanization, excessive collecting, destruction of natural habitats, and invasive species (Pitman and Jorgensen 2002). Many in situ and ex situ conservation strategies were developed within the past decade to maintain and preserve plant genetic resources. These developments on conservation strategies have been stimulated by universal concern regarding the reduction of valuable plant genetic resources (Paunescu 2009). Conservation strategies are largely based on the natural population management of valuable plant species. While ex situ conservation procedures are complementary in the preservation of plant species, in vitro procedures play a more important role than classical conservation procedures (Sarasan et al. 2006). In vitro clonal propagation and preservation strategies can distinctly contribute to maintaining natural populations via retransferring conserved plant species to the natural habitat (Holobiuc et al. 2009). In particuilar, efficient propagation and large-scale multiplication of wild plant species, which are difficult to propagate via traditional procedures, are provided by tissue culture methods. (Malda et al. 1999).
The medium composition plays an important role in the viability, development, regeneration and growth of plant cells, tissues and organs in in vitro cultures, and successful micropropagation of plant species depends on the optimization of culture medium (Gamborg et al. 1976). Many studies on tissue culture have been established to select the optimum medium composition among those currently in common use. The studies distinctly indicated that ionic compositons of culture media and the ratio of major ionic elements (among cations and anions, the balance of NH4+ and NO3−, usage of charcoal, different salts such as AgNO3, Fe-EDDHA, H3BO3) are important for in vitro germination, regeneration, growth and multiplication of plant species (Ozudogru et al. 2011,Yamamoto et al. 2012). However, it is difficult to determine the optimum nutrient composition for each species.
Graphs defined by vertices representing objects and edges representing relationships between objects are natural mathematical data structures for modeling structured objects. In this context, the most common questions are "How similar are the two vertices in a particular graph?" and "How similar are the two graphs to each other?". For example, in estimating protein function, it may be desirable to estimate whether a given protein is an enzyme. Computational approaches reveal protein function by finding proteins with a similar sequence, structure, or chemical properties. Modelling proteins with graphs and assigning similar functions to similar graphs is a highly effective method (Alvarez and Yan 2012). Graph kernel functions are formed to measure the similarity between the proteins and enzymes represented in this way. Roughly speaking, a kernel is a measure of the similarity between the structured objects and . In order to define kernel, the must be symmetrical and positive semi-defined mathematically. The kernel function should be defined between vertices for similarity measurements of vertices in a graph and should be defined between graphs for similarity measurements between graphs. In both cases, identifying a kernel that captures intrinsic semantics in the graph structure and that is highly efficient for evaluation purposes, is a challenge for this type of method. In this study, we introduce a new kernel function that measures similarity between vertex-weighted graphs. From a mathematical point of view, we define a symmetrical and positive semi-defined function , where and are vertex-weighted graphs representing experiments that are made using different active substances. Furthermore, we analyze the similarity of each graph representing experiments with a complete graph formed by the possible best scores among the performed experiments.
Variations at the biochemical, cytological, morphological, and molecular levels can be induced in in vitro cultures. Molecular marker systems are effective tools to determine and verify the genetic stability of in vitro propagated plants. Different polymerase chain reaction (PCR)-based marker systems have been widely used for genetic stability determination of in vitro cultures such as random amplified polymorphic DNA (RAPD;Ozudogru et al. 2011), amplified fragment length polymorphisms (AFLP;Gagliardi et al, 2007), simple sequence repeats (SSR;Bradaï et al. 2019) and inter simple sequence repeat (ISSR;Kaya and Souza 2017). We choose the marker techniques because of their reproducibility and simplicity. Furthermore, the ISSR marker system provides a useful, sensitive, specific and reproducible tool for the determination and validation of genetic stability among in vitro culture systems (Joshi and Dhawan 2007).
The main aims of the present study were to determine and optimize culture media using the graph kernel statistical analysis method for the genetically stable in vitro propagation of T. cilicicus, an endemic of Turkey. For this purpose, two different media (MS = Murashige and Skoog medium,Murashige and Skoog 1962, OM = Olive medium,Rugini 1993) were compared to investigate NH4+ and NO3− balance, charcoal, AgNO3, Fe-EDDHA, H3BO3 effects on clonal multiplication of T. cilicicus through shoot meristem tip culture. ISSR markers were used to determine the genetic stability of in vitro multiplicated T. cilicicus.
Materials and methods
Plant material and in vitro culture establishment
Plant materials belonging to the natural populations of T. cilicius were collected from Sandras Mountain (Muğla, Turkey,Fig. 1A). The legal authorization letter for sample collection was obtained from Mugla Metropolitan Municipality, Department of Agricultural Services (Doc. number: 10452259-622.03-E.930/15708). Surface sterilization of T. cilicicus shoot tips was performed according to surface sterilization protocol for Thymus spp. developed byOzudogru et al. (2011). The shoots (~1 cm) were washed under tap water for half an hour, and subsequently, they were treated with 70% ethanol for 5 min, 3.5% commercial bleach (Domestos®) for 15 min, then they were rinsed in distilled water at least three times. After surface sterilization, the shoot tips (~0.1 cm) were excised and transferred to semi-solid MS medium supplemented with 1 mg L-1 6-benzylaminopurine (BA), 20 g L-1 sucrose and 3 g L-1 phytagel.
Investigation of different medium compositions for regeneration and multiplication of shoot meristem
The shoot apical meristems (~0,5 - 2 mm in size) were isolated from in vitro grown T. cilicicus shoots (culture stating material,Fig. 1B-D) on semi-solid MS medium described above and they were transferred to MS or OM media (Rugini 1993) supplemented with different combinations of salt and/or plant growth regulators contents (Tab. 1). All cultures were incubated in a growth room under the standard culture conditions [25 ± 2 °C, 16-h photoperiod with cool daylight fluorescent lamps (50 μmol m−2 s−1)]. These apical meristems were isolated by eight different persons to decrease standard errors of manual ability. Ten apical meristems were used for each medium combination and each treatment was repeated at least three times.
Data analysis via graph kernel statistical method
The data of the in vitro meristem regenerations on different media previously described before were collected after four weeks. The averages of regeneration percentages, shoot numbers and shoot lengths were calculated with standard errors via IBM SPSS (V22.0) statistical program. The significant treatment differences were selected by a non-parametric statistical test, and the post hoc multiple comparisons test (Marascuilo and McSweeney 1977). Discrete data were subjected to ANOVA, followed by the least significant difference test at P≤0.05 to compare means (homology between values of regeneration percentages, shoot number and shoot length avarages were evaluated seperately and indicated different letters.
The graph kernel statistical index formula was created to determine the optimum medium composition taking into account all parameters for the best in vitro propagation of T. cilicicus (This method was used for the first time for in vitro propagation data analysis).
Graphs are natural mathematical concepts used in order to express structured data sets. In mathematics, a graph can be expressed via a tuple where is the set of vertices (or nodes) and is a set of edges (or links) which is the subset of . In a graph structure, the information of the components of a combinatoric system is encoded to vertices and the information of the relations between vertices is encoded to edges. If the edges are symmetrical, that is, if implies , then is called directed, otherwise undirected. Moreover, if , then is called a simple graph. Throughout this study, we will assume that a graph is simple and undirected. The vertices connected via an edge in are called adjacent.
In many daily life applications, a non-negative value called weight is assigned to each edge of . Edge weights can be determined based on metric distances, costs, or similarities. Networks modeled with edge-weighted graphs are widely used in genomics and system biology. There are only a few studies on vertex-weighted graphs as compared to edge-weighted network analysis. Like edge-weighted graphs, a vertex-weighted graph can be defined by assigning a non-negative value to each vertex of the graph (Knisley and Knisley, 2014).
Let be a vertex-weighted graph with the weight function and . The Laplacian matrix of is defined by the entities
where . Similar to the Laplacian operator defined in continuous spaces, the Laplacian operator defined on measures how a function's value in a vertex differs from vertices adjacent to .
In this study, for the comparison of vertex-weighted graphs, a graph kernel based on the Laplacian matrix described above is defined for the first time. The Laplacian graph kernel we defined created a hierarchy of nested subgraphs, enabling comparison of the graphs. Let and be two vertex-weighted graphs and their Laplacian matrices are and , respectively. Then the kernel function we use is
where and for the identity matrix , a parameter and is Moore-Penrose inverse of a matrix. The kernel function we defined is symmetrical and positive semi-definite. The Laplacian graph kernel we present captures the similarity between the general structures of the two graphs. However, this assumes that both graphs have the same size and are invariant in the permutations of the vertices.
While representing each experimental group with graphs, graphs with 30 vertices were used because there were 10 meristems in each petri and 3 different petri dishes. The vertices symbolizing the meristems from which shoots developed are related with each other with an edge and vertex weighted graphs are obtained. Vertex weighting function is defined as
where y is the number of dead shoots. Each case of the experiments conducted in this study is shown with vertex-weighted simple graphs. Graphs are effective mathematical structures for the analysis of data structures with many heterogeneity levels. For this reason, they encode a variety of relationships that classical statistical methods cannot capture. In the graph comparison apporach, each graph obtained in this study is compared using the graph kernel function, which models the best case. The graph that models the best case is a complete graph, that is, there is a link between each node. Also, since a weight is determined at the vertices of the graph, this complete graph model takes the maximum value within the weights. The complete graph model, with each vertex weight being the maximum of all cases, represents the best case, and the graphs obtained empirically are compared with the best case with the aid of the kernel function.
Determination of genetic stability using ISSR primers
The DNA samples isolated from the mother plant leaves (culture-starting material) and the shoots grown on the eight optimal media (according to graph kernel multiple propagation index, each regenerated shoot on each medium was analysed as individually with 24 primers) were compared to determine genetic stability using 24 ISSR primers (Kaya 2015). The DNAs were isolated by usingDoyle and Doyle’s (1987) CTAB protocol. Polymerase chain reactions were performed in a 25 μL reaction mixture containing 40 ng DNA, 0.4 mM primer, 0.4 mM of each dNTP, 2.5 mM MgCl2 and 1 unit Taq DNA polymerase. PCR conditions were as follows: after 3 min denaturation at 95 °C, the reactions were continued for 35 cycles of 15 sec at 95 °C; 30 sec at 54 °C; 3 min at 68 °C followed by a 10 min lag at 72 °C. PCR products were separated on 1.5% agarose gel at 80 V and were visualized under UV light via staining with 0.5 μg ml-1 ethidium bromide solution. The band profiles were scored as 1 (present) or 0 (absent) and all data were analyzed for the determination of genetic stability (Kaya and Souza 2017).
Results
The maximum multiple propagation index was obtained from meristems, which were regenerated on OM supplemented with 20 g L-1 sucrose, 1 mg L-1 kinetin (KIN) and 10 g L-1 charcoal (Tab. 1,Fig. 2A). The calculated averages of regeneration rate, shoot number, shoot length were 96.89%, 3 and 1.24 respectively on this medium combination. All seedlings derived from meristems that were grown on optimum medium combination determined via graph kernel multiple propagation index were easily rooted (Fig. 2B) and successfully acclimatized to greenhouse conditions (Fig. 2C).
The best result of vertex involved OM supplemented with 1 mg L-1 KIN and 10 g L-1 charcoal experiment weighted graph with 30 vertices and 406 edges. The mean of the vertex weights is 0.8422. The graph has one isolated vertex while those remaining are densely connected. However densely connected, the component is not a complete subgraph. The resulting vertex is weighted graph Laplacian matrix. This matrix denotes strong clustering characteristics on the graph. The maximum value in the matrix is 25 and minimum value is -1.7876 (Fig. 3A). The other graph theoretical vertex result of the experiment involved OM supplemented with 10 mg L-1, Fe-EDDHA and 10 g L-1 charcoal weighted graph with 30 vertices and 351 edges. The mean of the vertex weights is 0.8662. The graph has three isolated vertices and the remaining ones are densely connected. However densely connected, the component is not a complete subgraph. The result of vertex was weighted graph Laplacian matrix.. This matrix denotes strong clustering characteristics on graph. The maximum value in the matrix is 25 and minimum value is -2.333 (Fig. 3B). Graph theoretical vertex results of the experiment involved OM supplemented with 1 mg L-1 KIN and 1 mg L-1 AgNO3 weighted graph with 30 vertices and 300 edges. The mean of the vertex weights is 1.7777. The graph has five isolated vertices and the remaining ones are densely connected. However densely connected, the component is not a complete subgraph. The resulting vertex weighted graph Laplacian matrix. This matrix denotes weak clustering characteristics on graph. The maximum value in the matrix is 5.2 and minimum value is -2.333 (Fig. 3C).
While graph kernel multiple propagation indexes of all MS and OM media combinations had variable values from each other (Fig. 4A), optimum regeneration and shoot multiplication of T. cilicicus meristems were observed on OM, rather than MS medium, and the optimum medium composition for T. cilicicus meristems which was determined by multiscale graph kernel analysis, also contained kinetin and this allowed a 96.89% regeneration percentage and produced an average of 3 well formed shoots more than 1 cm long per regenerating explant (Tab. 1).
BA or KIN were used as growth regulator and the results indicated that MS medium supplemented with 6-benzylaminopurine was more effective in clonal multiplication than MS medium supplemented with kinetin. However, meristem regeneration, multiplication and development were more effective on OM supplemented with kinetin (Fig. 4B).
All medium combinations supplemented with charcoal had more positive effects on the growth and development of T. cilicicus meristem tissues than charcoal-free medium compositions (Fig. 4C). Moreover, the optimum medium composition determined via multiscale graph kernel analysis also contained charcoal (Tab. 1).
The Fe-EDDHA, AgNO3 or H3BO3 were also used for meristem regeneration of T. cilicicus, however, Fe-EDDHA had a more benefical effect on meristem regeneration than AgNO3 and H3BO3 (Fig. 4D).
Genetic stability of in vitro grown T. cilicicus meristems was determined by using 24 ISSR primers. The plants grown on the best eight proliferation media determined by the graph kernel multiple propagation index, were compared with the mother plant via ISSR primer. All assayed primers produced a total of 192 reproductive band profiles and none of these band profiles were polymorphic (Fig. 5).
Discussion
The methods that have emerged in the last decade in data mining have helped overcome the weaknesses of traditional statistical approaches. In the analysis of biological experiments in which many parameters are observed, the relationships between objects or individuals cannot be expressed by vectors. For this reason, mathematical structures called graphs stand out for the use of advanced statistical methods. Various algorithms need to be run in graph structures to obtain rich information from relationship data between objects or individuals. Several kernel function approaches are available in order to extract the information in the graphs in which the relations are encoded. The multiscale graph kernel function described in this study is defined for the first time in the literature and is used for the first time in the optimum proliferation medium determination process. Conventional graph kernel functions are defined by random walks, shortest paths, Fourier transforms, and take the global or local characteristics of graph data into account (Kriege et al. 2020). Besides, traditional statistical methods deal with the analysis of variances over certain parameters whereas the graph kernel multiple propagation index presented in this study is an indicator that examines both global and local features of graph data structures created by considering each of the parameters such as shooting length, shoot numbers, number of dead shoots and information about the relations among meristems having any shootings. ANOVA analyses related to the experimental results are also given in the study to demonstrate the effectiveness of this index.
Effect of different media on T. cilicicus meristem regeneration
The OM medium had a more positive effect on multiple propagation than MS medium and a reasonable explanation for this result is the reduced NO3- (NH4NO3, KNO3) salts as nitrogen sources in OM medium (Arab et al. 2014). At the same time, OM medium also contains a different nitrogen salt [Ca(NO3)2, 2.54 mM] as a nitrogen source. Many works to determine the potential utilities of different nitrogen sources such as NO3- and NH4+ have been undertaken and their results indicated that the different concentrations of different forms of nitrogen source in the nutrient media have produced very positive responses on somatic embryo development (Leljak-Levanić et al. 2004), plant recovery efficiency in ovule cultures (McCoy and Smith 1986) and shoot regeneration (Vinterhalter et al. 2007).
Effect of different cytokines on T. cilicicus meristem regeneration
Adenine type cytokinin plant growth regulators such as BA and KIN play different roles in many aspects of plant growth, development, anabolic and catabolic stimulation processes in plant cell metabolism (Quadri et al. 2012). Kinetin is beneficial for inducing cell development, proliferation and new shoot formation when supplemented to the culture medium (Castilho et al. 2019), and it has also been suggested for micropropagation of different Thymus species, for example, T. vulgaris, T. longicaulis (Ozudogru et al. 2011), T. cariensis (Ozudogru and Kaya 2012) and T. hyemalis (Nordine et al. 2013), T. persicus (Bakhtiar et al. 2016).
Effect of charcoal on T. cilicicus meristem regeneration
Charcoal effects on in vitro regeneration of plant tissues depend on the charcoal type, their activation type and also the plant species being cultured. In vitro culture medium supplemented with activated charcoal can have either an adverse or an advantageous effect on tissue growth and development and this depends on plant tissue, plant species, medium content and the aim of the study. The activated charcoal addition to in vitro culture media could have different effects in in vitro cultures, such as the provison of a degree of darkness, inhibition of undesirable substances, and plant growth regulator adsorption. A problem faced in tissue culture studies, especially with plant species containing phenolic compounds during their culture initial phase, is the browning of tissues and eventual death of the explants because of excessive polyphenol production caused by plant defense reactions. These phenolic compounds mostly have been known as being inhibitory or undesirable substances that should be eliminated from or avoided in in vitro culture conditions. Since activated charcoal adsorbs phenolic compounds, discoloration is prevented and polyphenol oxidase and peroxidase are renderedinactive. It also decreases browning of tissues and culture media, thus viability and regeneration of tissues increase (Pan and Staden 1998).
Effect of Fe-EDDHA, AgNO3 and H3BO3 on T. cilicicus meristem regeneration
The effects of Fe-EDDHA, AgNO3 and H3BO3 were tested for T. cilicicus meristem regeneration on MS and OM media containing BA or KIN (with or without charcoal) combinations (Tab. 1). For plant cells, one of the most essential microelements that carry out metabolic pathways such as chlorophyll biosynthesis is iron. Therefore, iron insufficiency restricts plant growth and development and also stimulates interveinal chlorosis (Guerinot 2001). Metabolic pathways such as photosynthesis, respiration, DNA, RNA and protein biosynthesis, detoxification and nitrogen fixation is usually based on iron redox enzymes including ferredoxin, cytochromes, ribonucleotide reductase, lipoxygenase, oxidases, catalases, superoxide dismutases, nitrite and nitrate reductases respectively (Curie et al. 2009).
Ethylene accumulation in in vitro culture vessels may cause growth inhibition, some abnormal plant forms, leaf senescence and leaf reduction in micropropagated plant cultures (Steinitz et al. 2010), but these effects generally depend on plant species, culture type, explant type and even vessel type (Jha et al. 2007). Since ethylene acts as an inhibitor for in vitro cultures, AgNO3 improves the growth and regeneration of different plant types when it is added to the culture medium (Sandra and Maira, 2013).
H3BO3 is generally used as a boron source for in vitro cultures and its defiency may cause different abnormalities such as the inhibition of metabolic activities and/or alteration of plant morphology. The possible function of Boron is the production of cell wall compounds such as uracil-diphosphate glucose, glucose-1phosphate and 6-phosphate gluconate (Matoh 1997).
In the current work, although the Fe-EDDHA had a more benefical effect on meristem regeneration than AgNO3 and H3BO3 (Fig. 4D), none of the medium combinations containing Fe-EDDHA, AgNO3 or H3BO3 was the optimum medium composition. These results almost resembled previous works on in vitro regeneration of Lagenaria siceraria (Saha et al. 2007), Cucurbita maxima (Lee et al. 2003), T. vulgaris (Ozudogru et al. 2011).
Genetic stability determination of in vitro grown T. cilicicus meristems
To determine the somaclonal variation, ISSR marker systems have been successfully used on some in vitro propagated plant species such as thyme (Ozudogru et al 2011), grapevine (Nookaraju and Agrawal 2012), sugarcane (Kaya and Souza, 2017). The previous reports support the current study that the plantlets derived from T. cilicicus organized meristems can be genetically stable after treatment under in vitro conditions. The ISSR analysis of in vitro grown T. cilicicus revealed no somaclonal variation among seedlings similar to in vitro propagated T. vulgaris and T. longicaulis (Ozudogru et al. 2011).
A micropropagation system that supports genetically stable clonal multiplication for T. cilicicus has been determined using the graph kernel statistical method. The results of the current study showed that the semi-solid MS medium supplemented with 20 g L-1 sucrose, 1 mg L-1 KIN and 10 g L-1 charcoal was the best for the proliferation of T. cilicicus meristems. Furthermore, the micropropagated plants were successfully rooted and acclimatized to greenhouse conditions. In this study, we present a graph kernel multiple propagation index that considers all possible parameters for determining an optimum proliferation medium. Such an index is used for the first time for the determination of the optimum proliferation medium. Moreover, such a kernel function can be applied in several subjects. This study of the micropropagation of T. cilicicus, an aromatic-medicinal plant species, indicates that in vitro propagation is feasible and practicable for rapid multiplication of economically important plant species, faster introduction of new cultivars with beneficial properties, and for rapid clonal propagation of healthy, bacterium-, fungus- and virus-free, healthy and genetic stable plant material.