INTRODUCTION
Hepatocellular carcinoma (HCC), one of the most common malignant tumors, is the fourth leading cause of cancer-related death worldwide, with an estimated 905,700 new cases and 830,200 deaths in 2020 (1). There are an estimated 1.4 million new liver cancer cases diagnosed in 2040, a 55 % increase from 2020 (2). Patients with HCC account for nearly 85–90 % of primary liver cancers, and the majority are diagnosed at the intermediate or advanced stages (3). Portal vein tumor thrombus (PVTT), a feature of the advanced HCC, is associated with a poor prognosis of HCC (4, 5). Evidence showed that the incidence of PVTT was approximately 44–62.2 % for HCC survivors, with a median survival time of 2–12 months for patients with HCC complicated by PVTT (6, 7). It is important to focus on effective treatment to improve the quality of life of patients with HCC complicated by PVTT.
Transcatheter arterial chemoembolization (TACE) is a therapeutic method that infuses chemotherapeutic drugs and embolic agents into the blood-supplying arteries of tumors, thereby leading to vascular embolization and ischemic necrosis of tumors, and it is considered the main local treatment for intermediate and advanced HCC (8). Sorafenib, an oral tyrosine kinase inhibitor, serves as a standard first-line systemic therapy for advanced HCC, inhibiting tumor growth via restraining the intracellular signaling pathways and extracellular receptors (9, 10). Previous studies reported that sorafenib with TACE has widely been utilized to treat patients with HCC complicated by PVTT or extrahepatic metastases (11–13). A randomized controlled trial (RCT) of sorafenib with TACE mentioned the progression-free survival improvement in unresectable patients with HCC (11). Salem et al. (14) found more than 40 % of patients in the control group received at least one TACE treatment prior to sorafenib, supporting the use of TACE in combination with sorafenib in patients with advanced HCC. To the best of our knowledge, the therapeutic strategy is also recommended in consensus guidelines in China and Korea (12, 15).
Although sorafenib plus TACE has been applied in clinical practice, this comprehensive treatment for patients with HCC complicated by PVTT remains controversial. Herein, we conducted a systematic review and meta-analysis on the basis of RCTs to comprehensively assess the effect of sorafenib plus TACE in treating patients with HCC complicated by PVTT.
METHODS
This systematic review and meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.
Search strategy
The literature was retrieved from PubMed, Cochrane Library, Embase, China National Knowledge Infrastructure, Wan-fang database, Web of Science, and CQVIP until 28th Sep 2022. The research terms included (liver OR hepatic OR hepatocellular) AND (cancer* OR neoplasm* OR tumor* OR tumour*) AND (transarterial chemoembolization OR TACE OR transcatheter arterial chemoembolization OR chemoembol i* OR emboli*) AND (sorafenib OR nexavar OR Raf 1 kinase inhibitor II’) AND (portal vein tumor thrombus OR portal vein thrombosis OR PVTT). Two authors ( H. Cao and Z. Feng conducted the article screening.
Inclusion and exclusion criteria
Inclusion criteria were as follows: i) study design: RCTs; ii) population: patients with HCC complicated by PVTT; iii) intervention: sorafenib plus TACE (group S+TACE); iv) comparison: TACE (group TACE); v) outcomes: time to progression (TTP), objective response rate (ORR), disease control rate (DCR), overall survival (OS), survival rate (SR), and grade 3 or 4 adverse events (hand-foot skin reaction, oral ulcer, diarrhea, ascites, hepatorenal syndrome, pleural effusion, gastrointestinal bleeding, and hepatic abscess); vi) articles in English and Chinese.
Exclusion criteria were as follows: i) reviews or meta-analyses, meetings or conference abstracts, case reports, and letters; ii) duplicated studies; iii) animal experiments; iv) studies that had incomplete data or whose data could not be extracted.
A total of 823 records were identified from the databases. After excluding 105 duplicate records, 384 irrelevant topics, 75 reviews or meta-analyses, 53 conference abstracts, 26 case reports, 1 animal experiment, and 25 studies that had incomplete data or whose data could not be extracted, 12 articles (16–27) assessed for eligibility were finally included.
Data extraction and quality assessment
Information was extracted from the 12 articles included (16–27) containing the first author, year of publication, the number of patients, groups, gender, age, follow-up time, Child-Pugh, Jadad score, and efficacy. The literature quality was assessed using the modified Jadad scale (28), divided into low quality (score of 1–3, four articles) and high quality (score of 4–7, eight articles). The quality assessment of the included articles was carried out by two authors (X. Li and S. Chen).
Stata 15.1 (Stata Corporation, College Station, TX, USA) was used for all statistical analyses. Relative risk (RR) and hazard ratio (HR) were used as the statistics for enumeration data. In this meta-analysis, 1,746 patients with HCC complicated by PVTT were grouped into the sorafenib plus TACE treatment group ( n = 458) and the TACE treatment group ( n = 1288) . Engauge Digitizer 10.8 software was used to extract data from the survival curve. The effect sizes were expressed with 95 % confidence intervals (CIs). The heterogeneity test was conducted for each effect size. The outcomes were analyzed by random-effect models when the heterogeneity statistic I2 was ≥ 50 % using the Q test, and otherwise, indicators were analyzed by fixed-effect models. The heterogeneity source was explored using a meta-regression analysis. Sensitivity analysis was performed for all outcomes. p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Characteristics of the included articles
The flow chart of the article screening is shown in Fig. 1. Totally 1,746 patients with HCC complicated by PVTT were enrolled in this study, of which 458 cases received the sorafenib plus TACE treatment (Group S+TACE), and 1,288 cases received the TACE treatment (Group TACE). The characteristics of the included studies are presented in Table I.

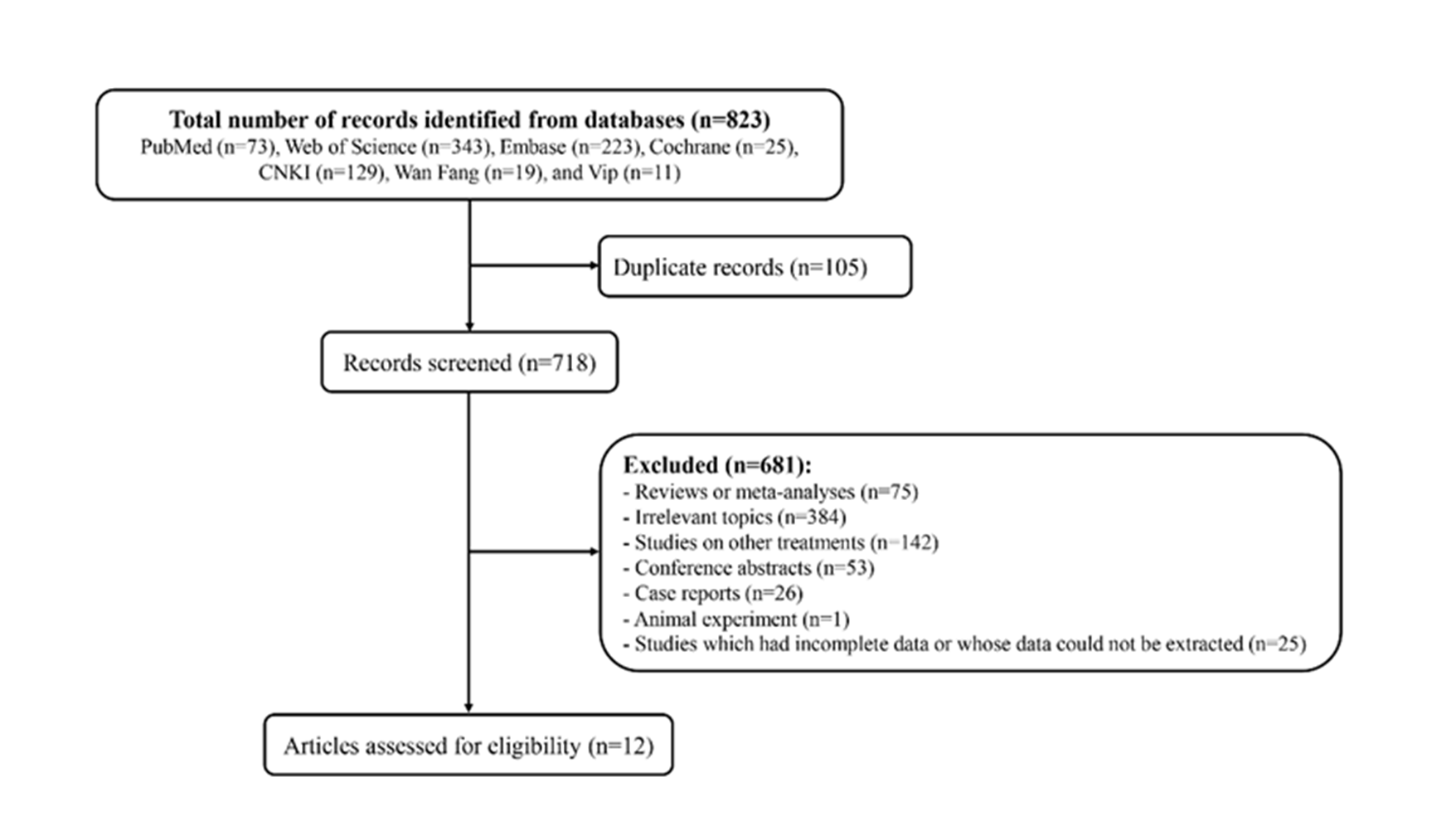
Fig. 1. The flow chart of the article screening.
Table I. The main characteristics of the articles included in this analysis
TACE – transcatheter arterial chemoembolization, S – sorafenib, NA – not applicable, SR – survival rate, CR – complete response, PR – partial response, SD – stable disease, TTP – time to progression, DCR – disease control rate, OS – overall survival.
a cases of age ≥ 50 and < 50 years old.
Impacts of sorafenib plus TACE on the efficacy of patients with HCC complicated by PVTT
The effects of sorafenib plus TACE on the efficacy of patients with HCC complicated by PVTT were presented in Table II and Fig. 2. The pooled results of ten articles (17, 18, 21–27) reporting OS, concluded that the OS in the S+TACE group was longer than that in the TACE group (HR: 0.596, 95 %CI: 0.507–0.685, p < 0.001; I2 = 0.0 %). Eight articles (18–21, 25–28) reporting ORR, concluded that the ORR in the S+TACE group was higher than that in the TACE group (RR: 2.101, 95 %CI: 1.555-2.839, p < 0.001; I2 = 0.0 %). DCR was mentioned in 8 studies (18–21, 25–28), and the overall results indicated that the DCR in the S+TACE group was better than that in the TACE group (RR: 1.547, 95 %CI: 1.126–2.126, p = 0.007; I2 = 79.6 %). Statistical differences were also found in TTP (HR: 0.379, 95 %CI: 0.205–0.553, p < 0.001; I2 = 4.5 %) and SR (RR: 1.416, 95 %CI: 1.183–1.694, p < 0.001; I2 = 83.8 %) between the two groups.
Table II. Impacts of sorafenib plus TACE on the efficacy of patients with HCC complicated by PVTT
HR – hazard ratio, RR – relative risk, TACE – transcatheter arterial chemoembolization, HCC – hepatocellular carcinoma, PVTT – portal vein tumor thrombus, OS – overall survival.

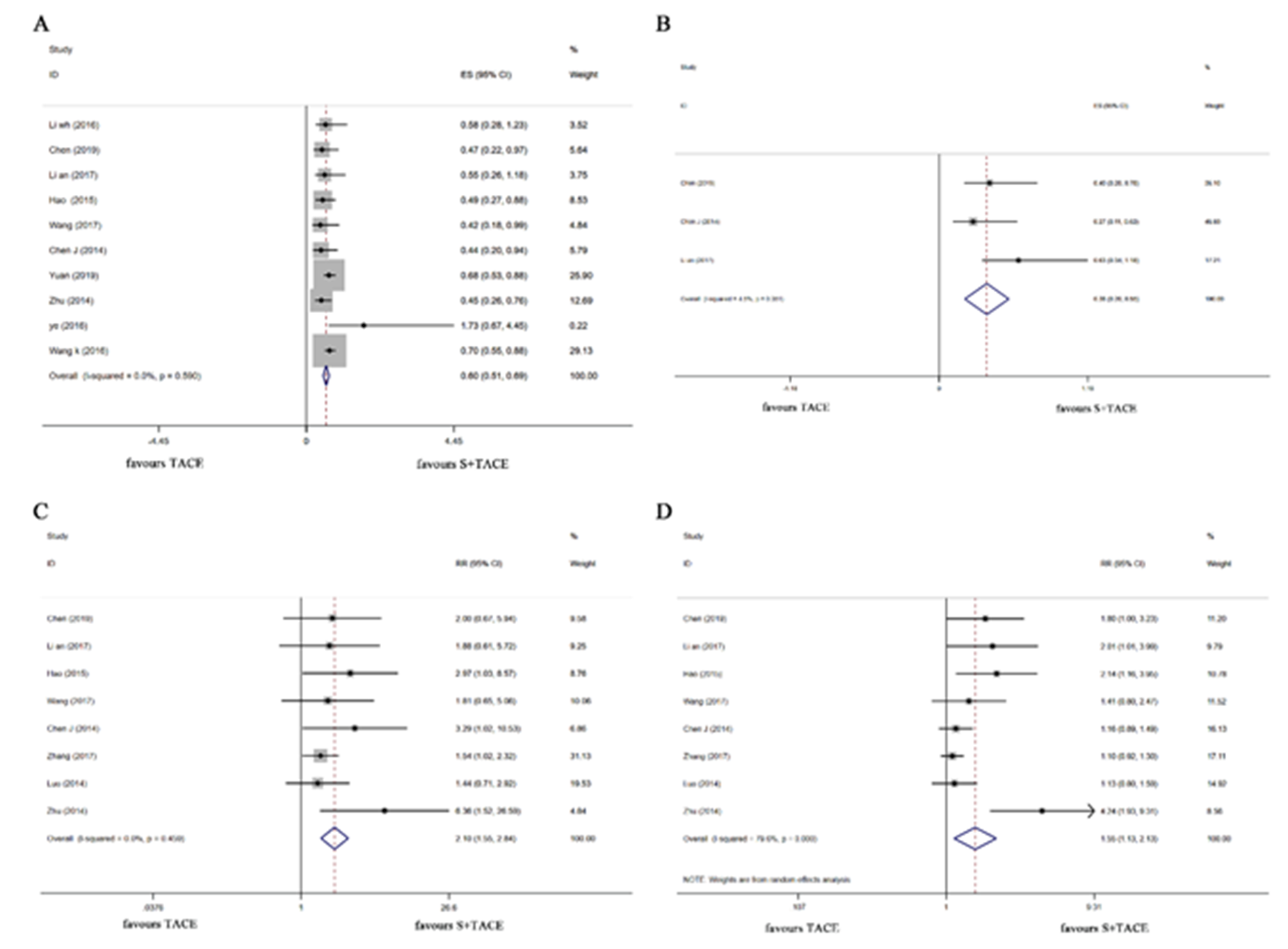
Fig. 2. Forest plots of sorafenib plus TACE vs. TACE for the efficacy of treating patients with cirrhosis complicated by PVTT . a) OS, b) TTP, c) ORR, d) DCR. TACE – transcatheter arterial chemoembolization, PVTT – portal vein tumor thrombus, OS – overall survival, TTP – time to progression, ORR – objective response rate, DCR – disease control rate .
The heterogeneity assessment was performed based on the sample size, literature quality, year of publication, and survival time. We found that the sample size, literature quality, and year of publication were not the source of heterogeneity. According to various survival times (Table II and Fig. 3), the differences were discovered in 6-month SR (RR: 1.349, 95 %CI: 1.015–1.793, p = 0.039; I2 = 80.3 %), 12-month SR (RR: 1.837, 95 %CI: 1.238–2.725, p = 0.003; I2 = 56.3 %) and >12-month SR (RR: 6.079, 95 %CI: 1.418–26.061, p = 0.015; I2 = 34.6 %) between the two groups.

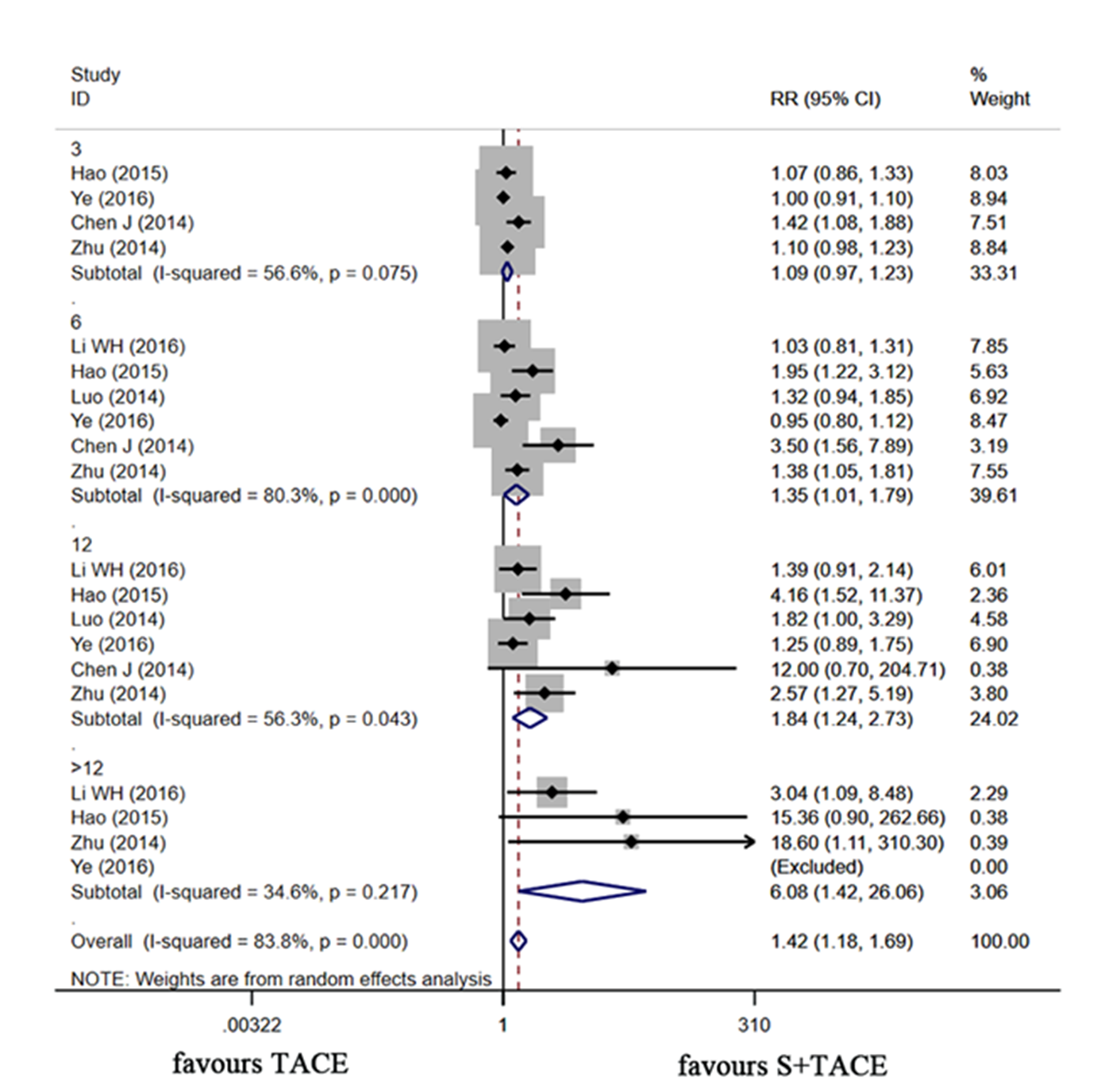
Fig. 3. Forest plot of sorafenib plus TACE vs. TACE alone for SR. TACE – transcatheter arterial chemoembolization , SR – survival rate, RR –relative risk .
Adverse events
In Table III, compared with the TCAE group, the higher odds of HFSR ( RR = 22.925, 95 %CI: 6.743– 77.935, p < 0.001), oral ulcer (RR = 4.949, 95 %CI: 1.521–16.103, p = 0.008), and diarrhea (RR = 5.525, 95 %CI: 2.416–12.635, p < 0.001) among patients with HCC complicated by PVTT were discovered in the S+TACE group. The marginal significance was found in ascites (RR = 1.787, 95 %CI: 0.957–3.339, p = 0.069), and gastrointestinal bleeding (RR: 1.987, 95 %CI: 0.996– 3.968, p = 0.052) between the two groups. The impacts of sorafenib plus TACE on different adverse events of patients with HCC complicated by PVTT are shown in Fig. 4.

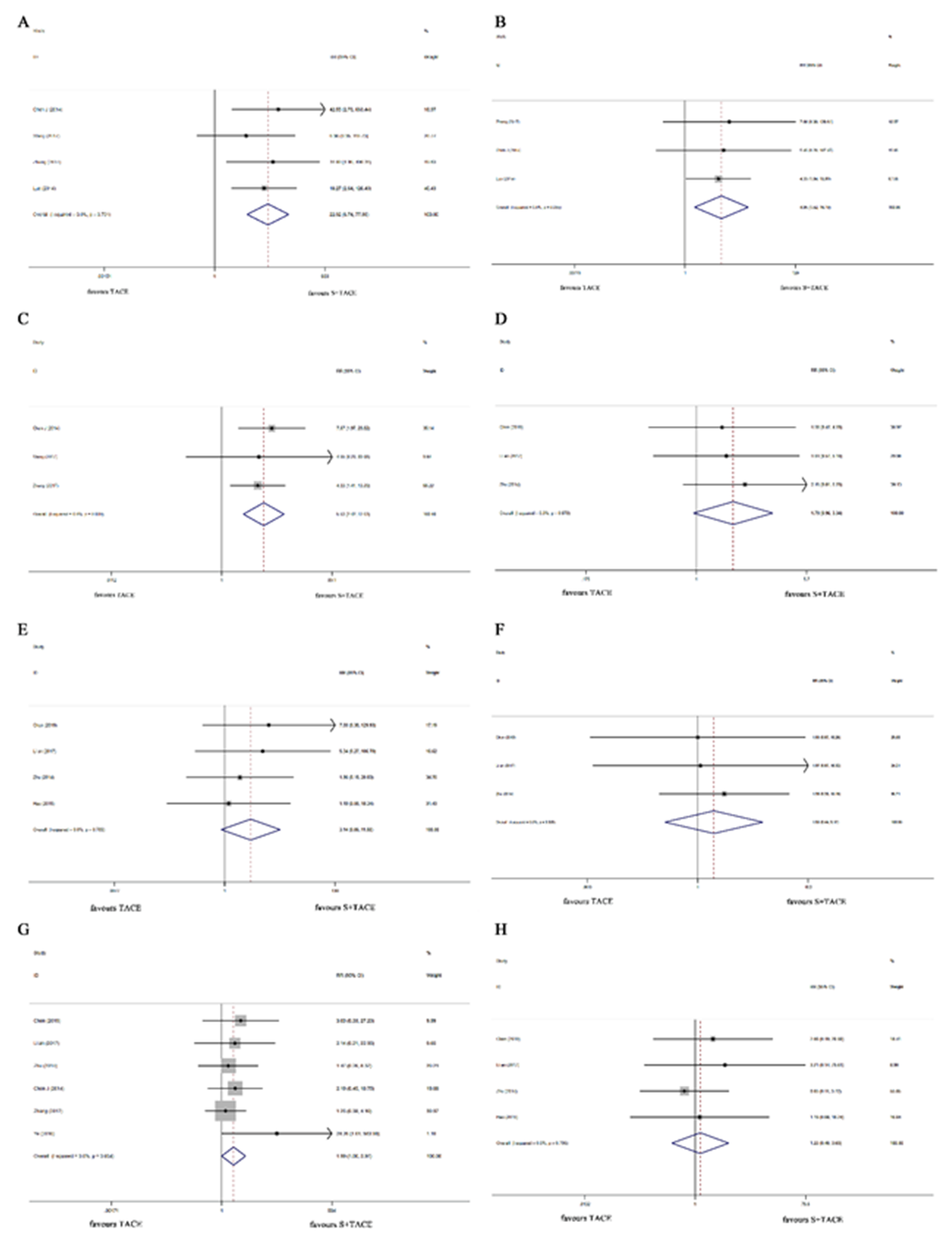
Fig. 4. Forest plots of sorafenib plus TACE vs. TACE alone for the adverse events in patients with HCC complicated by PVTT . a) HFSR, b) oral ulcer, c) diarrhea, d) ascites, e) hepatorenal syndrome, f) pleural effusion, g) gastrointestinal bleeding, and h) hepatic abscess. TACE – transcatheter arterial chemoembolization, HCC – hepatocellular carcinoma, PVTT – portal vein tumor thrombus, HFSR – hand-foot skin reaction, RR – relative risk.
Table III. Impacts of sorafenib plus TACE on the adverse events of patients with HCC complicated by PVTT
RR – relative risk, CI – confidence interval, TACE – transcatheter arterial chemoembolization, HCC – hepatocellular carcinoma, PVTT – portal vein tumor thrombus; HFSR – hand-foot skin reaction
Subgroup assessment
Two articles (23, 25) on PVTT grades were included. Wang et al. (23) explored several treatments for patients with HCC complicated by PVTT subtypes defined by Cheng's Classification (Type I: tumor invasion of segmental branches of the portal vein or above; Type II: the right/left portal vein; Type III: the main portal vein trunk; Type IV: the superior mesenteric vein) (29). The two studies found that the mean survival times for patients with type I, II, and III who received sorafenib plus TACE were 12.0 (6.6–17.4) months, 8.9 (6.7–11.1) months, and 7.0 (3.0–10.9) months, respectively. The mean survival times for patients with type I, II, and III who underwent TACE were 9.3 (5.6–12.9) months, 4.9 (4.1–5.7) months, and 4.0 (3.1–4.9) months, respectively. It was indicated that sorafenib plus TACE may be beneficial for patients with HCC complicated by type I, II, and III PVTT. Zhu et al. (25) classified PVTT types based on previous studies (30, 31). The types were as follows: type A – the main portal vein, type B – the first-order portal vein branch (the right or left portal vein), and type C – the second- or lower-order portal vein branches (segmental branches of the portal vein or higher). Their findings showed the median TTP of patients with HCC complicated by PVTT type A (0 months vs. 0 months), B (6 months vs. 3 months), and C (7 months vs. 5 months) between the sorafenib plus TACE group and the TACE group. The median OSs of different PVTT types were 3.0 (type A), 13.0 (type B), and 15.0 (type C) months in the sorafenib plus TACE group, and were 3.0 (type A) , 6.0 (type B) , and 10.0(type C) months in the TACE group. It was suggested that patients with HCC complicated by PVTT who received sorafenib plus TACE treatment had longer OS and TTP, especially in patients with HCC complicated by PVTT types B and C .
In the current meta-analysis with 12 RCTs, we explored the effect of sorafenib plus TACE on HCC with PVTT. Our findings show that the OS and TTP in the S+TACE group were longer than those in the TACE group. The ORR, DCR, and SR in the S+TACE group were higher than those in the TACE group . Compared with the TCAE group, the higher odds of HFSR, oral ulcer, and diarrhea among patients with HCC complicated by PVTT were discovered in the S+TACE group. The marginal significance was found in ascites and gastrointestinal bleeding between the two groups.
We found that the combination therapy (S+TACE) had longer TTP, higher ORR, and DCR than the only TACE treatment, indicating that the combined treatment was more effective than monotherapy. The short-term efficacy seemed to be similar in the two groups, whereas the long-term efficacy was different between the two groups. In the subgroup analysis, the short-term SR of 3 months seemed to be similar in the two groups, whereas the long-term SRs of 6, 12, and >12 months were higher in the S+TACE group than those in the TACE group. The OS also was longer in patients with HCC complicated by PVTT who received the sorafenib combined with TACE treatment, and this finding also has been supported by the research from Abdelrahim et al. (32). All above results demonstrated that the application of the combined therapy could extend the survival duration of patients with HCC complicated by PVTT . These findings were consistent with a previous report which suggested that sorafenib could prolong the OS in patients with HCC (33) . The positive effects of the combined therapy may be related to the blocking effects of sorafenib on neoangiogenesis and HCC growth.
Patients with HCC complicated by PVTT in the S+TACE group had higher incidences of HFSR, oral ulcer, diarrhea, ascites, and gastrointestinal bleeding between the two groups. It was suggested that compared with the TACE alone therapy, patients with HCC complicated by PVTT receiving the combined treatment had higher odds of developing adverse reactions, which may be controlled by changing the dosage of the drugs.
With the development of medical technologies, various therapeutic strategies have been applied to treat HCC with PVTT (34). A phase 3 RCT reported that patients receiving the TACE plus lenvatinib treatment had improved ORR and prolonged OS in comparison with lenvatinib monotherapy (35). Lu et al. (36) found that the OS of patients with HCC complicated by PVTT (main trunk) treated by irradiation stent placement plus TACE was longer than that of patients treated with sorafenib plus. Wang et al. (23) demonstrated that sorafenib plus TACE was beneficial for patients with HCC complicated by type I, II, and III PVTT. A retrospective study compared the efficacy of TACE combined with lenvatinib programmed death 1 (PD-1) inhibitor (TACE-L-P) with TACE combined with sorafenib plus PD-1 inhibitor (TACE-S-P) for treating HCC with PVTT (37). The mean OS and mean progression-free survival (mPFS) in the TACE-L-P group were longer than those in the TACE-S-P group. A previous study showed higher ORR, and longer mPFS in the lenvatinib group than those in the sorafenib group, while no difference was discovered in OS between the two groups (38). Guerrero et al. (39) explored the effect of anticoagulation vs. no treatment on all-cause mortality in patients with cirrhosis complicated by PVTT. The results suggested that regardless of the degree of thrombosis, patients with cirrhosis complicated by PVTT could consider taking anticoagulation and long-term maintenance therapy, as anticoagulation results in a greater survival benefit compared to recanalization. Overall, local or locoregional treatments combined with systemic therapy may be considered more effective treatment options for patients with HCC complicated by PVTT (34). Owing to the different etiologies, biological behaviors, and types of PVTT, different treatment strategies for patients with HCC complicated by PVTT should be developed individually in each country. Future clinical trials or cohort studies investigating treatment strategies should be stratified according to PVTT grades.
Our findings are susceptible to several important limitations. Readers should be cautious when interpreting our results due to following limitations: first, the publication bias assessment was not performed owing to the lack of available articles (less than 10), the reason for which may be that the test power is usually too low to distinguish chance from real asymmetry (40); second, four RCT studies analyzed in this SR were of low quality (scored 3 or lower on Jadad scale, which may influence the reliability of our findings; third, different chemotherapeutic agents and PVTT grades were not assessed, which may also affect the results. Further high-quality studies with large sample sizes are warranted to confirm these findings.
CONCLUSIONS
The current meta-analysis suggested that sorafenib plus TACE is superior to TACE monotherapy in patients with HCC complicated by PVTT . The combined therapy may increase the SR and extend the OS of patients with HCC complicated by PVTT . The incidence of adverse events including HFSR, oral ulcer, and diarrhea was high in patients who received the sorafenib plus TACE treatment, which may be associated with the side effects of the drug itself, and these adverse effects can be mitigated by suitable management. However, due to the publication bias and several studies being of low quality, our results need to be interpreted with caution. The sorafenib plus TACE treatment may be considered for patients with HCC complicated by PVTT. Future RCTs with well-designed, multicenter, large sample sizes are needed to further explore more effective treatment combinations.
Acknowledgments. – None.
Conflicts of interest. – The authors declare no conflict of interest.
Funding. – Zhejiang medicine and health science and technology project (No: 2022RC217).
Authors contributions. – Conceptualization, L.X. and C.Y.; methodology, L.X. and C.Y.; analysis L.X., S.C., H.C. and Z.F.; investigation, L.X., S.C., H.C. and Z.F.; writing, original draft preparation, L.X.; writing, review and editing, C.Y. All authors have read and agreed to the published version of the manuscript.
