Introduction
Consumers today are aware of their energy intake and its impact on health and are therefore concerned about the products they include into their diets. Besides, considering that the body sometimes produces excess oxidants that cause imbalance and oxidative damage to biomolecules and may eventually be responsible for the occurrence of several diseases, including cancer. Food products that provide psychological or physiological benefits in addition to the classical nutritional function are in demand. As a result, there is growing interest in foods containing functional ingredients and nutraceuticals that can benefit the body. In response to consumer demand, the food industry is attempting to fulfil these requirements by developing functional products with specific health benefits.
Yoghurt is one of the oldest fermented dairy products worldwide and is commonly produced from domesticated milk by lactic acid bacteria (especially Lactobacillus bulgaricus and/or acidophilus, as well as Streptococcus thermophilus) (Das et al., 2019; Ogunyemi et al., 2021). Furthermore, the active microorganisms in yoghurt, called probiotics, help to improve the balance between beneficial and undesirable bacteria in the intestinal tract. This stabilizes the gut microflora, lowers blood cholesterol, and improves immunomodulation, all of which are associated with a lower incidence of chronic diseases, such as gastrointestinal disorders and cancer (Ahmad et al., 2022). Yoghurt is considered a nutritious food that, in addition to its high digestibility and acceptability, improves lactose intolerance and continues to serve as an appropriate food vehicle for functional and nutritional ingredients for human wellness (Gahruie et al., 2015; da Silva et al., 2019). Fortifying food with natural additives is one of the best ways to boost the overall nutrient intake of food while minimizing adverse effects. The addition of additives, such as fruits, cereals, herbs, and plant extracts, to yoghurt can improve its rheological and antioxidant properties (Durmus et al., 2021; Wu et al., 2023; Ibhaze et al., 2022; Kiros et al., 2016; El-Sayed and Youssef, 2019).
Moringa oleifera Lam is one of the most cultivated cruciferous herbs in tropical and subtropical areas of Asia, Africa, and Central America. This crop is well-adapted to tough climatic conditions, allowing it to thrive across different geographical locations (Kou et al., 2018). Furthermore, the plant's vegetative parts have a long history of use as food, medicine, and water clarifier, making practically all plant parts versatile, particularly because of their high levels of dietary fiber, phenolic compounds, and macro- and micronutrients (Xu et al., 2019). M. oleifera extracts are used in traditional medicine in India, Malaysia, and Puerto Rico to treat anxiety, anaemia, diarrhoea, diabetes, and obesity (Ma et al., 2018; Luangpiom et al., 2013). Additionally, M. oleifera leaves are considered an excellent food source, rich in protein, vitamins, calcium, ascorbic acid, and antioxidant compounds such as flavonoids and phenols. Leaf extracts of M. oleifera have been shown to be useful in diabetes treatment, with the ability to lower blood sugar levels and improve antioxidant status (Olurishe et al., 2016; Pontual et al., 2012).
As a less expensive source of nutrients, M. oleifera can modulate the microbiota, just as prebiotics can improve cardiovascular health and enhance diets in developing nations (Fernandez and Marette, 2017). Therefore, M. oleifera may be more beneficial when paired with yoghurt to help people recover from both nutritional deficits and provide the body with the necessary nutrients (Ahmad et al., 2022). Taking all these factors into consideration, fortifying yoghurt with phenolic-rich additives, such as M. oleifera, seems to be an ideal way to maximize the benefits of consuming high phenolic compounds. Howbeit, yoghurt has unique properties that make it acceptable to consumers; therefore, it needs to be clarified whether the addition of M. oleifera will positively or negatively impact yoghurt. Given the dearth of understanding regarding the potential use of M. oleifera in yoghurt production, it is expected that specific aspects such as fermentation time, rheology, acidification, and physicochemical properties may well be altered (Dimitrellou et al., 2020). There is the need to learn more about the impact of M. oleifera in fortifying yoghurt quality towards improving human wellness. To supplement the existing information, this review compiles important information from relevant literature about M. oleifera fortifying yoghurt products, specifically the nutritional and production aspects. The therapeutic properties of the M. oleifera plant, yoghurt production, and its fortification (with M. oleifera powder and extracts) will be discussed, ending with limitations and directions for future studies.
Therapeutic effects of M. oleifera
M. oleifera possesses various phytochemicals, some with therapeutic properties, including antioxidant, antidiabetic, anti-inflammatory, and antitumor aspects. The aerial parts of M. oleifera plants and their bioactive components (Figure 1) reveal how physiological and environmental factors influence the existence of various compounds, including phenolic acids, flavonoids, tannins, glucosinolates, etc. Therapeutic effects of M. oleifera are presented in subsequent sub-sections, specifically antioxidant, antimicrobial, and anticancer aspects .
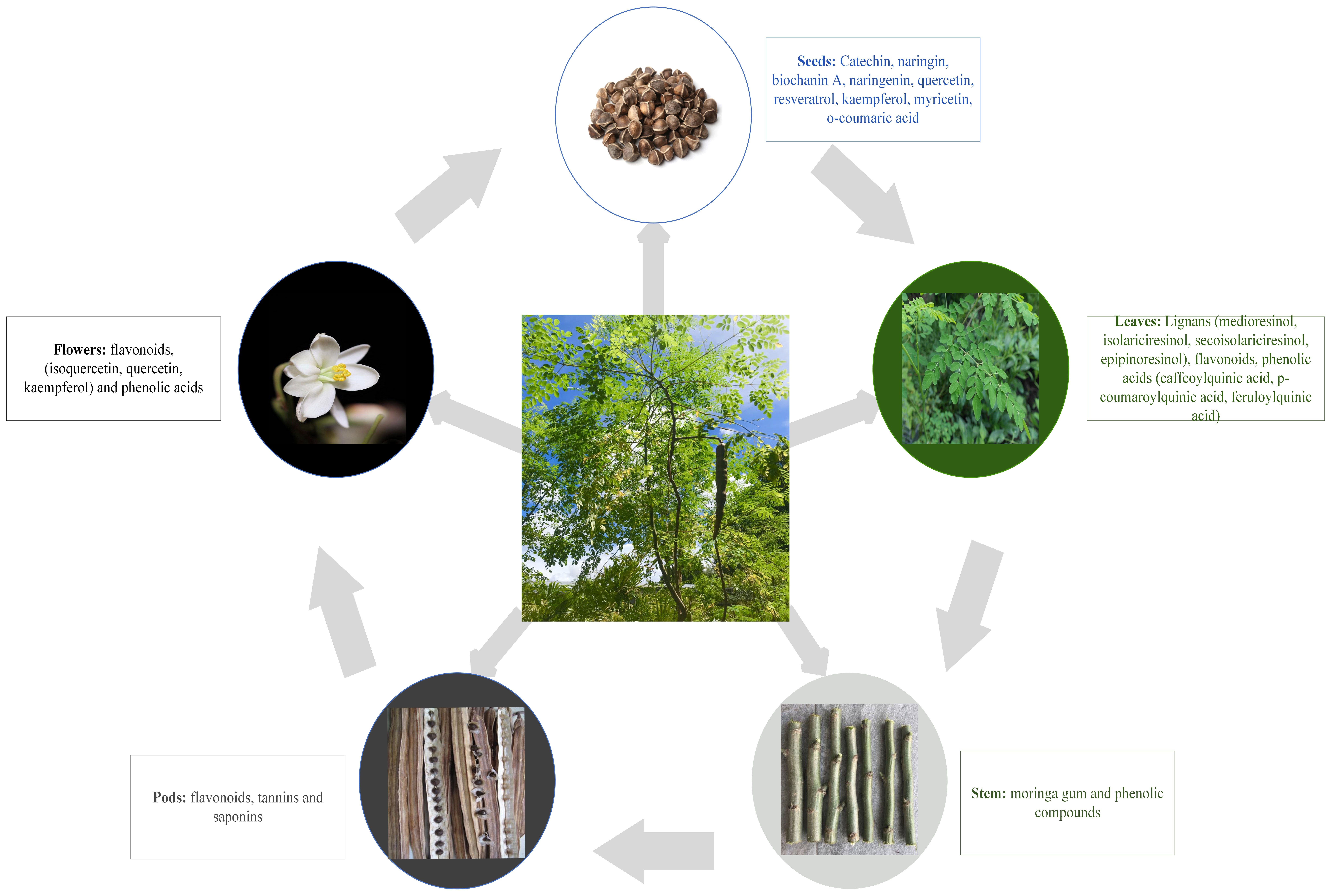
Figure 1. Aerial parts of M. oleifera plants and their bioactive components
Antioxidant activity
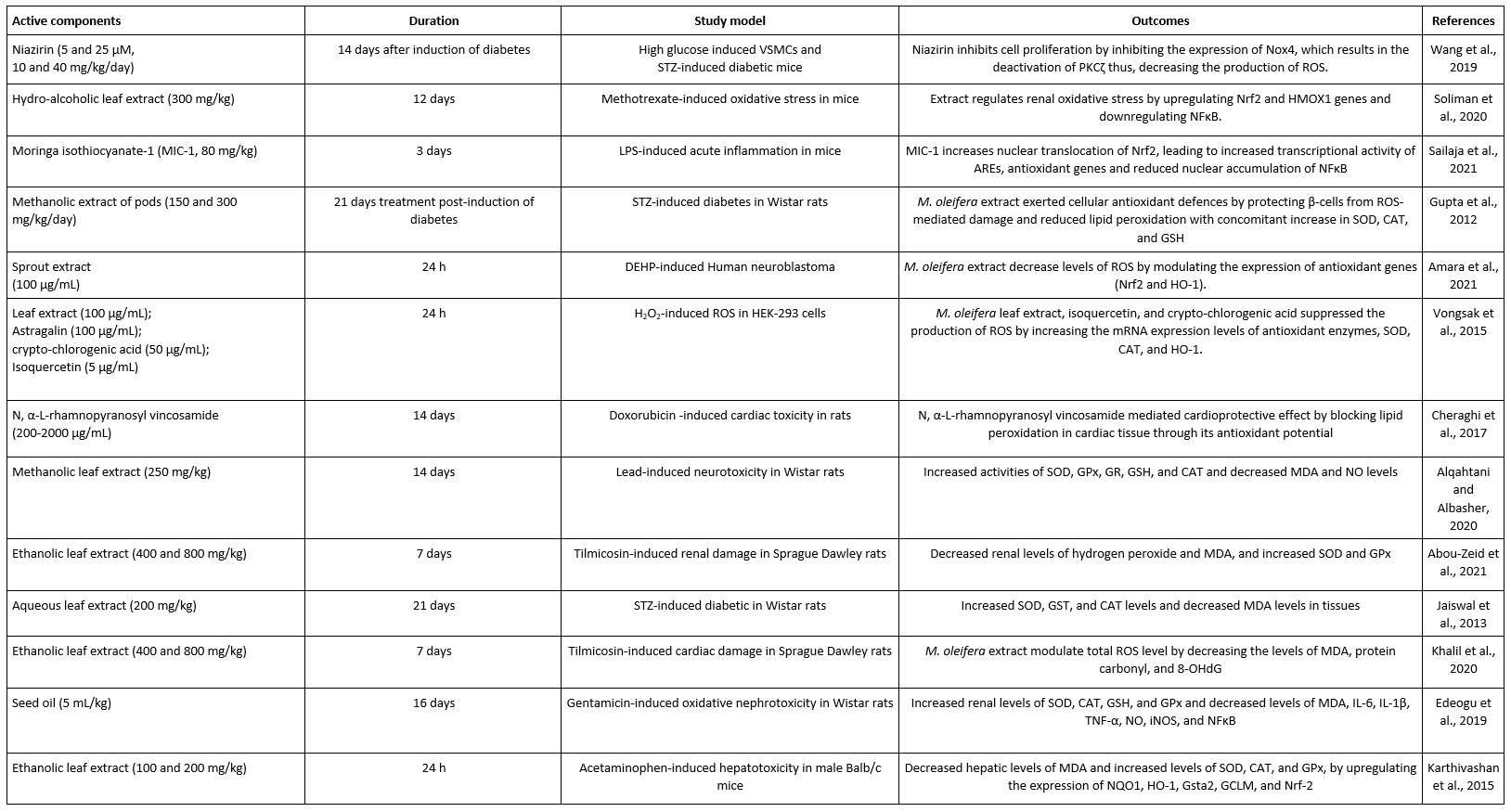
Biochemical reactions in the human body produce unstable free radicals like reactive oxygen species (ROS) that, in excess, can damage essential macromolecules such as proteins, lipids, carbohydrates, and nucleic acids. Thus, antioxidants would stabilize the free radicals and prevent such damage to macromolecules (Swati et al., 2018). M. oleifera contains antioxidant micronutrients such as zinc and selenium, vitamins A, C, and E, and other antioxidant pigments (α and β-carotene, xanthine, chlorophyll, lutein, and others) (Hodas et al., 2021). M. oleifera leaves, pods, and seeds contain such antioxidant compounds as rutin, quercetin, caffeoylquinic acid, and kaempferol. For example, M. oleifera leaves were reported to have 89.8 mg/100 g quercetin, 36.3 mg/100 g kaempferol, 2.9 mg/100 g isorhamnetin, and 129 mg/100 g total flavonoids, excluding apigenin and luteolin (Yang et al., 2008). Phenolic compounds could also be found in M. oleifera leaves, roots, flower, seed, and bark. Myricetin and quercetin (1530±10 µg/g, 985±4 µg/g) in the leaves, as well as gentisic acid and biochanin A (85±2 µg/g, 45±1 µg/g) in the roots, had significantly higher concentrations than others (Prabakaran et al., 2018). Elsewhere, the antioxidant capacity of methanolic extracts from the leaves, root, and stem bark of M. oleifera revealed IC50 values of 30, 16, and 38 μL, respectively. More so, in-vitro evaluation of the methanolic extracts of the leaves, root, and stem bark in the 2-deoxyguanosine assay model showed IC50 values of 40, 72, and 58 μL, respectively (Atawodi et al., 2010). To further contextualize this sub-section, Table 1 summarizes some recent reports of the antioxidant potential of M. oleifera (Wang et al., 2019; Soliman et al., 2020; Sailaja et al., 2021; Gupta et al., 2012; Amara et al., 2021; Vongsak et al., 2015; Cheraghi et al., 2017; Karthivashan et al., 2015; Edeogu et al., 2019; Khalil et al., 2020; Jaiswal et al., 2013; Abou-Zeid et al., 2021; Alqahtani and Albasher, 2020).
Table 1. Some reports about the antioxidant potential of M. oleifera
Antimicrobial activity
Practically all M. oleifera plant parts, from bark, roots, seeds, flowers, to leaves, would exhibit antimicrobial activities (Arora and Arora, 2021). Isolated from various parts of M. oleifera, both pterygospermin and isothiocyanates would exert both antibiotic and antifungal properties (Islam et al., 2021). Ethanolic extract of M. oleifera leaves/seeds would show antimycotic activities in vitro against Epidermophyton floccosum, Microsporum canis, Trichophyton rubrum, and Trichophyton mentagrophytes (Chuang et al., 2007). Antimicrobial activities of M. oleifera seed extracts were associative with the presence of moringin (4-(α-L-rhamnosyloxy) benzyl isothiocyanate) (Padla et al., 2012; Wen et al., 2022). Whilst crude chloroform extract of M. oleifera bark displayed promising antibacterial/antifungal activity (Nikkon et al., 2003), those of leaf (ethanol, methanol, and chloroform) would be anti-bactericidal, specifically against such gram-negatives as Pseudomonas aeruginosa and Shigella shinga, and gram-positives as Escherichia coli, Bacillus subtilis, Klebsiella aerogenes, Salmonella typhi, Staphylococcus aureus, and Streptococcus B haemolytica (Masurekar et al., 2015).
Anti-cancer activity
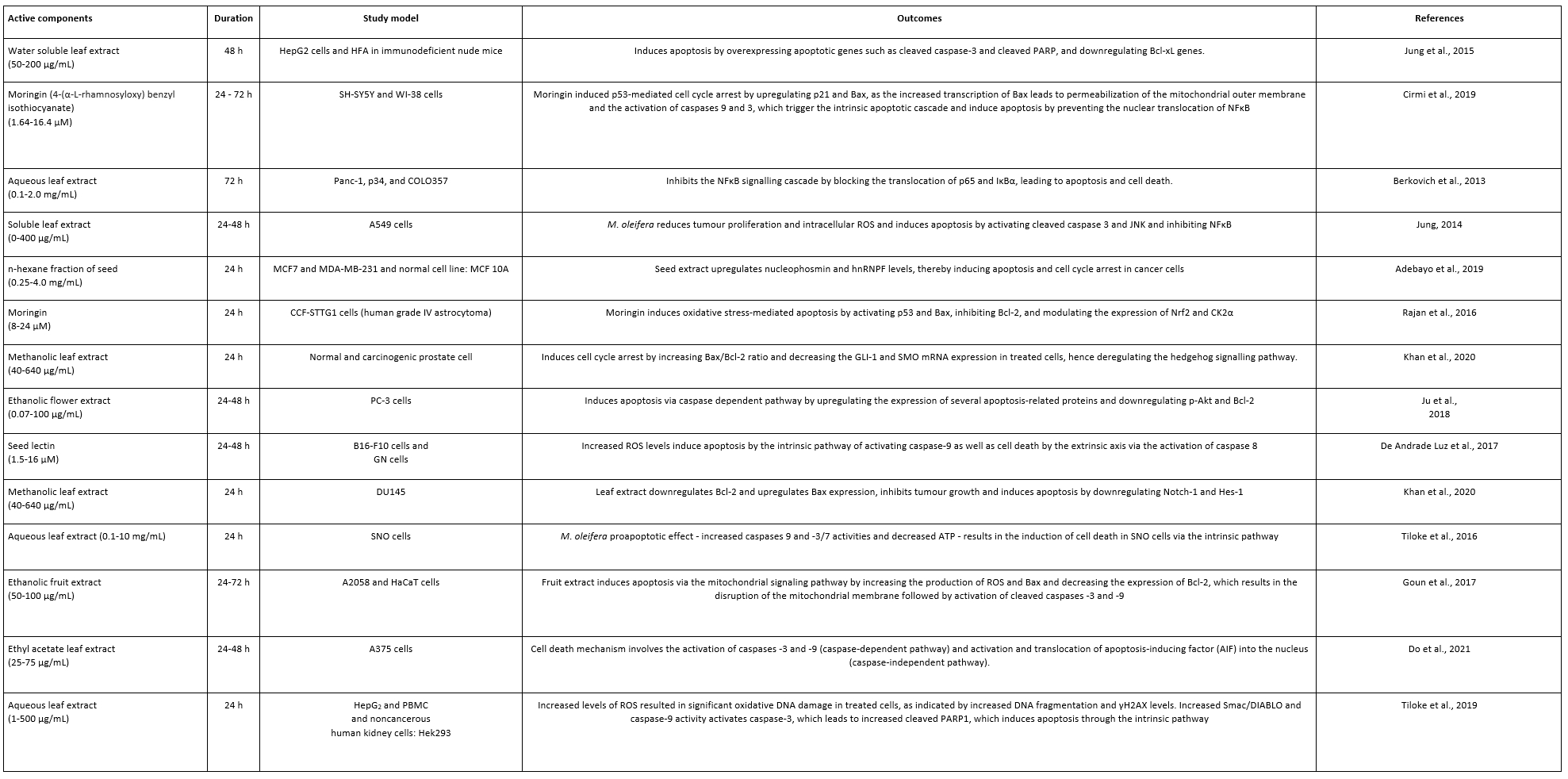
M. oleifera leaves/bark are considered as potential anti-cancer agents, as shown in Table 2. Extracts from M. oleifera leaf would inhibit the viability of hepatoma and acute lymphoblasts (Khalafalla et al., 2010). Such active ingredients as niazimicin, glucomoringin, glucosinolates, and β-sitosterol-3-O-β-D-glucopyranoside makes M. oleifera a promising anticancer candidate (Berkovich et al., 2013). Indeed, benzyl isothiocyanate promotes apoptosis by producing intracellular reactive oxygen species that lead to cancer cell death (Wu et al., 2021). Cold-water extract of M. oleifera leaf could be an anti-proliferative agent that inhibits cancer cell growth (Jung et al., 2015). Anti-proliferative effect may induce reactive oxygen species in cancer cells, which lead to apoptosis shown by upregulation of apoptotic pathway members, caspase 3 and caspase 9 (Hermawan et al., 2012). Elsewhere and through the activation of the intrinsic/extrinsic pathway, M. oleifera extracts would induce cell death in different tumour cells (Do et al., 2020; Madi et al., 2016; Akinlolu et al., 2021; Asaduzzaman et al., 2017; Das et al., 2021).
Table 2. Some reports about the anticancer potential of M. oleifera
Fortification of yoghurt with M. oleifera
Why is yoghurt fortification important?
Food fortification involves the addition of essential nutrients using staple foods as delivery vehicles to a target population especially where nutrients are minute or insufficient (Liyanage and Hettiarachchi, 2011). Notwithstanding the fortification purpose, both vehicle and fortifier must be compatible with the chemical/food matrix and avail nutritionally to achieve healthy food (Oyeyinka and Oyeyinka, 2018). Moreover, fortificants should not influence the taste, texture, colour, or flavour of the overall food product. Indeed, several factors can influence sensory, nutritional, and physicochemical properties of yoghurt, for example, the amount of fat in milk, the type of milk used (buffalo, goat, sheep, etc.), the production technique, additives and functional ingredients, and the starter culture used (Buttriss, 1997). Given the relatively short shelf life compared to other dairy products, such as cheese, the high water activity and rich nutritional composition make yoghurt vulnerable to spoilage microorganisms (Santos et al., 2018). Yoghurt contains free amino acids and bioactive peptides, which arise from the proteolytic activity of lactic acid bacteria during fermentation. Some lactobacilli can create bacteriocins and hydrogen peroxide, which is made proactive by certain bacteria cultures believed to synthesize and boost the presence of vitamin B in yoghurt (LeBlanc et al., 2015). Yoghurt stands out for its nutritional components, alongside prophylactics and therapeutic benefits, despite its slightly low pH that reduces pathogenic infection and gastric juice secretion (O’Connell and Fox, 2001). However, yoghurt remains a low source of phenolic compounds despite its therapeutic effects on casein, whey proteins, and traces of various antioxidant compounds (Niero et al., 2017; Dubrovskii et al., 2019).
Recently, there has emerged an increasing interest to develop unique-flavoured yoghurts, which might be seen as an ideal carrier of nutrients/functional ingredients in the human diet. Besides, the choice of adding different mineral salts (including organic and inorganic salts) to yoghurt, which adds to the complexities of food matrices, remains challenging. For instance, such salt addition tends to change yoghurt’s mouthfeel and pH. However, the popularity of functional yoghurts prepared with plant-derived components or additives such as fruits, vegetables, seeds, or even plant extract have risen (Roy et al., 2015; Alenisan et al., 2017; Hamed et al., 2020; Ahmad et al., 2022). Functional foods would possess various phytochemicals that facilitate their bioactivity, which helps to mitigate certain disease conditions/risks. Indeed, the addition of either fruits, herbs or plant extracts to yoghurt remains crucial, either as adaptogens (natural substances that increase the body’s resistance to stress) or as functional ingredients with additional functions other than supplying nutrients (Dubrovskii et al., 2019).
Functional impact of M. oleifera in yoghurt
M. oleifera contains several classes of phytochemicals besides minerals and carotenoids that can support the growth of lactic acid bacteria and exert antimicrobial and antioxidant scavenging activities. Antioxidants play an essential role in safeguarding against oxidative stress and maintaining a balance between the generation of active oxygen species and the quantity of endogenous antioxidants. Male albino rats exposed to lead acetate-induced oxidative stress received oral doses of green tea and M. oleifera yoghurt (88.2 mg/kg/day) for five weeks. The results showed a significant reduction in liver weight and levels of the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT), as well as a superior lowering effect on plasma total cholesterol, triglycerides, and low-density lipoprotein upon consumption of M. oleifera yoghurt (El-Ziney et al., 2017). Other groups of authors further elucidated that M. oleifera-fortified yoghurt had increased radical-scavenging activity of up to 40 % in a dose-dependent manner during three weeks of cold storage, as well as an increase in the expression of antioxidant proteins in human colon cells (Zhang et al., 2019). Such results might be attributed to the formation and degradation of mineral and phenolic compounds based on the interactions between lactic acid bacteria and compounds present in M. oleifera.
Furthermore, a study on the influence of probiotic yoghurt supplemented with M. oleifera leaf powder (4.3 g) as a source of micronutrients in the gut, oral, breast milk, and vaginal microbiotas of pregnant women (n = 56) in Tanzania revealed that M. oleifera-probiotic yoghurt provided a safe and inexpensive food for pregnant women without adversely changing the gut and oral microbiota, as well as improving the gut microbiota of new-borns (Bisanz et al., 2015). A previous study on the potential use of M. oleifera probiotic yoghurt for heavy metal exposure in vulnerable populations, including pregnant women (n = 60) and children (6–10, n = 44), found that probiotic yoghurt consumption had a protective effect against increases in mercury (3.2 nmol/litre; P = 0.035) and arsenic (2.3 nmol/litre; P = 0.01) levels in pregnant women’s blood and no significant changes in children’s blood (Bisanz et al., 2014).
Chemical composition of M. oleifera-fortified yoghurt versus non-fortified yoghurt at different concentrations or extracts is shown in Table 3. Hassan et al. (2016) claimed that after adding 0.5 %, 1 %, 1.5 %, and 2 % M. oleifera leaf powder to yoghurt made from buffalo milk, the optimal supplementation ratio was 0.5 %. Compared to plain yoghurt, the authors observed that adding M. oleifera to yoghurt increased total solids, fat, total protein, and amino acids, especially alanine, leucine, tyrosine, and glutamic acid. The increase in the amino acid content of fortified yoghurt may be related to the numerous nutrients in the M. oleifera plant, as well as the fact that during fermentation and storage, due to the action of lactobacilli, proteolysis increases, leading to the formation of bioactive peptides that enhance the activity of streptococci. On the contrary, Akajiaku et al. (2018) reported that adding different proportions of M. oleifera leaf powder to yoghurt had no significant changes on total solids and ash, while significantly increasing the percentage of total protein (Table 3).
Table 3. Chemical composition of M. oleifera-fortified yoghurt versus non-fortified yoghurt at different concentrations or extracts
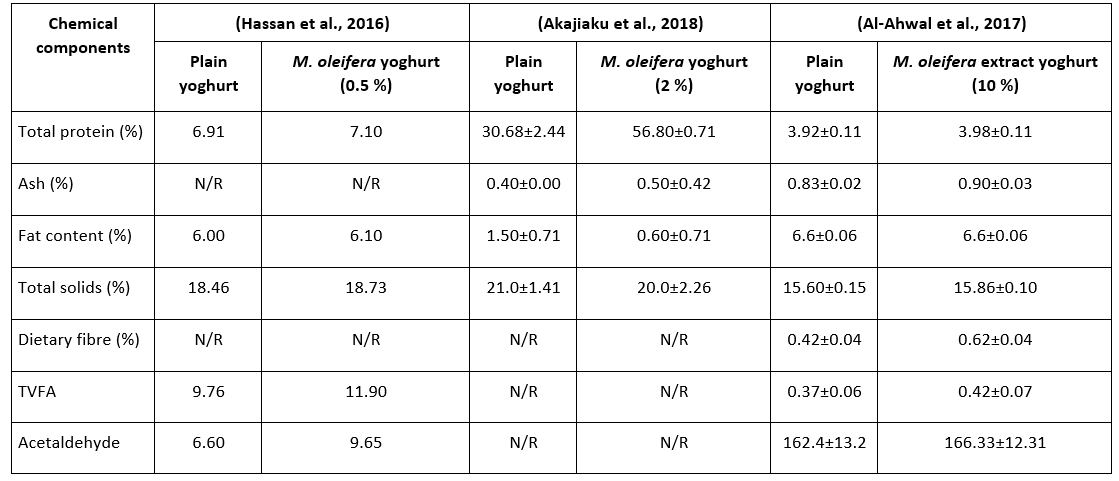
N/R means not reported; TVFA means total volatile fatty acids (0.1N NaOH 10 g-1 of Yoghurt); Acetaldehyde (mole 100 g-1 of yoghurt).
Bikheet and co-authors also observed that the addition of ethanol and water extracts of M. oleifera (1, 3, and 5 %) to yoghurt enhanced its vitamin C and mineral contents (Fe, Ca, K, and P), total solids, total flavonoids, total phenols, total proteins, and antioxidant capacity after production and during storage (Bikheet et al., 2021). This result is further corroborated by a different study that found that adding M. oleifera to yoghurt increased the antioxidant scavenging activity, total protein, dietary fibre, volatile fatty acids, and acetaldehyde composition of the fortified yoghurt either fresh or during storage (Al-Ahwal et al., 2017). In addition, yoghurt supplementation by M. oleifera has been reported to improve its textural and chemical properties (El-Gammal et al., 2017). Elsewhere, a study on low-fat yoghurt reported that the addition of M. oleifera powder resulted in an increase in the water holding capacity (WHC) with a significant decrease (p<0.05) in the syneresis when compared to the control sample during the first week of storage. The increase in water holding capacity was postulated to be due to the interaction between the particles of the M. oleifera powder and the casein matrix in the yoghurt. However, the study showed a significant increase (p<0.05) in syneresis during the second week of storage (Adepoju and Selezneva, 2020). Cardines et al. (2018) prepared yoghurt supplemented with different M. oleifera seed extracts at concentrations of 0.5 and 1.5 % (v/v). Seed extract enhanced the acidification of yoghurt and led to higher consistency indices than the control yoghurt. The fortified yoghurts were characterized by higher protein content and significantly lower syneresis, which depicted the compacted networks of the three-dimensional network of aggregated casein micelles (Figure 2). Elsewhere, the addition of permeated (5, 10, and 15 mL/L) and concentrated (1, 2, and 3 mL/L) seed extracts of M. oleifera to yoghurt revealed that concentrated additives presented viscoelastic behaviour, improved protein and fat compared to plain yoghurt, and significant pH decrease with storage. By adding 2 mL/L, the produced yoghurt had increased firmness, as confirmed by compact/homogenous microstructure and reduced susceptibility to syneresis (Quintanilha et al., 2021).
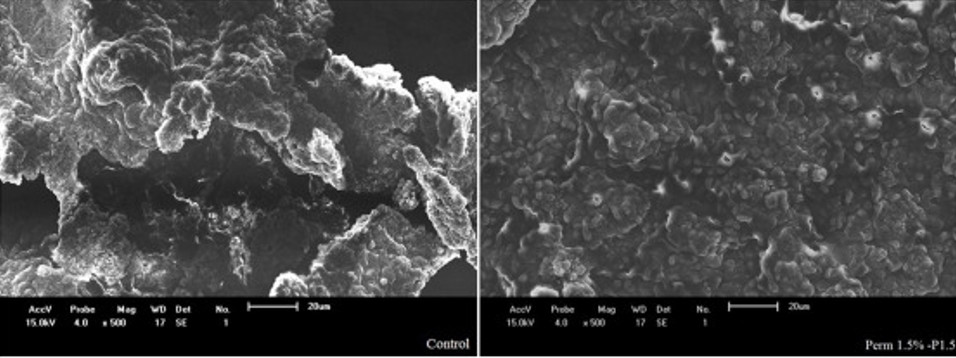
Figure 2. SEM of plain yoghurt (left) with permeated seed extract fortified yoghurt (right) (Source: Cardines et al., 2018)
The antimicrobial effects of 1 and 2 % ethanolic extracts of pomegranate peels and M. oleifera leaves on the viability of E. coli in yoghurt were investigated. It was found that 2 % ethanolic extracts of pomegranate peels and M. oleifera produced peak inhibition against E. coli during the storage period (AM et al., 2019). Fortification of yoghurt with 0.05, 1.0, and 2 % Moringa extract improved the growth of Streptococcus thermophilus, Bifidobacterium longum, and Lactobacillus acidophilus during fermentation, decreased syneresis by up to 21 %, increased WHC by 17 %, and increased viscosity (Zhang et al., 2019). Similarly, M. oleifera would support the growth of probiotic cultures of Lactobacillus rhamnosus GR-1 and Lactobacillus acidophilus (Hekmat et al., 2015). Besides, the polyphenols present in M. oleifera leaf prevented spoilage by decreasing the pH of yoghurt and inhibiting the microbial (fermentative) spoilage caused by yeasts and moulds (Georgakouli et al., 2016). To further support the above discourse, a summary of the biochemical and functional changes in yoghurt is presented in Table 4.
Table 4. Preparation of functional yoghurt with M. oleifera and its properties
Advantages of M. oleifera in comparison to other natural antioxidants
Considering the capacity of M. oleifera to fortify yoghurt, understanding the advantages associated with it is important. For example, a clinical study on malnourished individuals found that consuming 10 g of powdered M. oleifera leaves per day for six months resulted in a quicker recovery rate (36±16.54 days) and an increase in average weight gain of 8.9±4.3 gkg-1 per day with no significant increase (p˃0.05) in haemoglobin level. Thus, it is recommended as an effective alternative for addressing malnutrition in children (Zongo et al., 2013). Another study on the effect of using M. oleifera leaves powder (25 g/ day) to improve anaemia in children with iron deficiency anaemia aged 6-24 months for six months revealed a significant decrease in anaemia prevalence in the intervention group by 53.6 % and an increase in mean haemoglobin (10.9 g/dL) versus the control group (13.6 %, Hb 9.4 g/dL) (Shija et al., 2019)
Moreover, several authors have used M. oleifera as a fortifier in yoghurt production, with the primary purpose of improving the organoleptic, nutritional, and functional aspects. Most beneficial features of such fortification include increased antioxidant properties, amino acid content, increased textural qualities of the fortified yoghurt, increased total viability count of both lactic acid bacteria and probiotic strain Lactobacillus rhamnosus, and increased nutritional quality of the fortified yoghurt. Similar to M. oleifera, several fruit pulps, cereals, vegetables, and extracts have been introduced to yoghurt to improve its functional and nutritional qualities, serving as a gelling, water-binding, and thickening agent (Vénica et al., 2020; Nandakumar et al., 2021; Illupapalayam et al., 2014; Barakat and Hassan, 2017). A study on the sensory and chemical properties of yoghurt fortified with pumpkin fiber (0.5, 1.0, and 1.5 %) revealed increased viscosity and lowered syneresis, but protein decreased with an increase in fibre concentration (Bakirci et al., 2017). Another study on the fortification of yoghurt with fenugreek and M. oleifera seed flour (0.1 and 0.2 %, respectively) reported the latter with higher total phenolic content, antioxidant capacity, antibacterial activity, and mineral content (Ca, P, K, and Fe). In contrast, fenugreek seed flour yoghurt obtained significantly higher microbial viability count than plain yoghurt and M. oleifera yoghurt (Dhawi et al., 2020). Shokery et al. (2017) prepared yoghurt supplemented with green tea extract and M. oleifera leaves extract at 1 and 0.9 %. The workers found increased syneresis with M. oleifera, while green tea decreased it. However, both bio yoghurts were characterized by higher contents of phenolic acids and improved antioxidant activities than plain yoghurt, which may offer range of yoghurt products with additional health benefits to consumers. Thus, compared to other widely used plant antioxidants, M. oleifera can improve the properties of yoghurt and yield extra nutritious products.
Yoghurt production process using M. oleifera
A block diagram of yoghurt production with M. oleifera is shown in Figure 3. It can be argued that streptococci possess higher aerotolerance compared to lactobacilli, which, given the well-established growth ratio of 4:1, would suggest a recognized synergistic interaction, especially when fermentation kicks off. Further, Lactobacillus avails the needed peptides that enhance the growth of streptococci because of its greater and more significant proteolytic activity. More so, the initial fermentation taken over by the cocci is believed to depress the redox potential, producing formate (methanoate), CO2, and pyruvic acid as by-products. Methanoate would, therefore, encourage the growth of lactobacillus given by decreased oxygen, which in tandem accelerates the whole fermentation process. Post-fermentation, the yoghurt’s temperature is rapidly dropped to about 4-5 °C via chiller/heat exchanger to stop the cultures’ fermentative activity, inhibit enzyme activity, and initiate the cold gelatinization of the curd.
M. oleifera can also be added after fermentation, followed by cooling, before serving (Kuikman and O'Connor, 2015). When probiotic yoghurt was refrigerated at 4 °C, the addition of M. oleifera had growth-promoting effects on Lactobacillus rhamnosus GR-1, a probiotic bacterium (Van Tienen et al., 2011). Moreover, Lactobacillus rhamnosus GR-1’s growth may be boosted by M. oleifera, but potentially only at a greater concentration ( M. oleifera at 1 %) and when sugar is present (Hekmat et al., 2015). Compared to the control, the supplementation of yoghurt with M. oleifera extract would reduce the fermentation period in a dose-dependent manner (Zhang et al., 2019). Besides, increased fermentative activity of bacterial starter cultures may corroborate the flavonoid, phenolic, and organic acid contents of M. oleifera-enriched yoghurt (Rodríguez-Pérez et al., 2016). To achieve health benefits, bacterial colony formation must exceed 106 CFU mL-1, which makes the potential growth-promoting effects of M. oleifera crucial to extending the probiotic yoghurt’s shelf-life. Combined with other adjuncts, M. oleifera leaf powder would improve yoghurt’s nutritional/sensory properties (Kechagia et al., 2013). The effects of adding different adjuncts (banana, avocado, and sweet potatoes) showed improved taste and overall acceptability of M. oleifera-enriched yoghurt (Kuikman and O'Connor, 2015).
Despite specific standardized thresholds, such as milk fat content, the overall quality of yoghurt varies globally. Among the very common yoghurt categories, especially those based on fat level, are skimmed, low-fat, and high-fat yoghurt. Yoghurt styling and nutritional qualities are greatly influenced by additives such as sweetening and flavouring agents, preservatives, and functional ingredients. M. oleifera and its extracts have served as functional ingredients that enrich yoghurts (Bikheet et al., 2021) and have an added value during yoghurt production. Raw M. oleifera leaves can be washed, dried in an oven, and ground to make powder or extract. The extract can then be boiled or macerated in water. The milk base is heated in a homogenizer at 65–70°C and 15–20 MPa pressure to reduce the fat globule diameter and improve the mixing of the extract with milk casein (Adepoju and Selezneva, 2020). Moreover, the milk base is pasteurized to destroy pathogens, denature whey protein, inactivate milk enzymes, reduce redox potential, and eliminate inhibitory substances at 85 °C for 20-30 min or 90-95 °C for 5 min, chilled to 40-45 °C, which is ideal for lactic acid bacteria growth. 2-4 % of the bulk starter or the amount specified on the commercial starter culture for direct vat inoculation or direct vat set at 40-43°C are used to inoculate the milk base (Nagaoka, 2019). Yoghurt fermentation starts with the action of bacterial cultures, which could either be a mixed culture comprising of Streptococcus thermophilus (S. thermophilus) and Lactobacillus delbrueckii subsp. bulgaricus (L. bulgaricus) or other bacteria mixtures, on pasteurized milk. By acting on complex macromolecules in milk, such as proteins, fats, and carbohydrates, S. thermophilus and L. bulgaricus carry out three key metabolic processes, namely glycolysis, proteolysis, and lipolysis, converting them into simpler and readily absorbable nutrients (Buttriss, 1997). In addition, the fermentation process is carried out in an aseptic vat or containers at the optimum temperature for lactic acid bacteria (40-43 °C) until the pH drops to 4.5-4.7, followed by rapid cooling to stop any further decrease in pH.
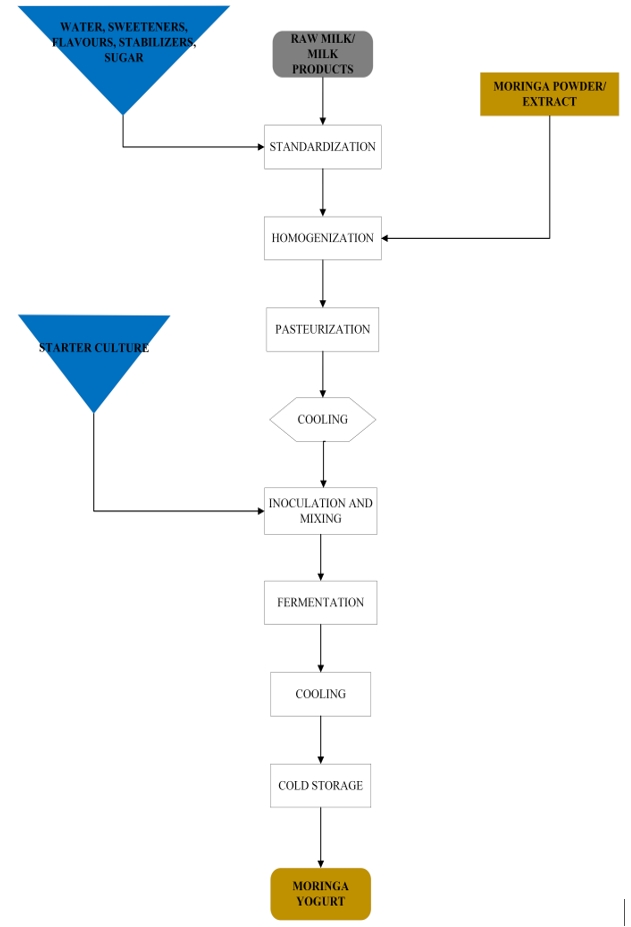
Figure 3. Block diagram of yoghurt production with M. oleifera
Toxicology and safety
There is a need for additional synthesis of relevant information on the safety and toxicity of M. oleifera in human studies, especially in relation to effective dose intake. Nevertheless, there are an eclectic number of studies on the therapeutic effects of M. oleifera in different preclinical models, and animal studies, when properly conducted, provide useful indicators of safety in humans. Oral administration of M. oleifera leaf extract at supra-supplementation levels of 3000 mg kg-1 to rats did not result in any hepatorenal toxicity or haematological alterations after acute exposure. However, the authors claimed that M. oleifera is genotoxic at a dose of 3000 mg kg-1 (Asare et al., 2012). Another study on the acute (5000 mg kg-1) and subacute toxicity (40-1000 mg kg-1) of M. oleifera extract in rats revealed an increase in liver enzymes ALT and ALP (p<0.001) with no adverse histopathological changes, but the consumption of the leaves should not exceed a maximum of 70 g per day to avoid accumulative toxicity of essential elements over extended periods (Asiedu-Gyekye et al., 2014).
A double-blinded, randomized, placebo-controlled trial evaluating the efficacy of feeding M. oleifera leaf capsules to early postpartum patients to increase breast milk volume revealed there was no significant difference in breast milk volume on the third day of postpartum between the intervention and the control group (73.5 vs. 50 mL, p = 0.19). However, the amount of breast milk in the intervention group was 47 % higher than that in the control group (Fungtammasan and Phupong, 2022). Another randomized crossover study examining the effects of M. oleifera leaf extract (500 mg dry extract) on plasma glucose concentrations and antioxidant status in healthy volunteers showed that M. oleifera significantly improved antioxidant capacity in humans, without causing hypoglycemia (Ngamukote et al., 2016). Studies on the potential toxicity of M. oleifera are currently lacking, and some information in the literature is debatable. Hence, it is necessary to conduct studies on the toxicokinetic, acute, sub-chronic, and chronic toxicity, as well as studies on the allergenicity, immunotoxicity, and neurotoxicity of M. oleifera.
Limitations of incorporating M. oleifera to yoghurt and future prospects
Although several studies have successfully created one form of M. oleifera-enriched yoghurt or another (refer to Table 4) , striking a balance on how the bioactive ingredients of M. oleifera would be incorporated per serving of yoghurt remains debatable. The main challenge is related to the observed sensory properties when M. oleifera extract/leaves are incorporated into yoghurt especially at high concentrations (Trigo et al., 2022). As achieving the recommended dietary allowance of this bioactive ingredient in functional product such as yoghurt without producing an off-taste, not only proves challenging, the rich polyphenolic content of M. oleifera may equally be associated with the astringent taste that occurs in milk products (O’Connell and Fox, 2001). Fortification of yoghurt with M. oleifera extract, even at a high concentration of 2.5 % w/v yoghurt, showed no adverse effect on lactic acid bacteria; however, the sensory properties of the final product were poorly accepted (Rupa and Vijay, 2022). Potential solutions might include the initial blanching of the leaves before drying, encapsulation of the extracts, as well as introduction of fruit flavours and fruits into yoghurt.
Higher concentrations of M. oleifera, which could increase syneresis and reduce firmness/viscosity of fortified yoghurt, could be a minor challenge during storage (Shokery et al., 2017). Achieving a balance between fortification levels using this bioactive ingredient is required, especially within the dietary recommended limits to ensure the sensory property of the final M. oleifera-fortified yoghurt becomes acceptable. Thus, the optimum fortification level of M. oleifera in yoghurt needs further investigation, with increased focus on sensory, physico-chemical, and antioxidant properties. Furthermore, more chronic toxicity studies using M. oleifera on hepatic, renal, hematopoietic, cardiac, and reproductive changes are warranted. This is because there are currently limited human studies on the toxicity levels of M. oleifera, especially considering that yoghurt can be appealing to different consumers of different age groups and for different benefits .
Importantly, the M. oleifera plant continues to be a rich source of micronutrients, macronutrients, and bioactive compounds that facilitates the growth of lactic acid bacteria with promising effects on antioxidant and rheological properties of yoghurt. Moreover, the low phenolic content of yoghurt not only makes it a potential food vehicle, but also, a target for food fortification with plant ingredients. Owing to its nutritional, antioxidant, and antimicrobial properties, M. oleifera can be positioned as a functional ingredient in dairy technology to help alleviate the situations of malnutrition in low-income countries. Technological challenges such as syneresis and sensory properties involving a M. oleifera fortified yoghurt, however, still needs to be addressed.
Acknowledgements
The research funding from the Ministry of Science and Higher Education, RF (Ural Federal University Program of Development within the Priority-2030 Program).
Obogaćivanje jogurta biljkom Moringa oleifera: prehrambeni i proizvodni aspekti
Sažetak
Jogurt je hranjivi mliječni prehrambeni proizvod proizveden pomoću bakterija mliječne kiseline koji pospješuje probavu i apsorpciju hranjivih tvari, te održava crijevnu floru. Međutim, riječ je o namirnici niskog sadržaja fenola. Budući da se jogurt smatra visokovrijednim prehrambenim proizvodom koji ljudski organizam učinkovito opskrbljuje hranjivim tvarima, razvoj jedinstvenih jogurta obogaćenih sastojcima biljnog podrijetla postaje sve popularniji. Moringa oleifera važna je fitoterapeutska biljka poznata po svojim antioksidativnim svojstvima, s obzirom na prisutnost širokog spektra fenolnih spojeva. Obogaćivanje jogurta dodatkom M. oleifera stoga bi poboljšalo mineralni i fenolni profil te pospješilo rast probiotičkih bakterija. Ovaj rad daje pregled prehrambenih i proizvodnih mogućnosti biljke M. oleifera za obogaćivanje jogurta. Terapeutska svojstva biljke M. oleifera ukazuju na visoki potencijal njezine primjene u obogaćivanju jogurta u svrhu poboljšanja nutritivnih i funkcionalnih svojstava. S obzirom da proizvodnja jogurta zahtijeva pridržavanje specifičnih standardiziranih pragova u pogledu okusa, bogat sadržaj polifenola M. oleifera koji daju oporost, zahtijeva provedbu istraživanja kojima bi se pronašle mogućnosti za prevladavanje ovog problema.
Ključneriječi: Moringaoleifera; antioksidans; jogurt; obogaćivanje; funkcionalna hrana
References
https://doi.org/10.1016/j.jaubas.2017.05.001
Das, P.K., Asha, S.Y., Siddika, M.A., Siddika, A., Tareq, A.R.M., Islam, F., Khanam, J.A., Rakib, M.A. (2021): Methanolic extract of Moringa oleifera leaves mediates anticancer activities through inhibiting NF-𝜅B and enhancing ROS in Ehrlich ascites carcinoma cells in mice. Journal of Advanced Biotechnology and Experimental Therapeutics 4, 161-170. https://doi.org/10.5455/jabet.2021.d116
da Silva, S.C., Fernandes, I.P., Barros, L., Fernandes, Â., Alves, M.J., Calhelha, R.C., Pereira, C., Barreira, J.C.M., Manrique, Y., Colla, E., Ferreira, I.C.F.R., Barreiro, M.F. (2019): Spray-dried Spirulina platensis as an effective ingredient to improve yoghurt formulations: Testing different encapsulating solutions. Journal of Functional Foods 60, 103427.https://doi.org/10.1016/j.jff.2019.103427
https://doi.org/10.1111/jfbc.13338
Nikkon, F., Saud, ZA., Rahman, M.H., Haque, E. (2003): In vitro antimicrobial activity of the compound isolated from chloroform extract of Moringa oleifera Lam. Pakistan Journal of Biological Sciences 6 (22), 1888-1890.https://doi.org/10.3923/pjbs.2003.1888.1890
O’Connell, J.E., Fox, P.F. (2001): Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. International Dairy Journal 11 (3), 103-120. https://doi.org/10.1016/S0958-6946(01)00033-4
