Introduction
Individuals' healthy nutritional needs and demands for functional foods are increasing day by day. Fermented dairy products can be considered as essential functional foods due to their unique characteristics, ingredients, and nutritional value (García-Burgos et al., 2020). Kefir is a traditional fermented dairy product containing over 50 species of potential probiotic microorganisms. It contains lactic acid bacteria (Lactobacillus, Lactococcus, Leuconostoc, and Streptococcus), yeasts ( Kluyveromyces, Candida, Saccharomyces, and Pichia), and acetic acid bacteria (Magalhães et al., 2011; Dong-Hyeon et al., 2019). Various beneficial effects of kefir and its microbiota have been reported, such as cholesterol-lowering, blood pressure regulation, antimicrobial, antioxidant, anticarcinogenic, anti-inflammatory, wound healing, immunomodulatory, anti-allergenic, anti-diabetic, and anti-obesity properties on health (Dong-Hyeon et al., 2019). In the literature, there are many studies in which fruits, ingredients, aromas, prebiotics, phenols, selenium, B 12, folate, dietary fibre, inulin, maltodextrin, and some plant extracts are included in order to enrich kefir. Dietary fibre and phenols are the essential ingredients to develop health benefits in functional foods (Goncu et al., 2017; Dinc et al., 2018).
Coffee bean is a raw material that contains high amount of dietary compounds and has been used as raw material in coffee and chocolate industry. Coffea arabica (Arabica) and Coffea canephora (Robusta) are the most common coffee varieties in the world. Coffee silverskin (CSS) is a by-product that can be obtained from the coffee beans after the roasting process and consists of 1-2 % of the processed batch. It is poor in fat and carbohydrates while having high content (60 %) of soluble dietary fibre. Thus it is accepted as a significant source of functional food components. Chemically, CSS consists of cellulose, hemicellulose, proteins, fats, polyphenols, minerals, and various compounds such as melanoidins formed due to the Maillard reaction that occurs as a result of heating during the coffee roasting process. CSS is a complex carbohydrate composed of a high amount of dietary fibre (56-62 %) (Bessada et al., 2018). Its antioxidant properties are based on the presence of chlorogenic acid in the dietary fibre matrix. CSS is an ingredient with antioxidant properties and can be used as a new functional food supplement, as it supports the growth of Bifidobacteria as a prebiotic (Borrelli et al., 2004; Esquivel and Jiménez, 2012).
In this study, CSS materials that belong to 2 coffee varieties (Arabica and Robusta) were used in kefir production as a food supplement. Effects of CSS on kefir production were investigated via physicochemical properties, viable microbial counts, and in-vitro viability of the kefir culture under simulated in vitro gastrointestinal conditions during 28 days of storage.
Material and methods
Materials
A freeze-dried kefir culture, Danem Kefir ® (Danem Kefir Co., Isparta, Turkey) consisting of L. kefiri, L. kefiranofaciens subsp. Kefiranofaciens, L. kefiranofaciens subsp. Kefirgranum, L. parakefiri, L. acidophilus, L. casei, L. reuteri, L. bulgaricus, L. helveticus, L. fermentum, Leuconostoc mesentereoides, Lactococcus lactis, Streptococcus thermophilus, Bifidobacterium bifidum, Acetobacter pasteurianus, Kluyveromyces marxianus, Saccharomyces cerevisiae, and Kluyveromyces lactis), ultra-high temperature (UHT) bovine milk with 3.0 % fat (Sutas Dairy Co., Bursa, Turkey), and coffee CSS were used in the kefir productions. The CSS varieties ( Coffee arabica and Coffee robusta) were supplied from the coffee bean roasting plant (Bayramefendi Osmanlı Kahvecisi Co., Istanbul, Turkey). The CSS was grounded by a coffee grinder (Delonghi Kg 49, Italy) and sieved (60 mm) before using in the kefir production.
Methods
Kefir culture and coffee silverskin (CSS) preparation
Before kefir production, commercial freeze-dried kefir culture Danem Kefir ® (0.5 g) was initially activated according to the manufacturer's instruction. For this reason, the kefir culture was added to 1 L UHT milk and incubated at 25 ºC for 24 h in a glass jar. The activated kefir culture was inoculated (3.0 %) into sterile whole UHT milk (200 mL, 9.71 % total dry matter, 3.00 % fat). Kefir samples were previously prepared by CSS incorporation of Arabica and Robusta varieties in concentrations of 0 (Control), 0.5, 0.75 and 1.0 %. The kefir fermentation was performed at 25 ºC for 24 h. After the incubation, the final kefir products were stored at +4 ºC and analysed at weekly periods during the 28 days of storage. For each measurement, duplicate analyses were carried out.
Physicochemical characterization
CSS and kefir samples were evaluated physiochemically for titration acidity (lactic acid equivalent; AOAC, 2005), pH (S220-K Seven Compact, Mettler Toledo, Milano, Italy; AOAC, 2005), total dry matter (AOAC, 2005) and total dietary fibre (AOAC, 1995) before and after kefir fermentation (Day 0 and 1) and during the storage period (Day 7, 14, 21 and 28).
Microbial viability
10 mL of kefir samples were homogenized with 90 mL of 0.1 % peptone water. Serial dilutions in peptone water were prepared, and transferred onto specific agar media. TAMB (Total Aerobic Mesophilic Bacteria), yeasts, coliforms, and E. coli in the samples were enumerated on Plate Count Agar (PCA; CM0325, Oxoid), Rose Bengal Chloramphenicol Agar (RBC; CM0549, Oxoid), Violet Red Bile Agar (VRB; CM0968, Oxoid) and Chromocult TBX Agar (CM0945, Oxoid), respectively. For lactobacilli and lactococci enumeration, de Man Rogosa Sharpe Agar (MRS; CM0361, Oxoid) and M17 Agar (CM0785, Oxoid) were used and supplemented with 0.1 % cycloheximide (SR0222, Oxoid) to prevent yeast growth, respectively. The incubation periods with PCA, M17 agar, and MRS agar were at 30°C for 48 h, RBC agar at 22-25 °C for 5 days, VRB agar at 37 °C for 24 h and TBX agar 44 °C for 24 h under aerobic conditions (Irigoyen et al., 2005). The typical colonies were counted after incubation, and the results were expressed as log cfu/mL.
Survival of kefir culture in simulated in vitro GI conditions
The viability of kefir microbiota under the simulated gastrointestinal conditions was determined using in vitro digestive enzymatic extraction method with slight modifications (Nazzaro et al., 2009). For the simulation of gastric digestion, the simulated gastric media (GM) consisted of 1 g/L peptone, 8 g/L NaCl, 0.2 g/L KCl, 0.24 g/L KH 2PO4, 1.44 g/L Na 2HPO 4 with 3 g/L pepsin (P7000, Sigma-Aldrich) were prepared, and its pH was adjusted to 2.0 with 1 M HCl (Merck, Germany). For the simulated intestinal digestion, the simulated intestinal media (IM) consisted of 1 g/L peptone, 8 g/L NaCl, 0.2 g/L KCl, 0.24 g/L KH 2PO4, 1.44 g/L Na 2HPO 4 with pancreatin (1 g/L; 1.07130.1000 Merck, Germany) and bile salts (4.5 g/L; B8756, Sigma-Aldrich, USA) in 0.1 M NaHCO 3 were prepared. Both solutions were filtered through a sterile syringe filter (0.22 µm, Millipore, Germany). 1 mL kefir sample was transferred into 9 mL of GM. The mixture was incubated at 37 °C for 60 min. Then, the pH of GM was adjusted to 7.2 with 1 M NaHCO 3 to stop the enzymatic reaction. 2 mL simulated GM was suspended in 18 mL of IM and then incubated under aerobic conditions at 37 °C for 2 h. The number of viable cells was determined after 2 h incubation by plating on MRS agar for lactobacilli (at 30 °C for 48 h), M17 agar for lactococci (at 30 °C for 48 h), and Rose Bengal Chloramphenicol Agar for yeasts (at 22-25 °C for 5 d) after preparation of the serial dilutions. The typical colonies were counted after incubation, and the results were expressed as log cfu/mL.
Statistical analysis
Results were presented as the mean ± standard deviation (SD) of 2 measurements. The data was performed using the SPSS statistical software (SPSS 16.0, Chicago, IL). The level of statistical significance among the means was evaluated with analysis of variance (ANOVA) by considering probability level of 5% ( p<0.05).
Results and discussion
The pH and total acidity
Carbohydrates are converted to some organic acids and their metabolites by homo- and heterofermentative lactic acid bacteria and the yeasts. Thus, variation in pH value is accepted as an indicator of the microbial activity level. The mean pH and total acidity values of sterilized whole milk before inoculation with kefir culture were 6.46 and 0.15 %, respectively. As seen from Figure 1, the reduction in pH (from 5.39-6.47 to 4.33-4.41) was observed in the samples fermented for 1 day, whereas the pH remained constant (4.32-4.41) for longer periods of storage. At the beginning of fermentation, total acidity values of kefir samples were between 0.15-0.40 % and changed between 0.67-1.03 % at the end of the storage period depending on the microbiological activity (Figure 1). Generally, the total acidity values remained unchanged during the storage period for all kefir samples (p>0.05). As expected, the changes observed in the pH and acidity were probably related to a decrease in LAB counts that are responsible for the production of fermentation metabolites such as organic acids, ethanol and CO 2. The production rate of the fermentation metabolites tends to reduce in the presence of yeasts available in the kefir beverage. Similar relation was also observed by some previous researchers (Guzel-Seydim et al., 2000; Irigoyen et al., 2005; Kok-Tas et al., 2013; Gul et al., 2015; Barukčić et al., 2017; Goncu et al., 2017; Kabakci et al., 2020). pH was significantly affected by the CSS supplementation (p<0.05), whereas it was not observed significant changes statistically by the gradual elevation of CSS supplementation (p>0.05).
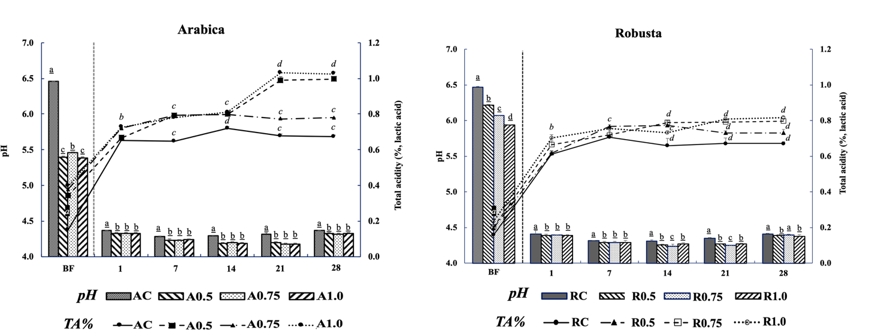
Figure 1. Changes in pH and total acidity values of kefir samples during storage
(A: Arabica; R: Robusta; C: Control; TA: Total Acidity; BF: Before Fermentation); The values are given in the graphs are mean value± SD. The mean values represented by bar graphs with different letters are significantly different, statistically (p≤0.05)
Supplementation of CSS for 0.5 % was found enough to develop a metabolic activity for the kefir starter cultures. On the other hand, the domination of pH despite the elevated CSS levels (1.0 and 1.5 %) was probably due to the buffer capacity of the milk proteins available in kefir. Increase in acidity and therefore decrease in pH are the expected ambient changes seen in the kefir production; however, a tendency of decrease in pH can be related to buffering capacity of high content of milk proteins despite the decomposition of lactose and additional prebiotic effect of CSS (Balabanova and Panayotov, 2011).
According to the Codex standard (2003), a distinctive characteristic of kefir production is titratable acidity in addition to microbiological criteria and defined as min 0.6 % (w/w, lactic acid) in the product. The titratable acidity values of the kefir samples produced in our research were consistent with this Standard (2003) and the previous kefir studies (Guzel-Seydim et al., 2000; Barukčić et al., 2017; Goncu et al., 2017; Hikmetoglu et al., 2020; Kabakci et al., 2020). When compared to the control samples, supplementation of CSS caused approximately 0.7 % (as lactic acid) acidity after the 7 days of storage. Effects of prebiotic supplementation on the total acidity and the stimulation of LAB have also been observed by the previous researchers. It was found that prebiotic effect of CSS (Iriondo-DeHond et al., 2019) caused higher or similar acidity levels in the kefir samples in our study (Guzel-Seydim et al., 2000; Barukčić et al., 2017; Goncu et al., 2017; Hikmetoglu et al., 2020; Kabakci et al., 2020).
Total dry matter and dietary fibre
Before inoculation with kefir culture, the total dry matter and dietary fibre contents of CSS (Arabica and Robusta) were 94.36-95.24 % and 60.70-54.90 %, respectively. The dry matter content of kefir samples slightly changed from 9.71-10.06 % to 10.87-11.54 %, and the control samples had the lowest dry matter content because they were not supplemented by CSS (Figure 2).
The changes observed in the total dry matter contents slightly increased depending on the gradual elevation of CSS (0.5, 0.75, and 1.0 %) and were not significant, statistically (p>0.05). Similarly, the kefir products that were supplemented with the apple and lemon fibres (Goncu et al., 2017) and the supplements (Irigoyen et al., 2005; Gul et al., 2015), caused to slight increases in the total dry matters. The changes determined in the dry matter content were also reflected in the chemical constituents of the kefir that was produced by cow and buffalo milk. The microbiological and chemical changes occurred in the kefir, but the fermentation process did not cause significant changes in the total dry matter content. Only slight differences in the minor constituents and proteins were related to reducing of water content due to evaporation (Tomar et al., 2020).
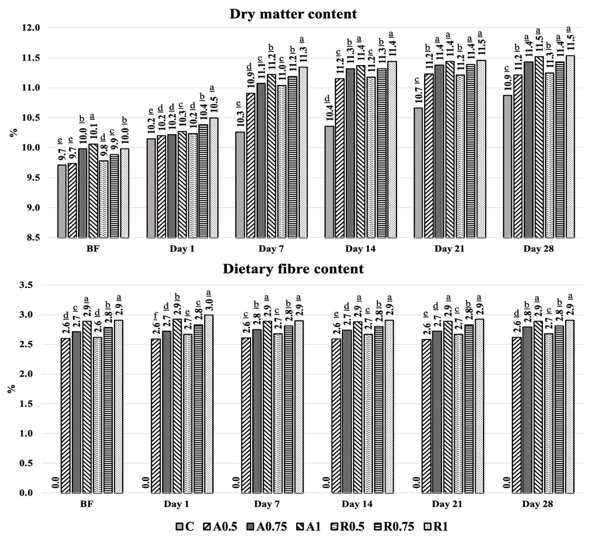
Figure 2. Changes in the total dry matter and dietary fibre contents of kefir samples during fermentation and storage
(A: Arabica; R: Robusta; C: Control); The values are given in the graphs are mean value± SD. The mean values represented by bar graphs with different letters are significantly different, statistically (p≤0.05).
Dietary fibre is the main constituent of CSS and responsible for its prebiotic characteristic. CSS consists of 45-80 % dietary fibre while the soluble content of the dietary fibre in CSS is 10-16 % (Borrelli et al., 2004; Ballesteros et al., 2014; Iriondo-DeHond et al., 2019). As expected, the total dietary fibre content in kefir samples (2.62-2.91%) was significantly higher than in the control samples (Figure 2). In general, depending on the supplementation ratio and variety of CSS used, total dietary fibre content increased in the kefir samples after CSS supplementation. However no significant changes in total dietary fibre were observed during storage period, statistically ( p>0.05).
Changes in microbial dynamics of kefir samples
Kefir samples were evaluated in terms of TAMB, coliform bacteria and E. coli loads in addition to lactobacilli, lactococci, and yeast counts. During 28 days of storage at 4 ºC, E. coli and coliform bacteria were not detected in any of the examined samples. When the gradual elevation of CSS content on TAMB counts were compared, the Arabica-1.0 % (12.93 log cfu/mL) and Robusta-1.0 % (11.49 log cfu/mL) samples had the highest TAMB counts (p<0.05). We concluded that CSS showed more significant effect than the control samples on the TAMB counts (9.04 and 10.64 log cfu/mL), respectively.
Previous studies showed that 3 groups of microorganisms co-exist in the kefir grains: the LAB group that including Lactobacillus and Lactococcus, acetic acid bacteria, and yeasts (Magalhães et al., 2011). In our research, lactobacilli and lactococci counts increased during the fermentation period within 24 h. At the end of 28 days of storage at +4 °C, the kefir samples enriched with Arabica and Robusta varieties of CSS (0.75%) reflected the highest lactobacilli (8.34 and 8.28 log cfu/mL) and lactococci (8.39 and 8.36 log cfu/mL) counts as given in Figure 3 and 4, respectively. Whereas, lactobacilli, and lactococci counts of the control samples were lower than the other samples and changed between 7.45-7.65 and 7.83-8.02 log cfu/mL at the end of storage.
According to the Codex Alimentarius standard (2003), kefir should contain a minimum 7 log cfu/g count of microorganism and 4 log cfu/g count of yeast (Codex Alimentarius, 2003). On the other hand, it has been declared that it can contain 10 2-10 3 cfu/g molds and <3 MPN (most probable number)/g E. coli in a maximum of 2 samples out of 5 by Turkish Food Codex Communiqué on Fermented Milk Products (2009). If the microbial counts detected in our research are compared to the standards mentioned above and the previous references, counts of lactobacilli and lactococci in our study were between 7.45-8.34 log cfu/mL and 7.83-8.39 log cfu/mL at the end of storage period and are consistent to the previous references (Kok-Tas et al., 2013; Goncu et al., 2017; Hikmetoglu et al., 2020; Kabakci et al., 2020) in which kefir has been found to contain 10 8 cfu/mL lactobacilli and 10 9 cfu/mL lactococci.
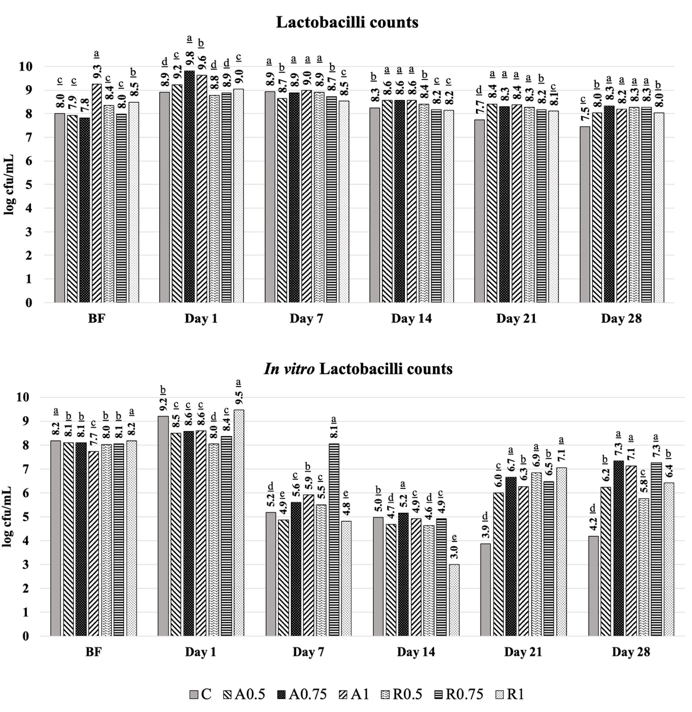
Figure 3. Viability of lactobacilli counts after in vitro GI digestion during 28 days of cold storage
(A: Arabica; R: Robusta; C: Control; BF: Before Fermentation); The values are given in the graphs are mean value± SD. The mean values represented by bar graphs with different letters are significantly different, statistically (p≤0.05).
It has been found that CSS supplementation did not act to the viability of the starter bacteria in yoghurt ( Streptococcus salivarius var. thermophilus and Lactobacillus acidophilus) as mentioned by Bertolino et al., (2019). However, counts of the starter bacteria in the reported research were elevated above the level (7 log cfu/mL) that has been recommended by Codex Alimentarius by CSS enrichment. Similarly, lactobacilli and lactococci counts were determined as 8 and 9 log cfu/mL in the kefir samples that were prepared by addition of starter bacteria and the granulated kefir culture during 21 days of cold storage (Kok-Tas et al., 2013). If effect of CSS variety on the counts of lactobacilli and lactococci was considered, the viability rates of lactobacilli and lactococci were between 84.98-87.01 and 79.99-87.40 % in the Arabica CSS added samples and 88.94-94.10 and 82.37-92.44 % in the Robusta CSS added samples during the storage, respectively. Briefly, the viability of lactobacilli and lactococci was influenced positively by the CSS supplementation in the kefir. The samples supplemented with Robusta CSS caused to higher viability rates (%) for lactobacilli and lactococci than the Arabica CSS. As reported by Goncu et al., (2017), lactococci counts reduced 0.68-2.08 log cycles due to sensitivity to low pH in the kefir.
In vitro viability of kefir microbiota during storage
The main characteristics of the probiotic microorganism can be summarized under three main items. In order to provide health benefits, probiotic bacteria should consist of live microorganisms at the level recommended by Codex Alimentarius. The proposed level of live probiotic microorganisms should be kept until their consumption and following the consumption their viability should be maintained throughout the gastrointestinal system up to the colon.
The fermented probiotic foods should contain probiotic microorganisms at the level of 10 8-10 9 cfu/g or mL at the end of the fermentation process in order to provide their expected health benefits (Lo Curto et al., 2011; Kok-Tas et al., 2013; Goncu et al., 2017; Hikmetoglu et al., 2020; Kabakci et al., 2020). The viability of probiotic bacteria available in the commercial food products should be maintained throughout the digestive tract. There are very difficult ambient conditions such as high acidity and bile salts to limit their viability in the gastrointestinal system. Their tolerance is also affected by the presence of prebiotics or non-digestible carbohydrates in the upper digestive tract. Thus, dairy products such as kefir are defined as convenient food matrices that can play a role as a good probiotic carrier (Lo Curto et al., 2011).
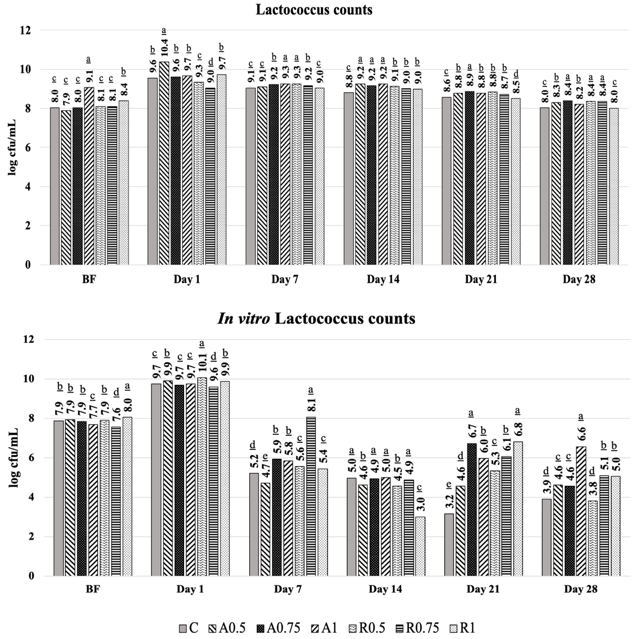
Figure 4. Viability of lactococci counts after in vitro GI digestion
(A: Arabica; R: Robusta; C: Control); The values are given in the graphs are mean value± SD. The mean values represented by bar graphs with different letters are significantly different, statistically (p≤0.05).
In vitro viability counts of lactobacilli were 8.50-9.19 and 7.92-9.47 log cfu/mL at the beginning of storage period for Arabica and Robusta CSS added kefir samples, while they were 4.19-7.33 and 5.24-7.25 log cfu/mL at the end of storage (Figure 3), respectively. The samples supplemented with Arabica and Robusta CSS had higher in vitro lactococci viability level (9.67-9.89 and 9.60-10.06 log cfu/mL) at the beginning of the storage, whereas they were determined as 3.89-6.57 log cfu/mL and 3.80-5.10 log cfu/mL at the end of storage period (Figure 4), respectively. CSS is a by-product of roasting step in coffee bean processing and is a rich source in terms of melanoidins that are the compounds formed during the roasting process. It has been mentioned that compounds that have a lower molecular weight and are produced by melanoidins, exhibit better solubility during in vitro gastrointestinal digestion; thus, can be defined as a component of soluble maillardized dietary fibre. As stressed by Borrelli et al., (2004), due to high soluble dietary fibre content, CSS can be used as a commercial prebiotic food component, because it supports to growth of Bifidobacteria while activity of Lactobacillus spp. is not affected by CSS under in vitro conditions.
Dietary fibre is a food component with prebiotic character and has high water retention capacity. It promotes to growth of the probiotic bacteria, and reduces absorption of energy components such as fat and sugar while it passes through the intestine. On the other hand, insoluble dietary fibre is a compound that has a low water-holding capacity and accelerates the movement of food in the digestive system (Ballesteros et al., 2014). In terms of in vitro viability, the highest values of lactobacilli and lactococci counts were determined in the Arabica CSS-added kefir samples as 7.33 and 6.57 log cfu/mL at the end of storage. Similar results were obtained for lactobacilli (7.25 log cfu/mL) in Robusta CSS-added samples. B ut, lactococci counts were not affected by Robusta CSS supplementation significantly and determined as 5.10 log cfu/mL. It was determined that the expected viability counts for the probiotic products were provided by 0.75 and 1.0 % Arabica CSS supplementation for in vitro lactobacilli counts (7.33 and 7.12 log cfu/mL) in the kefir samples. Lactobacilli counts were lower than the Arabica added ones and 7.25 with 6.41 log cfu/mL for the same supplementation ratios of Robusta CSS.
When the lactococci growth levels were evaluated, it was determined that the lactococci counts were below the level of <7 log cfu/mL in Arabica and Robusta CSS-added kefir samples. CSS is one of the food by-products with high water and oil holding capacity (Ballesteros et al., 2014), and it can reduce the water activity in the product that is added into. Water activity levels that are needed for the growth of lactic acid bacteria in the kefir culture are 0.95-1.0 (Goncu et al., 2017). In this respect, due to the increase in water holding capacity under in vitro model digestive system conditions, lactobacilli numbers were decreased to 1.25 and 1.10 log cfu/mL in 0.75 % of Arabica and Robusta CSS added kefir samples. When the both CSS varieties were compared, the lowest decrease in lactococci numbers was observed in the samples with Arabica CSS (1.0 %) and Robusta CSS (0.75 %) as 3.17 and 4.51 log cfu/mL.
Under in vitro simulated digestive system media, one of the most critical criteria to be considered regarding activity of the probiotic bacteria is resistance to low pH and high pepsin concentration in the mimic stomach conditions. However, pH level in the stomach can rise to 4-6 depending on the buffering capacity of the constituents available in the food after consumption, and following, it is stabilized to 2.5-3.5 pH (Faye et al., 2012). Meanwhile, the probiotic bacteria pass to the duodenum in where protease enzymes are abundant, and they can survive in the presence of bile salts (Ekmekcioglu, 2002). By the end of the storage period, when the effects of in vitro digestion on lactobacilli were considered before and after in vitro digestion, it was determined that the survival rate of lactobacilli was between 84.98-87.01 % and changed to 73.37-85.45 % for Arabica CSS added samples, while they were determined as 88.94-94.10 % and decreased to 67.64-86.86 % in the Robusta CSS added samples. The survival rate of lactococci decreased to 46.66-67.42 from 79.90-87.40% in the Arabica CSS added kefir samples, while it lowered to 37.78-53.08 from 82.37-92.44% in the Robusta added kefir samples. On the other hand, protein and fat contents are important for the microbial growth. The hard-textured foods such as cheese or viscous dairy products such as yoghurt and kefir have high protein contents responsible for buffering capacity. In protein rich foods, viability of probiotic bacteria is affected positively. If the fat content in the same food is high, the microbial growth is supported stronger, because fat is a source of energy for the microbial development (Ortakci et al., 2012).
Higher viability rates of the LAB are generally related to sub-lethal acidic stress occurred in the first 24-hour of the fermentation as found by Faye et al., (2012) and Xie et al., (2009). Similar conditions were provided in our study in where stomach pH can be adapted easily in vitro digestion conditions. Besides, the yeasts that survive under in vitro simulated conditions can develop together with LAB, synergistically. Briefly, interaction between the LAB and the yeast available in the kefir culture causes the high viability rates in our kefir samples are thought.
Changes in yeasts counts
The most common microorganisms available in the kefir flora are lactic acid bacteria ( Lactobacillus, Leuconostoc, Streptococcus and Lactococcus ssp.), fermentative yeasts ( Kluyveromyces, Saccharomyces, Candida, and Torulopsis), and acetic acid bacteria (Magalhães et al., 2011; Kok-Tas et al., 2013; Dong-Hyeon et al., 2019). Yeasts stimulate LAB by forming metabolites such as CO 2, pyruvate, propionate, while LAB forms galactose, a by-product of lactose metabolism that will be assimilated by yeasts.
By the end of storage, yeast counts in the kefir samples supplemented by Arabica and Robusta coffee silverskin are 2.75 log cfu/mL and below the value reported (3.0 log cfu/mL) in Codex Alimentarius (2003) and Turkish Food Codex Fermented Products Communiqué (2009). In previous studies on kefir (Irigoyen et al., 2005; Kok-Tas et al., 2013; Goncu et al., 2017; Kabakci et al., 2020); yeast counts have been reported to vary between 5-8 log cfu/mL in general. Compared to these studies, the yeast counts found in our study are lower. On the contrary, there are also studies that yeast counts of the kefir samples are lower than the yeast counts determined in our study. For example, in studies conducted by Grønnevik et al., (2011), Cetinkaya and Elal Mus (2012), and Delgado-Fernandez et al., (2019), it was observed that kefir samples had yeast counts between 2-3 log cfu/mL.
Low yeast counts obtained in our study may be caused by differences in kefir grain composition used in kefir production or starter culture microbiota. Yeasts may also stabilize the LAB population in cheese and yogurt (Viljoen et al., 2003), why it is possible that they may have played a role as a "yeast extract" that contains the vitamins for the growth of LAB. The yeasts are also thought to act as promoters of bacterial growth (Liu et al., 2009). The high number of lactic acid bacteria in kefir samples caused to decrease the yeast count more than expected. It is thought that incubation period for kefir production was not sufficient to develop the yeast count, and thus, the yeast count was lower (2.75 log cfu/mL) than expected at the end of the incubation period (24-hour). Also, the roasted coffee beans contain galacto- and arabino-oligosaccharides. During the roasting process, fructooligosaccharides can be formed as a source of energy by the raw coffee silverskin due to some complex chemical reactions (Iriondo-DeHond et al., 2019). The galacto-oligosaccharides are non-digestible bioactive compounds that are metabolized by bacteria that possess β-galactosidase and have in vitro immunostimulatory effects on the growth of Lactobacillus spp. and Bifidobacterium spp. (Guarino et al., 2020). Simova et al., (2002) stated that the number of LABs in kefir flora varies between 80-90% and yeasts between 10-20%. Borrelli et al., (2004) and HadiNezhad et al., (2001) reported that soluble dietary fibre sources with the prebiotic character support better the growth of some bacterial species ( Bifidobacterium and Lactobacillus). Mitterdorfer et al., (2001) stated that yeast species prefer glucose, fructose, sucrose primarily, and fructooligosaccharide may contribute to the viability of yeasts (Oh et al., 2013).
Conclusion
Primarily, new data whether CSS belonging to the both coffee varieties ( Arabica and Robusta) can be used as a new supplement for kefir production was obtained in our research. It was found that the recommended level of viable probiotic bacteria to provide therapeutic effect (10 6 g cfu/mL minimum) was obtained by CSS supplementation for lactobacilli and lactococci counts at the end of the fermentation and 28 days of storage. After the in-vitro gastrointestinal digestion, it was determined that enrichment with 0.75 and 1.0 % of Arabica-CSS caused to increase in the survival rate of lactobacilli, however, lactococci and yeasts were not able to resist the available GI conditions, and thus they lost their viability during the storage period. It was concluded that the CSS led to protection and survival for lactobacilli without affecting the kefir fermentation. However, it is thought that further studies are required regarding resistance and stability of CSS against those bacteria.
Acknowledgements
This study was supported by the Scientific Research Council of Bursa Uludag University, Turkey (Project No: OUAP(SBF)-2019/10). As a new product, kefir with coffee silverskin has a patent application to Turkish Patent Institute with the number 2019-GE-396055. The authors would like to specially thank Osmanli Kahvecisi Incorporated Company for providing the project raw material, coffee silverskin for the kefir production.
Utjecaj dodatka srebrne pokožice zrna kave na in vitro preživljavanje kefirne kulture tijekom skladištenja
Sažetak
Probiotički mliječni proizvodi moraju u trenutku konzumacije sadržavati određenu količinu živih probitičkih bakterija. Kefirna kultura sadrži različite vrste mikroorganizama čiji se broj, ovisno o brojnim čimbenicima, može mijenjati tijekom skladištenja. U ovom je istraživanju ispitivan utjecaj dodatka novog tipa funkcionalnog sastojka - srebrne pokožice zrna kave (eng. CSS, coffee silverskin) na raspoloživost mikroorganizama koji čine kefirnu kulturu. U tu svrhu je CSS dobiven iz dvije vrste kave ( Arabica i Robusta) dodavan uzorcima kefira u tri različite koncentracije (0,5, 0,75 i 1,0 %) na početku procesa fermentacije. Utvrđeno je kako je prije svega dodatak CSS dobivenog od Robusta vrste značajno utjecao na preživljavanje bakterija mliječne kiseline (laktobacila i laktokoka) koja je iznosila 88,00-94.10 %, odnosno 82,37-92,44 %. Tijekom 28 dana skladištenja na 4 °C utvrđeno je kako obogaćivanje kefira s CSS dobivenim iz obje vrste kave povećava preživljavanje laktobacila i laktokoka u uvjetima in vitro probave, što je uvjetovano količinom dodatka. Također, broj plijesni padao je tijekom skladištenja.
Ključne riječi: kefir, srebrna pokožica zrna kave, Arabica, Robusta, dijetalno vlakno, in vitro raspoloživost
References
Guzel-Seydim, Z.B., Seydim, A.C., Greene, A.K., Bodine, A.B. (2000): Determination of organic acids and volatile flavor substances in kefir during fermentation. Journal of Food Composition and Analysis 13 (1), 35-43.https://doi.org/10.1006/jfca.1999.0842
HadiNezhad, M., Duc, C., Han, N.F., Hosseinian, F. (2013): Flaxseed soluble dietary fibre enhances lactic acid bacterial survival and growth in kefir and possesses high antioxidant capacity. Journal of Food Research (5), 152-152.https://doi.org/10.5539/jfr.v2n5p152
Kok-Tas, T., Seydim, A.C., Ozer, B., Guzel-Seydim, Z.B. (2013): Effects of different fermentation parameters on quality characteristics of kefir. International Journal of Dairy Science 96(2), 780-789.https://doi.org/10.3168/jds.2012-5753
