Introduction
Allium L. (Amaryllidaceae, Allioideae, Allieae) has a disputed generic delimitation and species boundaries, which is compounded by the proliferation of its synonyms (Choi et al. 2012, Nour et al. 2022). It is one of the most taxonomically complex and species-rich genera, with approximately 1063 species (Sennikov and Lazkov 2023). Members of the genus exhibit high morphological diversity in their floral parts (Friesen et al. 2006). Allium is a bulbous plant with a short rhizome, membranous tunics, and linear leaves. Its inflorescence is a multiflowered umbel, and the floral parts are strongly withered once the capsules have matured. Capsules are trigonous and typically contain ovate to drop-shaped seeds; however, the shape of the seeds varies greatly depending on the characteristics of the capsule (Fritsch 2001). The genus is dispersed across Africa; southwestern, middle, and central Asia; Europe; and North and South America (Friesen et al. 2006, Khassanov 2018). Allium was classified into 15 subgenera and 67 sections using the internal transcribed spacer region (ITS) (Friesen et al. 2006). Allium species have a high rate of endemism, particularly with respect to the Mediterranean area, which is regarded as the main Allium diversity center (Brullo et al. 2019). Bedair et al. (2023) reported that A. barthianum Asch. & Schweinf., A. blomfieldianum Asch. & Schweinf., A. mareoticum Bornm. & Gauba and A. trifoliatum Cirillo are Mediterranean endemic species; these species are included in this study.
In the Flora of Egypt, El Garf (2000) recognized 20 Allium species, and Boulos (2009) recorded 29 Allium taxa (21 wild, four cultivated species, and four subspecies). According to the classification of Friesen et al. (2006), those taxa occupied four subgenera and six sections: Allium subg. Allium L. ( Allium sect. Allium L. and A. sect. Codonoprasum Rchb.), A. subg. Amerallium Traub. ( A. sect. Briseis (Salisb.) Stearn. and A. sect. Molium G.Don ex Koch.), A. subg. Cepa Radic ( A. sect. Cepa L.), and A. subg. Melanocrommyum Webb et Berth. ( A. sect. Melanocrommyum). Allium crameri Asch. & Boiss. and A. mareoticum are endemic to Egypt (Abdelaal et al. 2018), while A. barthianum and A. blomfieldianum are near-endemic (Bedair et al. 2023). A restricted distribution was observed for A. artemisietorum Eig & Feinbrun, A. desertorum Forssk., and A. papillare Boiss. (from Egypt to Jordan), A. erdelii Zucc. (from Northeast Libya to Syria), A. sinaiticum Boiss. (from Sinai, Egypt, to Northwest Saudi Arabia), and A. spathaceum Steud. ex A.Rich. (from East Sudan to North Somalia) (POWO 2021). Although A. spathaceum was recorded in the Egyptian Flora as a rare species collected earlier from Gebel Elba, in the southeastern corner of Egypt, it was included in this study (Täckholm 1974).
The taxonomic significance of the quantitative and qualitative traits of Allium seeds has been studied. Some studies focused on a particular region, including those in Europe. Cesmedziev and Terzijski (1997) investigated the spermoderm structures of 18 Bulgarian species of A. subg. Codonoprasum. Eight Allium species, following five subgenera and five sections, were examined in Poland by Bednorz et al. (2011), revealing the presence of unusually raised anticlinal walls. Moreover, sixty-two Allium taxa from Türkiye were classified into four subgenera, and nine sections were examined to determine the taxonomic relevance of the epidermal cell shape; sculpturing of periclinal walls; and position, shape, and undulation type of anticlinal walls (Celep et al. 2012).
Several studies were carried out on the Asian Allium; Lin and Tan (2017) investigated 38 species belonging to seven subgenera and 19 sections from Xinjiang, China. Baasanmunkh et al. (2020) also described 48 species from Uzbekistan, Kyrgyzstan, and Mongolia, representing seven subgenera and 24 sections. There were differences in seed size and shape, the arrangement of epidermal cells, and the different anticlinal and periclinal wall traits between the species and sections. These differences were shown in both studies. The Iranian Allium was examined based on 20 species classified into four subgenera and 11 sections studied by Neshati and Fritsch (2009). Veiskarami et al. (2018) investigated 23 species from two subgenera that compose the A. ampeloprasum L. alliance. Khorasani et al. (2020) examined thirteen species and five sections. The authors found that the most influential traits were seed shape, periclinal wall sculpture, anticlinal wall undulations, and type of verruca.
Other publications address the testa ultrastructure of 88 taxa belonging to 15 sections in A. subg. Melanocrommyum was investigated by Fritsch et al. (2006). Yusupov et al. (2022) studied 95 worldwide species belonging to 14 subgenera and 58 sections. The latter reported that the description of the periclinal wall shape and the anticlinal wall curvature could indicate the evolutionary state of a species. A new technique called unsupervised machine learning was implemented to analyze the seed coat patterns of approximately 100 species of Allium that have been previously described (Ariunzaya et al. 2022). The authors classified the studied taxa according to their anticlinal wall pattern: irregularly curved, irregularly curved to nearly straight, straight, S-type, U-type, U- to Ω-type, and Ω-type. The periclinal walls were also divided into five types: granule, small verrucae, large verrucae, marginal verrucae, and verrucate verrucae. Some phylogenetic studies of Allium correlate the morphological characteristics of seeds with the evolutionary status of each species (Choi et al. 2012, Yusupov et al. 2022).
The present study provided a detailed seed morphological description of 22 Allium taxa – using stereomicroscopy and scanning electron microscopy (SEM) – to provide information on the native, endemic, and near-endemic species in Egypt and address the significance of seed traits for infrageneric classification. This work provides the first description of the seeds of 13 Allium taxa, including A. artemisietorum, A. barthianum, A. blomfieldianum, A. crameri, A. desertorum, A. erdelii, A. mareoticum, A. papillare, A. roseum subsp. tourneuxii Boiss., A. sativum L., A. sinaiticum, A. spathaceum, and A. trifoliatum. Additionally, this study reports for the first time a comparative investigation of dorsal seed surface traits against ventral traits, revealing conspicuous differences for most species and highlighting the most informative diagnostic seed traits for distinguishing taxa.
Plant material
Twenty-two Allium species were investigated using fresh or dried materials from their mature seeds. The voucher specimens were deposited in two herbaria: the Cairo University Herbarium (CAI), located at the Botany Department, Faculty of Science, Cairo University, Egypt; the Agricultural Research Center Herbarium (CAIM), located at the Flora and Phytotaxonomy Research Department, Horticultural Research Institute, Cairo, Egypt; and the South Valley University Herbarium, Qena, Egypt (On-line Suppl. Tab. 1). The studied taxa were identified according to Boulos (2009). The nomenclature and synonyms used were revised according to the International Plant Names Index (IPNI 2022).
Seed macro- and micromorphology
Seeds were washed with 70% ethyl alcohol to remove dust or any attached floral parts. For the macromorphological study, the seeds were examined using an Olympus stereomicroscope supported with a 1 cm ocular micrometer and photographed to measure the following seed size parameters: length, width, length/width (L/W) ratio, area, and seed shape.
The dorsal and ventral sides of the seeds were mounted on a copper stub with double-sided tape for micromorphology. The samples were then gold-coated in a fine-coat JEOL JFC-1100E (Japan) ion sputtering device for five minutes. The seeds were investigated using a JEOL JSM-IT200 SEM (Tokyo, Japan) at the Faculty of Science, Shatebi Building, Alexandria University, Alexandria, Egypt. Thirty-seven quantitative and qualitative traits were investigated. Diagnostic quantitative characteristics were evaluated using ImageJ (1.51j8). The number of epidermal cells was counted at a magnification of 700×, equivalent to a field view area of 182.9 × 137.1 µm. The terminology used comes from Barthlott et al. (1981) and Yusupov et al. (2022).
Data analysis
The quantitative data were analyzed using Minitab Ltd. version 19.1 (64-bit). Descriptive statistics were calculated for each variable on both seed surfaces. Different comparisons were conducted using one-way analysis of variance (ANOVA). The P values were considered significant at < 0.05. A post hoc analysis of twenty-two taxa was conducted for each variable using Tukey’s test for pairwise mean comparisons. The results are represented as letters, where means that do not share similar letters are significantly different. Furthermore, a post hoc analysis of the interaction between the seed dorsal and ventral surfaces was performed for each taxon via Tukey’s test for pairwise mean comparisons. A P value of a trait less than 0.05 is represented by an asterisk (*) to indicate a significant difference between the two surfaces.
A principal component analysis (PCA) was conducted based on 26 quantitative traits for the studied taxa without any predefined groups. The eigenvalues and percent variances of 21 principal components and the character loadings of the first two axes were analyzed according to the seed morphometric characteristics. In addition, a discriminant analysis was performed to indicate the most discriminant traits for separating sections of Allium. Multivariate analysis was performed using Past software version 4.03 (Hammer et al. 2001).
Results
The descriptive morphometric measurements of Allium taxa seed traits for dorsal and ventral seed surfaces are illustrated in On-line Suppl. Tab. 2. The quantitative macromorphological characteristics of the examined seeds are summarized in On-line Suppl. Tab. 3, and the micromorphological characteristics of the seed coat are exemplified in On-line Suppl. Tab. 4. The qualitative seed morphological characteristics of the studied Allium taxa are described in On-line Suppl. Tab. 5. The scanning electron micrographs of the investigated samples, illustrating the whole seeds and the detailed features of testa cells, are also exemplified in Figs. 1–5.
The seeds of the examined taxa were dull, shiny, or glossy black. The seed length varied from 1.54 mm ( A. blomfieldianum) (Fig. 2: 5A, 5D) to 3.70 mm ( A. crameri) (Fig. 2: 7A, 7D). The narrowest seeds (0.88 mm) were observed in A. artemisietorum (Fig. 1: 2A, 2D), and the widest seeds (2.55 mm) were observed in A. cepa (Fig. 2: 6A, 6D).
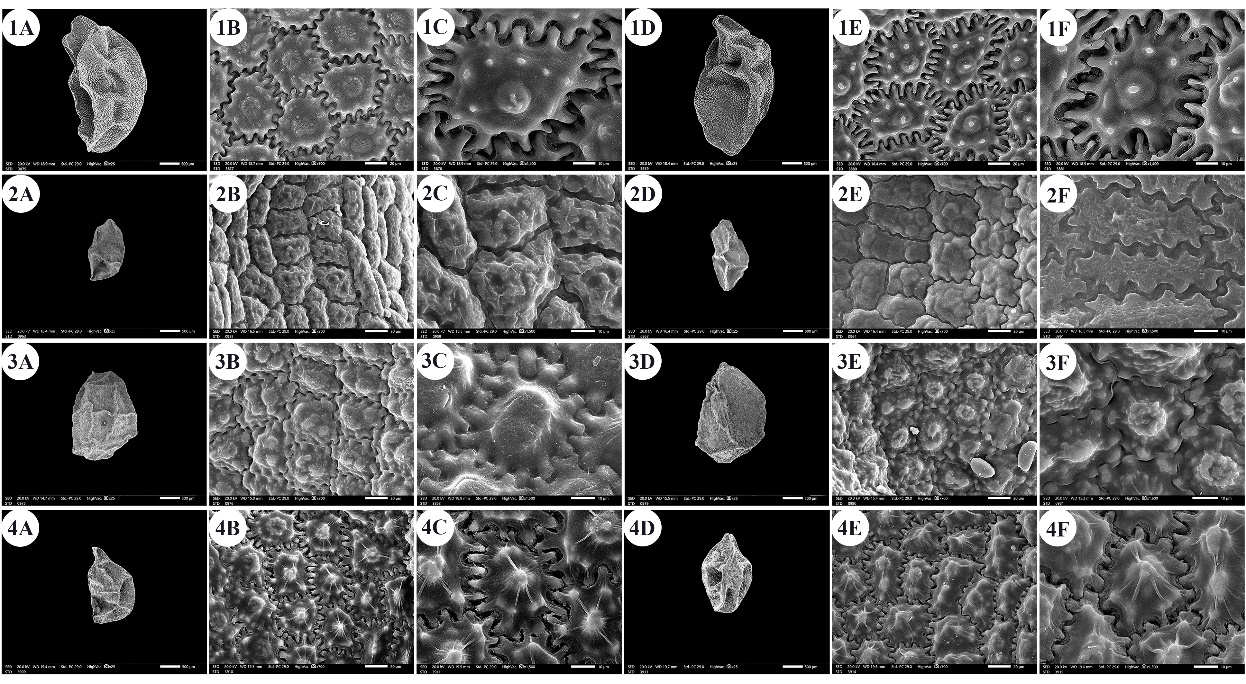
Fig. 1. A –F: scanning electron micrographs of Allium seeds. A –C: dorsal surface, D –F: ventral surface. 1 – A. ampeloprasum, 2 – A. artemisietorum, 3 – A. aschersonianum, 4 – A. barthianum. Scale bars: A, D = 500 µm; B, E = 20 µm; C, F = 10 µm.
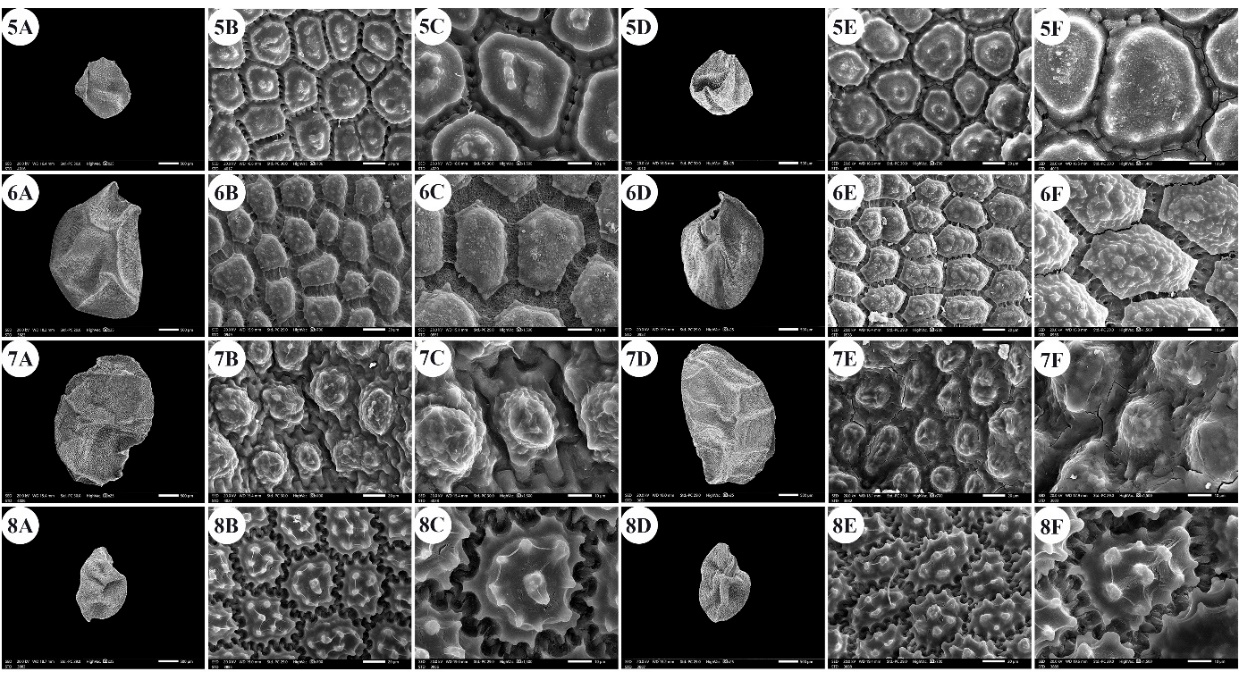
Fig. 2. A –F: scanning electron micrographs of Allium seeds. A –C: dorsal surface, D –F: ventral surface. 5 – A. blomfieldianum, 6 – A. cepa, 7 – A. crameri, 8 – A. curtum. Scale bars: A, D = 500 µm; B, E = 20 µm; C, F = 10 µm.
The seed length/width ratio ranged from 1.01 in A. blomfieldianum to 2.40 in A. sinaiticum (Fig. 5: 19A, 19D). The smallest seed area (1.045 mm2) was recorded for A. artemisietorum, and the highest (7.148 mm2) was recorded for A. crameri (On-line Suppl. Tab. 3). The seeds were widely elliptical, elliptical, widely ovate, ovate, while some taxa had mixed shapes, widely elliptical and elliptical (On-line Suppl. Tab. 5).
The number of epidermal cells per unit area ranged from 12 to 48 cells in A. neapolitanum Cirillo and A. pallens L. (Fig. 4: 13E, 14B). Notably, the epidermal cell count at the dorsal surface was greater than that at the ventral surface (On-line Suppl. Tab. 2). The minimum epidermal cell length was recorded for A. spathaceum (18.54 µm) (Fig. 5: 20E, 20F), and the maximum length was 99.97 µm for A. crameri (Fig. 2: 7E, 7F). The lowest epidermal cell width was 21.22 µm in A. desertorum (Fig. 3: 9E, 9F), and the greatest width was 97.18 µm in A. neapolitanum (Fig. 4: 13B, 13C). However, the epidermal cell length/width ratio varies within species, where the cell length is sometimes greater than the cell width and sometimes otherwise. This ratio ranges from 0.31 (as in A. spathaceum; Fig. 5: 20E, 20F) to 2.42 (as in A. cepa; Fig. 2: 6B, 6C). Its area ranged from 582.90 µm2 in A. pallens (Fig. 4: 13B, 13C) to 5785.0 µm2 in A. crameri (Fig. 2: 7E, 7F). The epidermal cell shape varied from orbicular to widely elliptic, elliptic, oblong, or polygonal with 4 to 8 edges. The cells may be arranged in a jigsaw-like pattern or side-by-side. The epidermal cells may be close to each other with no intercellular space, as in A. neapolitanum and A. spathaceum (Fig. 4: 13B and Fig. 5: 20E, respectively), or distant where the intercellular space length may reach up to 13.45 µm, as in A. cepa (Fig. 2: 6B, On-line Suppl. Tab. 4).
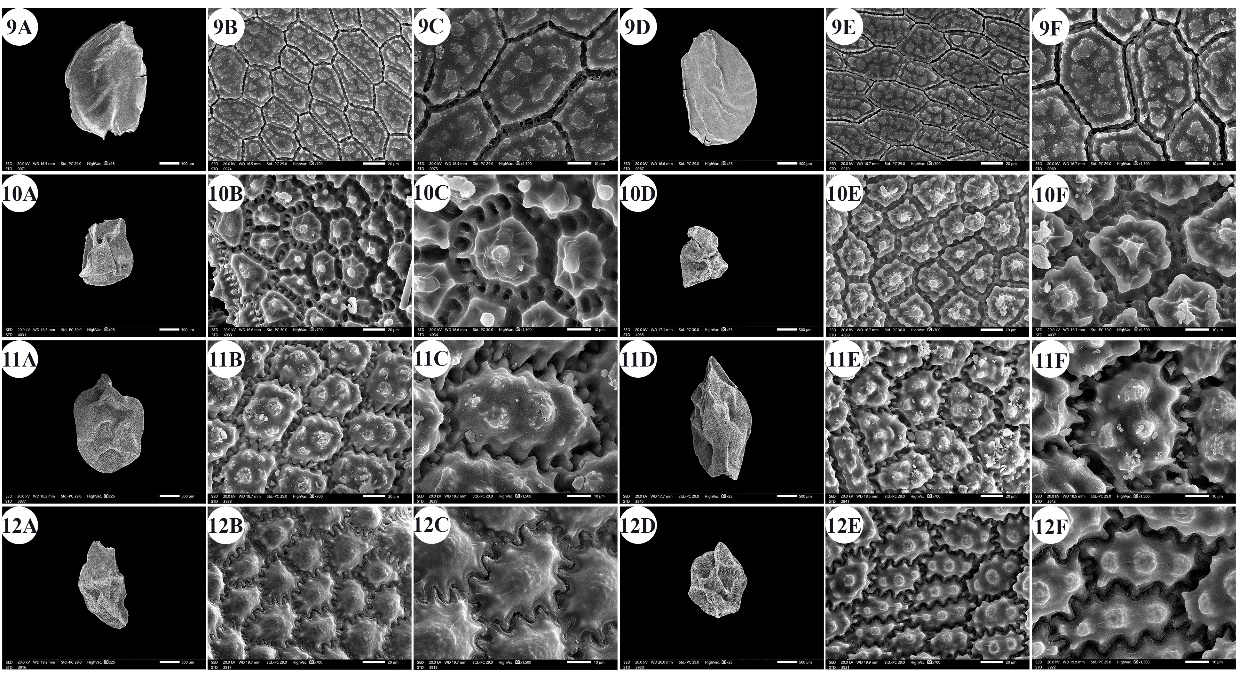
Fig. 3. A –F: scanning electron micrographs of Allium seeds. A –C: dorsal surface, D –F: ventral surface. 9 – A. desertorum, 10 – A. erdelii, 11 – A. kurrat, 12 – A. mareoticum. Scale bars: A, D = 500 µm; B, E = 20 µm; C, F = 10 µm.
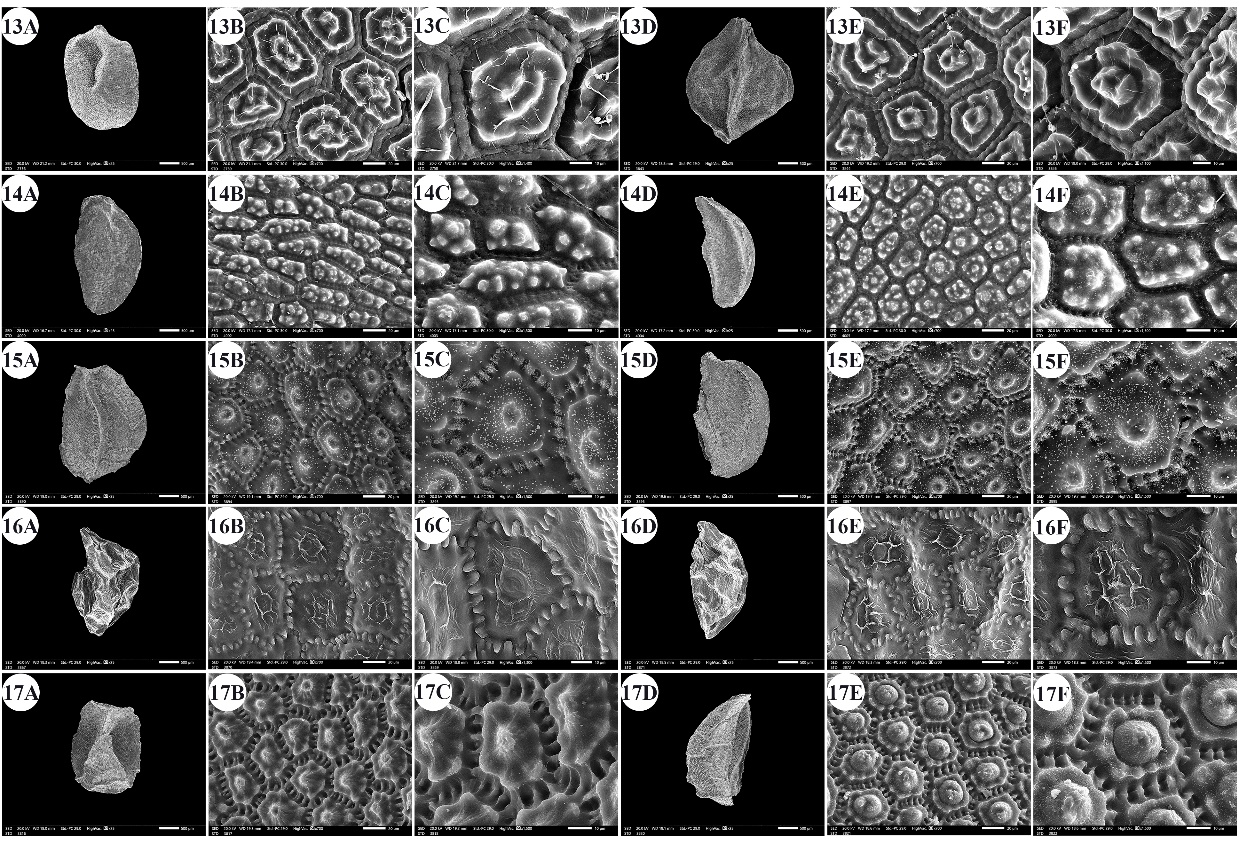
Fig. 4. A –F: scanning electron micrographs of Allium seeds. A –C: dorsal surface, D –F: ventral surface. 13 – A. neapolitanum, 14 – A. pallens, 15 – A. papillare, 16 – A. porrum, 17 – A. roseum subsp. tourneuxii. Scale bars: A, D = 500 µm; B, E = 20 µm; C, F = 10 µm.
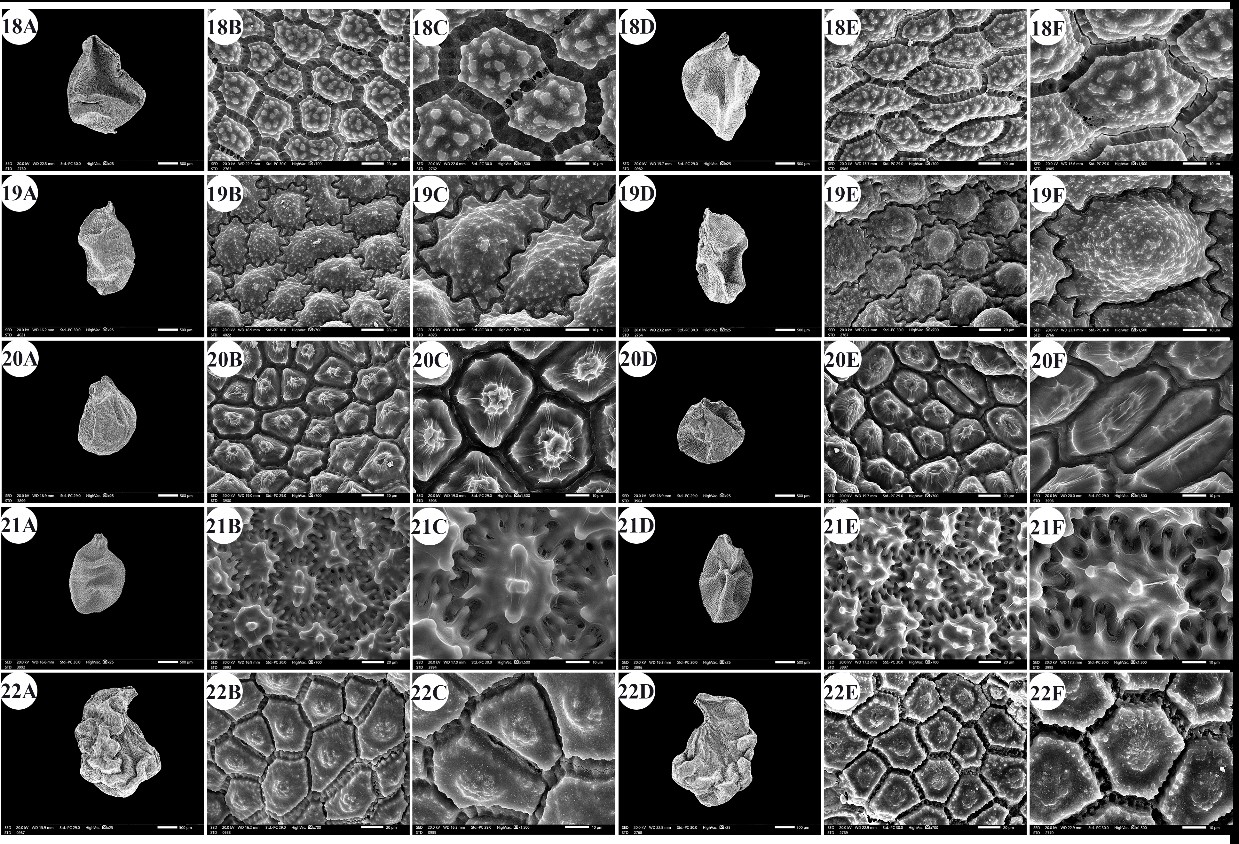
Fig. 5. A –F: scanning electron micrographs of Allium seeds. A –C: dorsal surface, D –F: ventral surface. 18 – A. sativum, 19 – A. sinaiticum, 20 – A. spathaceum, 21 – A. sphaerocephalon, 22 – A. trifoliatum. Scale bars: A, D = 500 µm; B, E = 20 µm; C, F = 10 µm.
The anticlinal wall (AW) may be straight, irregularly curved, or undulating with various forms of undulation elements, such as S-type, U-type, or Ω-type (Omega-type) (On-line Suppl. Tab. 5). The count of undulation elements per cell varied from 8 ( A. crameri) (Fig. 2: 7B, 7C) to 33 (as in A. pallens; Fig. 4: 13B, 13C). The length of the undulation elements fluctuates from 1.29 µm (as in A. artemisietorum; Fig. 1: 2B, 2C) to 14.35 µm (as in A. crameri) (Fig. 2: 7B, 7C), and the width ranges from 1.99 µm (as in A. trifoliatum; Fig. 5: 22E, 22F) to 11.20 µm (as in A. crameri; Fig. 2: 7B, 7C). The L/W ratio of the undulation elements ranged from 0.40 (as in A. spathaceum; Fig. 5: 20B, 20C) to 2.60 (as in A. barthianum; Fig. 1: 4B, 4C). The distance between each of the two undulation elements varied from 0.58 µm in A. spathaceum (Fig. 5: 20E, 20F) to 17.58 µm in A. crameri (Fig. 2: 7E, 7F) (On-line Suppl. Tab. 4). The cell boundaries were channeled in most taxa or raised in A. neapolitanum (Fig. 4: 13C, 13F) and A. papillare (Fig. 4: 15C, 15F). The relief of the intercellular space or cell boundary was scabrate ( A. artemisietorum; Fig. 1: 2C, 2F), A. erdelii, A. porrum L., A. roseum subsp. tourneuxii, A. pallens, A. papillare, A. spathaceum, and members of A. subg. Melanocrommyum), striate ( A. neapolitanum; Fig. 4: 13C, 13F), reticulate with a broad mesh of connecting threads ( A. cepa; Fig. 2: 6C, 6F, and A. sativum; Fig. 5: 18C, 18F), or with a narrow mesh of thin connecting threads in the remaining species. The curvature of the periclinal wall is generally convex, except for that of A. desertorum, which was flat (Fig. 3: 9C, 9F), and that of A. porrum, which was flat and concave toward the center (Fig. 4: 16C, 16F). All the studied taxa had verrucate periclinal walls, while verrucae were absent for A. artemisietorum (Fig. 1: 2C, 2F), A. papillare (Fig. 4: 15C, 15F), and A. porrum (Fig. 4: 16C, 16F). Allium artemisietorum and A. papillare have densely granulated periclinal walls (On-line Suppl. Tab. 5).
Data analysis
One-way ANOVA, which was utilized to test the variation between the dorsal and ventral seed surfaces, revealed a significant difference in the epidermal cell count for most taxa except A. subg. Cepa ( A. cepa) and A. subg. Melanocrommyum ( A. aschersonianum Barbey and A. crameri) showed a non-significant difference between the surfaces. The distance between two undulation elements showed non-significant variation for both surfaces of the studied taxa, except for A. artemisietorum and A. roseum subsp. tourneuxii. The highest number of traits showing significant differences between the two surfaces was recorded in A. erdelii; these traits included epidermal cell count per unit area, epidermal cell length, width, the L/W ratio, undulation element width, and the L/W ratio. In contrast, A. cepa demonstrated insignificant variation between the two surfaces except in the epidermal cell area (On-line Suppl. Tab. 4).
One-way ANOVA was used to assess the significant differences in the variation among taxa. For A. sect. Allium, the studied traits were significant for each pair of taxa; however, the epidermal cell L/W ratio (dorsal surface) was insignificant for all taxa. The two species of A. sect. Codonoprasum are very different in terms of the number of epidermal cells per unit area, the length of epidermal cells (dorsal surface), the number of undulation elements per cell, the width of undulation elements, and the L/W ratio of undulation elements. There were also non-significant differences in the following traits among the members of A. sect. Molium: epidermal cell length, epidermal cell width (ventral surface), epidermal cell L/W ratio, epidermal cell area (ventral surface), undulation element length, and distance between two undulation elements (ventral surface). The other traits exhibited significant variation between each pair of species. The two species comprising A. sect. Melanocrommyum were significantly different in the following traits: seed length and area, undulation element length (dorsal surface), undulation element width, undulation element L/W ratio (ventral surface), and the distance between two undulation elements (dorsal surface) (On-line Suppl. Tab. 4).
PCA explained 99.96% of the total variation in the first two components (Fig. 6, On-line Suppl. Tab. 6).
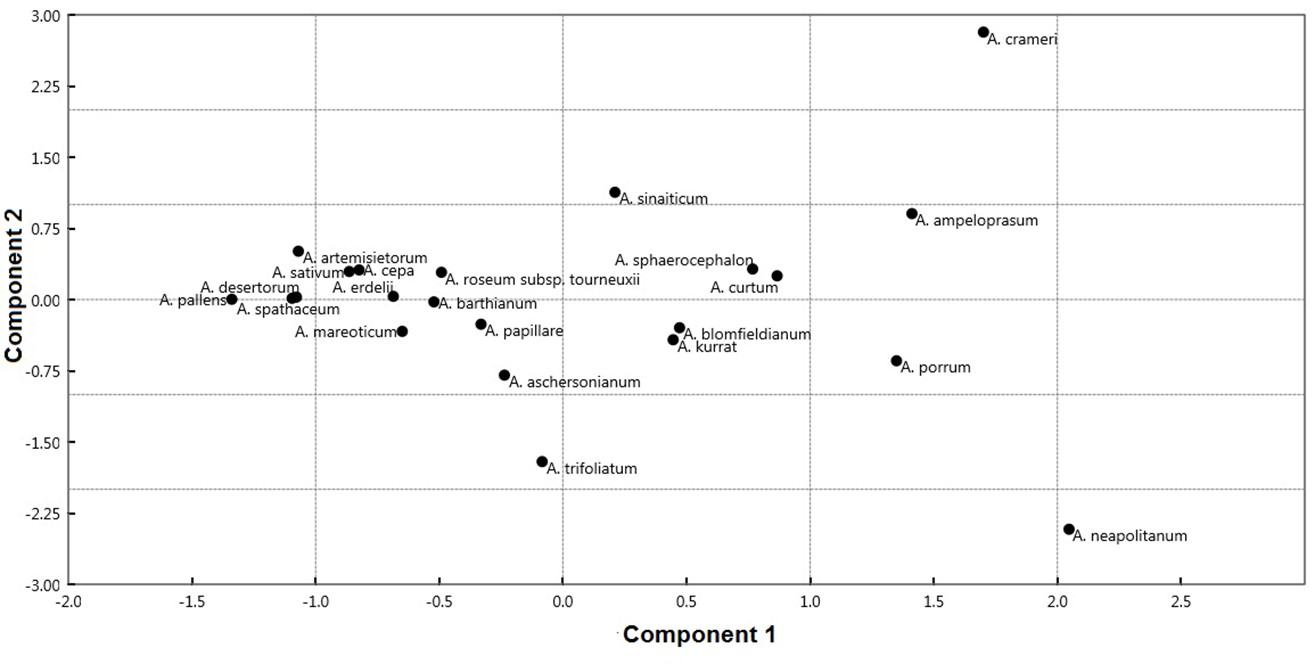
Fig. 6. Scatterplot of the first two axes from principal component analysis (PCA) of the 22 Allium taxa based on analyzed seed morphological traits.
The first component exhibited 87.90% of the total variation. This component separates some species of A. sect. Allium ( A. ampeloprasum, A. curtum Boiss. & Gaill., A. kurrat Schweinf. ex K.Krause, A. porrum, A. sinaiticum, and A. sphaerocephalon L.); A. crameri of A. sect. Melanocrommym; and A. sect. Molium ( A. blomfieldianum and A. neapolitanum) in the right side from the other taxa of A. sect. Allium ( A. artemisietorum, A. barthianum, A. mareoticum, and A. sativum); A. sect. Briseis ( A. spathaceum); A. sect. Cepa ( A. cepa); A. sect. Codonoprasum ( A. desertorum and A. pallens); A. aschersonianum of A. sect. Melanocrommym; and A. erdelii, A. papillare, A. roseum subsp. tourneuxii; and A. trifoliatum of A. sect. Molium. Species on the right side were characterized by larger epidermal cell area ranging from 2013.37 to 3821.03 mm2, whereas the left side taxa have smaller values ranging from 880.10 to 1557.04 mm2. The contribution of the individual measured traits to the first and second components are illustrated in On-line Suppl. Tab. 7. The discriminant analysis results showed that seven sections of Allium were successfully identified. Twenty seed traits represent the most important variables in discriminating the studied taxa (Fig. 7A and 7B). These characteristics are used to perform an artificial key for 22 Allium taxa.
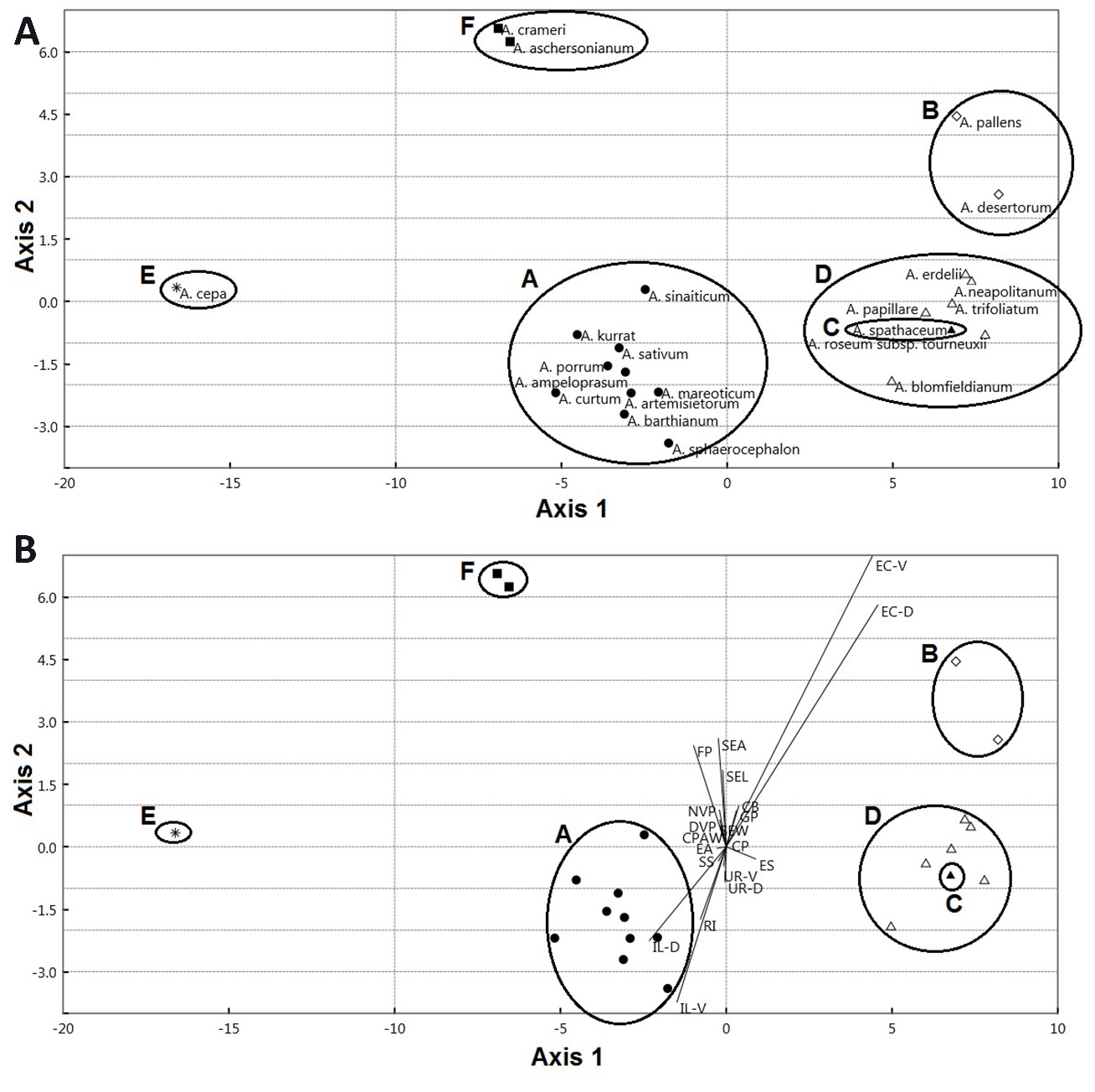
Fig. 7. Scatterplot (A) and biplot (B) of the first two axes from Discriminant Analysis for the sectional classification of Allium. A – A. sect. Allium, B – A. sect. Codonoprasum, C – A. sect. Briseis, D – A. sect. Molium, E – A. sect. Cepa, F – A. sect. Melanocrommyum. Abbreviations: SEL – seed length, SEW – seed width, SEA – seed area, EC-D – epidermal cell count/unit area (dorsal), EC-V – epidermal cell count/unit area (ventral), IL-D – intercellular space length (dorsal), IL-V – intercellular space length (ventral), UR-D – undulation element L/W ratio (dorsal), UR-V – undulation element L/W ratio (ventral), SS – seed shape, ES – epidermal cell shape, EA – epidermal cell arrangement, CPAW – curvature pattern of the anticlinal wall, CB – cell boundary, RI – relief of intercellular space (cell boundary), CP – curvature of the periclinal wall (PW), FP – fine relief of the PW, DVP – diameter of verrucae on PW, NVP – number of verruca on PW, GP – P/A of granules on PW.
Artificial key of Allium taxa based on the most significant seed quantitative traits and qualitative macro- and micromorphological traits
1.a. The epidermal cell arrangement is a jigsaw-like pattern………………………………… 2
b. The epidermal cell arrangement is a side-by-side pattern ……………..………….…… 12
2.a. The undulation pattern of the anticlinal wall is Ω-type …………………………………. 3
b. The undulation pattern of the anticlinal wall is otherwise ……...…………………....….. 5
3.a. The seed length is 3.4–3.7 mm, and the seed area is 6.08–7.14 mm2……..….. A. crameri
b. The seed length is 1.95–2.76 mm, and the seed area is 1.81–4.81 mm2 ……….......….... 4
4.a. The intercellular space length is 0.23–0.93 µm, and the periclinal wall is sparsely granulated …………………………………………………...………….… A. aschersonianum
b. The intercellular space length is 2.04–5.99 µm, and the periclinal wall has no granules…………………………………………………………………….….….… A. curtum
5.a. The undulation pattern of the anticlinal wall is S-type undulation ….... A. artemisietorum
b. The undulation pattern of the anticlinal wall is U-type undulation ………………….….. 6
6.a. Polygonal epidermal cells, and the curvature of the periclinal wall is flat and centrally concave without verrucae ……………………………..…………….…...………..... A. porrum
b. Orbicular, widely elliptic, or elliptic epidermal cells, and the curvature of the periclinal wall is convex with verrucae …………………………………….......……..…..……………… 7
7.a. Ovate seed shape, the seed length is 3.4–3.58 mm, the seed width is 1.99–2.3 mm, and the seed area is 4.84–5.63 mm2 ........................................................................... A. ampeloprasum
b. Widely elliptic or elliptic seed shape: the seed length is 1.7–3.0 mm, the seed width is 0.88–1.83 mm, and the seed area is 1.10–3.58 mm2 ………………………………………… 8
8.a. The seed length is 2.49–3.0 mm, the seed width is 1.55–1.83 mm, and the seed area is 3.16–3.58 mm2 ………………………………….………………………………….... A. kurrat
b. The seed length is 1.76–2.10 mm, the seed width is 0.88–1.44 mm, and the seed area is 1.10–2.32 mm2…………………………………………………………………………..…..... 9
9.a. The epidermal cell count per unit area is 30–31 cells…………...……...... A.mareoticum
b. The epidermal cell count per unit area is 17–27 cells …………………...…...…..……. 10
10.a. The number of periclinal wall verrucae is ≤ 15 …............................ A.sphaerocephalon
b. The number of periclinal wall verrucae is ˃ 15 ……..…………………...…..……..… 11
11.a. The intercellular space length is 3.4–8.92 µm, and the undulation element L/W ratio is 1.1–2.60 ……………………………………………...………………………... A.barthianum
b. The intercellular space length is 1.24–2.05 µm, and the undulation element L/W ratio is 0.8–0.99 …………………………………………...…………………………..... A.sinaiticum
12.a. The anticlinal wall is straight to irregularly curved ……….……….……….…..……. 13
b. The anticlinal wall is undulated …………………………………………...…………. 15
13.a. The relief of the intercellular space is a narrow mesh of thin connecting threads, the epidermal cell count per unit area is 33–35 cells, the intercellular space length is 1.75–2.78 µm, and the periclinal wall is non-granulated …………….……...….……………… A. desertorum
b. The relief of the intercellular space is reticulate with a broad mesh of connecting threads, the epidermal cell count per unit area is 21–28 cells, the intercellular space length is 5.94–13.4 µm, and the periclinal wall is granulated ………………….……………..……... 14
14.a. The seed area is 4.2–6.09 mm2…………………………………………………... A. cepa
b. The seed area is 3.2–3.3 mm2………....…...……………………….………… A. sativum
15.a. The undulation pattern of the anticlinal wall is U-type …………….……....………… 16
b. The undulation pattern of the anticlinal wall is S-type …………...…...………...……. 18
16.a. Raised cell boundary, and the periclinal wall is without verrucae ....………. A. papillare
b. Channeled cell boundary, and verrucate periclinal wall ……………………………… 17
17.a. The seed length is 1.56–1.64 mm, the seed area is 1.25–1.75 mm2, the epidermal cell count per unit area is 32–35 cells, and the periclinal wall has one large central dome ………………………………………………………………………………………... A. erdelii
b. The seed length is 2.24–2.45 mm, the seed area is 2.62–3.37 mm2, the epidermal cell count per unit area is 23–24 cells, and the periclinal wall has one small central dome ...................................................................................................... A. roseum subsp. tourneuxii
18 a. The epidermal cells are close to each other (intercellular space is absent) ………….... 19
b. The epidermal cells are distant from each other (intercellular space is present) …....... 20
19.a. Raised striate relief of cell boundary, the seed length is 2.61–3.16 mm, seed width is 1.77–2.54 mm, seed area is 3.84–5.36 mm2, and the epidermal cell count per unit area is 12–14 cells ……………..........................................................................................…. A. neapolitanum
b. Channeled scabrate relief of cell boundary, the seed length is 1.84–1.99 mm, seed width is 1.42–1.48 mm, seed area is 2.08–2.15 mm2, and the epidermal cell count per unit area is 33–34 cells .................................................................................................…… A. spathaceum
20.a. The seed length is 1.54–1.77 mm, and the seed area is 1.48–2.03 mm2………………………………………………………………………... A.blomfieldianum
b. The seed length is 2.56–3.16 mm, and the seed area is 2.72–4.14 mm2……………... 21
21.a. Ovate seed shape, the epidermal cell count per unit area is 43–44 cells, the undulation element L/W ratio is 0.72–0.95, and the periclinal wall has many small domes …………………………………………….…………………………………….…. A.pallens
b. Elliptic seed shape, the epidermal cell count per unit area is 27–28 cells, the undulation element L/W ratio is 1.23–1.47, and the periclinal wall has one small central dome …………………..….……….......................................................................……. A. trifoliatum
Discussion
A high degree of seed morphological diversity has been observed in the genus Allium, as scanning electron microscopy (SEM) can clearly illustrate the details of the seed testa (Neshati and Fritsch 2009, Bednorz et al. 2011, Celep et al. 2012, Veiskarami et al. 2018, Baasanmunkh et al. 2020, Khorasani et al. 2020, Yusupov et al. 2022). Yusupov et al. (2022) noted that Kruse (1988) reported the inter- and intraspecific variation of Allium, indicating that some seed testa traits are section- and species specific. Our results follow the latter seed morphological studies, indicating the most informative diagnostic traits for distinguishing Allium taxa at the species level. Discriminant analysis is a very informative method for evaluating the utility and importance of the studied morphological traits by determining which traits were most useful for maximizing differentiation between the studied groups (Temunović et al. 2024). These traits are summarized as seed length, width, area, and shape, epidermal cell count/unit area, intercellular space length, undulation element L/W ratio, epidermal cell shape and arrangement, curvature pattern of the anticlinal wall, cell boundary, relief of intercellular space, curvature and fine relief of the periclinal wall, diameter and number of verrucae on PW, and the presence/absence of granules on the periclinal wall.
Fritsch et al. (2006) recognized that S-like, U-like, and Ω-like are the most common anticlinal wall undulation modes and, the present study revealed the same types of undulations. A limited amount of variation in the different parts of a single seed surface was observed also by Fritsch et al. (2006); this variation was restricted to the presence or absence of granules on the periclinal wall of epidermal cells and their size. This study reported for the first time a detailed comparative investigation of the quantitative traits of dorsal and ventral seed surfaces. The variation between the dorsal and ventral seed surfaces exhibited a conspicuous difference for 19 out of 22 species in terms of epidermal cell count/unit area. Additionally, there was significant variation in undulation element width (seven species); the epidermal cell L/W ratio; the area; the count of undulation elements per cell (six species); undulation element length and the undulation element L/W ratio (five species); epidermal cell length, width, and intercellular space length (four species); and the distance between two undulation elements (two species).
Fritsch (2001) described the seed sizes of the different subgenera but reported numerous exceptions: A. subg. Allium is roughly light-grained; A. subg. Melanocrommyum is heavy-grained, whereas A. subg. Amerallium ranges from small to large-grained. In the present study, the seed size of A. subg. Allium is 1.61–3.59 × 0.89–2.30 mm, A. subg. Amerallium is 1.54–3.47 × 1.21–2.55 mm, A. subg. Cepa is 2.54–3.26 × 1.98–2.55 mm, and A. subg. Melanocrommyum is 1.95–3.70 × 1.34–2.40 mm.
Our observations revealed that the epidermal cell shape was not different in A. subg. Amerallium to that in other subgenera. This study agrees with the findings of Yusupov et al. (2022): the seeds are broad to narrowly ovoid, and the anticlinal wall undulation type is mostly straight to arched. Species of A. sect. Molium, on the other hand, are arched to the S-type. Yusupov et al. (2022) mentioned that Kruse (1988) described A. sect. Molium as having wide, depressed channel-like anticlinal walls and prominent verrucose structures on their periclinal walls. However, the present study revealed that two out of five species had raised walls: A. neapolitanum and A. papillare. Celep et al. (2012) measured the seed size of A. subg. Amerallium to be in the range of 2.5–3.90 × 1.0–1.86 mm, and the seed L/W is 2.10–2.64. Our results differ slightly for the seed minimum length, which is 1.54–3.47 × 1.21–2.54 mm, 1.01–1.99. The smallest seed length in the subgenus was detected for A. blomfieldianum (1.54–1.77 × 1.21–1.59 mm), and the widest seed was A. neapolitanum (2.61–3.16 × 1.77–2.54 mm). They also reported that Meikle (1985) performed similar measurements for A. neapolitanum. The latter researchers also reported that A. sect. Molium shares a polyhedral cell with a striate or rugulate intercellular region covered by many small verrucae coalescing to a marginal ledge and with mostly one verruca in the center of the periclinal wall. This finding is compatible with our description, as the relief of the cell boundary was striate in A. neapolitanum, scabrate in A. erdelii and A. papillare, or had a narrow mesh of thin connecting threads in A. blomfieldianum and A. trifoliatum.
According to Fritsch et al. (2006), the epidermal cells of A. subg. Melanocrommyum demonstrated less variation than the other subgenera. The latter authors reported similar results for members of A. sect. Melanocrommyum because they had anticlinal walls that were Ω-like undulations and convex periclinal walls with verrucate sculptures. Some species have agranulous periclinal walls, while others, such as A. aschersonianum, do not have any granules. In addition, Celep et al . (2012) revealed that periclinal walls contain several or more verrucae.
The multivariate analyses revealed that members of A. subg. Allium have a wide range of variation characterizing the subgenus. This finding is congruent with the finding of Fritsch (2001) that A. subg. Allium is the most diverse and rich subgenus of the genus Allium. Minor dissimilarities in epidermal cell arrangement were observed between the members. Although the seed shape and the anticlinal and periclinal walls of the members of A. sect. Allium were variously undulated, these results are consistent with those of Neshati and Fritsch (2009), Veiskarami et al. (2018), and Baasanmunkh et al. (2020). Bednorz et al. (2011) revealed the presence of unusually raised anticlinal walls in some species of A. sect. Allium. In contrast, a channeled pattern was observed for the investigated members of A. sect. Allium.
Allium ampeloprasum is a polymorphic species complex sometimes treated as a wild leek without considering any subspecies, and sometimes as a cultivated leek with subspecies or varieties (Dey and Khaled 2013, Guenaoui et al. 2013). In the present study, A. ampleoprasum was treated as a wild accession, while A. kurrat and A. porrum were recorded as cultivated culinary species; this follows Boulos's (2009) work on the Egyptian flora. Allium kurrat and A. porrum have not been observed naturalizing in other habitats far from their cultivation fields (Mifsud and Mifsud 2018). Allium ampeloprasum, A. kurrat, and A. porrum exhibited significant differences between pairs of species in terms of seed length, L/W ratio, area, epidermal cell count per unit area, intercellular space length (dorsal surface), undulation element length and width, the L/W ratio, and the distance between two undulation elements (dorsal surface). Moreover, there were non-significant differences in the following traits: seed width, epidermal cell size parameters, intercellular space length (ventral surface), count of undulation elements per cell, and distance between two undulation elements (ventral surface). The epidermal cells of the three species exhibit a jigsaw-like arrangement, and the anticlinal walls exhibit a U-type undulation mode with channeled cell boundaries. Allium ampeloprasum and A. kurrat share many characteristics, but the latter species has more verrucae (˃15) on the periclinal walls. Allium porrum was distinguished from the other two species by its variably polygonal epidermal cells with 5 to 7 edges; the periclinal wall is peripherally flat, centrally concave, and wrinkled but lacking verrucae. The description of A. porrum is well matched with that of Lin and Tan (2017).
Conclusions
The present study carried out a detailed seed macro- and micromorphological investigation of 22 Allium taxa, including 11 species that are described for the first time; six of them are endemic species: A. barthianum, A. blomfieldianum, A. mareoticum, A. trifoliatum (Mediterranean endemic species), A. crameri and A. mareoticum (endemic to Egypt). Our results highlighted the most informative diagnostic traits for distinguishing Allium taxa. These traits are summarized as seed length, width, area, and shape, epidermal cell count/unit area, intercellular space length, undulation element L/W ratio, epidermal cell shape and arrangement, curvature pattern of the anticlinal wall, cell boundary, relief of intercellular space, curvature and fine relief of the periclinal wall, diameter and number of verrucae on PW, and the presence/absence of granules on the periclinal wall. The count of undulation elements per cell, the undulation element length and width, the L/W ratio, and the distance between two adjacent undulation elements were measured for the first time in this study. This study reported for the first time a detailed comparative investigation of the quantitative traits of dorsal and ventral seed surfaces. Dorsal and ventral seed surface variations exhibited conspicuous differences in most species. Multivariate analysis revealed that members of A. subg. Allium have a wide range of variation characterizing the subgenus. Allium subg. Allium is the most diverse and rich subgenus of the genus. Allium ampleoprasum is treated as a wild accession, while A. kurrat and A. porrum are recorded as cultivated culinary species in the Egyptian flora because they have not been observed naturalizing in other habitats far from their cultivation fields. Allium ampeloprasum, A. kurrat, and A. porrum exhibited significant differences between pairs of species; as a result, they are treated herein as distinct species. Additional regional studies should be conducted on the seeds of native, endemic, and near-endemic Allium taxa for the better understanding of the variation within the genus. Finally, Allium merits being subjected to integrated investigations through different approaches to resolve the taxonomic problems of the genus.
