Introduction
Kefir contains most of the nutrients from milk, provides positive contributions to the digestive system and exerts anti-mutagenic and anti-carcinogenic properties (Guzel-Seydim et al., 2006; Maalouf et al., 2011). Therapeutic effects of kefir are associated to the probiotic bacteria content in the kefir grain microbiota and the metabolic products yielded by these bacteria (Guzel-Seydim et al., 2005). Lactobacillus kefir, Lb. kefiranofaciens, Lb. kefirgranum and Lb. parakefir, many lactic acid bacteria and yeast that affect the characteristic product feature are present in kefir grains (Guzel-Seydim et al., 2021; Koçak et al., 2021). However, these microorganisms are not found in the commercial starter culture used in the production of industrial kefir (Davras et al., 2018).
In recent years, there is an increasing trend of consumption towards alternatives to cow milk and its products due to better therapeutic properties. Especially the consumption of goat and donkey milk as well as their products have been growing (Bhardwaj et al., 2020). The direct consumption of donkey milk, which has become more prominent with its effect on human health, is gradually increasing. Donkey milk contains lower levels of fat, protein, and inorganic salt, but higher level of lactose than cow milk, so it shows a composition close to human milk (Öztürkoğlu Budak and Gürsel, 2012). The high level of lysozyme, a natural antimicrobial agent, and its resistance to gastric pH, enables this protein to reach the intestinal system and has a selective effect on intestinal bacteria (Yvon et al., 2019). Similar to goat milk, donkey milk contains less saturated fatty acids and more unsaturated fatty acids.
Recently, Yıldız et al. (2016) studied the effect of donkey milk and DMK on biochemical parameters in blood serum of mice with Ehrlich acid solid tumour. They observed no significant changes in blood serum values of mice fed kefir and water for 10 days, so they recommended the feeding time longer than 10 days. Esener et al. (2018) found that tumour volume decreased in animals fed with donkey milk kefir but increased in animals fed with donkey milk. The authors also reported that kefir made by using donkey milk induced apoptosis, suppressed proliferation, and decreased co-expression of iNOS and eNOS, however, these activities were not observed in animals fed donkey milk.
In recent years, studies on donkey milk and donkey milk products increased rapidly. However, studies on the evaluation of donkey milk as a fermented milk product are limited.
Therefore, this research aims to characterise the chemical, physical, microbiological and sensory properties of DMK and CMK by comparing them during 21 days of cold storage.
Materials and methods
Materials
Donkey milk was obtained from 20 donkeys in a private production farm in Isparta, Turkey. Cow milk was obtained from producers raising Holstein cows in Burdur, Turkey. Both types of milk samples were transported to the laboratory within 30 minutes by maintaining the cold chain. Both types of milk were used for kefir production. The kefir grain was provided from the Dairy Plant settled at the Faculty of Agriculture, Ankara University, Ankara. Milk samples and kefir grain was brought to the laboratory under cold conditions storage in dry ice. Both were also kept in cold storage until manufacturing.
Kefir production
Kefir production was carried out at the laboratory of Food Processing Department, Burdur Food, Agriculture and Livestock Vocational School, Mehmet Akif Ersoy University. Each batch was produced from 2 L fresh milk samples, which were first pasteurized at 87±1 °C for 20 minutes, then cooled to 25±1 °C and transferred to sterile glass jars divided into four equal parts. Following inoculation with 3 % kefir grain, all the milk samples were incubated for approximately 18-24 hours until the pH value was reached 4.5±0.1 (Şendoğan et al., 2021). At the end of the fermentation, the grains were removed via a sterile plastic filter, and the kefir beverage was kept in the refrigerator for one night; then, the samples were filled in sterile glass jars (each sample 500 mL) and stored under the refrigerator conditions (+4 °C) during 21 days. Analysis of kefir samples was performed on days 1, 7, 15, and 21 of cold storage.
Methods
Chemical and physical analysis of milk and kefir samples
The titratable acidity, total solids, fat, ash, protein were estimated according to the AOAC (2012) international method. Lactose analysis was performed according to method TS 13359 on an HPLC system (Anonymous, 2008). The pH value was measured by electrode immersion with a pH meter (Mettler-Toledo, Greifensee, Switzerland). The density of milk samples was determined according to Anonymous (1983). The viscosity of kefir samples was determined by using a rotational viscometer (Model RVDV‐II, Brookfield Engineering Laboratories, UK) with an RV2 disk spindle at 100 rpm speeds, at 15±1 °C. The amount of carbon dioxide in kefir samples was determined by titration according to the Connizorai method (Anonymous, 1983). All chemicals were of analytical grade and obtained from Merck or Sigma-Aldrich, Germany.
Scanning Electron Microscope (SEM) (Fei quanta feg 250 Model) was used to observe microbiota in kefir serum structures. First, the samples were centrifuged at 4100 rpm for 10 min (Nuve, NF 800R model, Turkey) to obtain the serum part of kefir. Then, the serum part of kefir samples was directly taken into sterile tubes without any filtration. After that, the samples were lyophilized (Bluewave, BW-100 Freeze Dryer, China) and ready for SEM inspection. Before the study, no coating was applied to the samples. During the analysis, the low-vacuum mode was used, and different parts of the samples were investigated.
Analysis of volatile aroma compounds in kefir samples
The method modified by Yılmazer and Seçilmiş (2006) was used for the determination of volatile aroma compounds. Four g of kefir samples were put into the headspace system. The analysis was performed with the Agilent 7697A Headspace (Agilent 7890A and GC 5975C MS) device. The column temperature program was as follows; after waiting 5 minutes at 35 ºC, it was reached 240 °C with an increase of 5 ºC per minute and kept at this temperature for 5 minutes. Other applications were as follows: detector temperature 250 °C, injector temperature 240 °C, flow rate 10 psi (He), Needle 90 °C, transfer line 120 °C, vial oven 85 °C, thermostat time 5 minutes, pressurize time 0.5 minutes, the injection time 0.08 minutes and the withdraw time 0.5 minutes.
HPLC analysis of amino acids in kefir samples
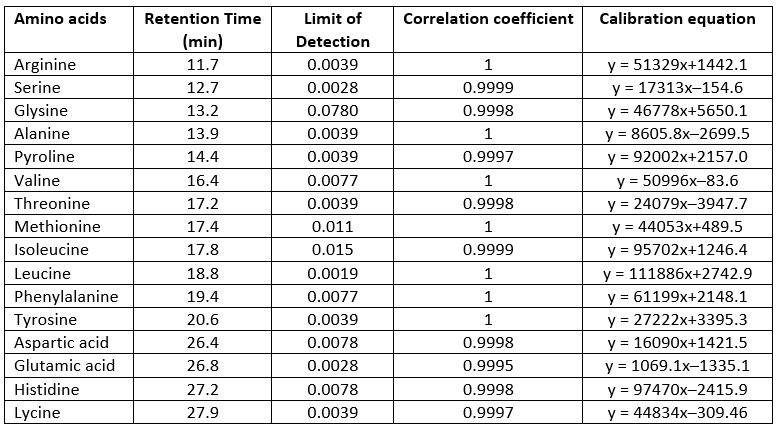
Amino acids were determined according to the method of Köse et al. (2011) by a modification. For this purpose, 25 g of kefir samples were weighed, and 25 mL of 0.1 M HCl was added. The homogenized mixture was centrifuged at 4000 rpm at 4 °C for 20 minutes. The upper phase was removed, then 100 µL of 2 N NaOH, 150 µL of saturated sodium bicarbonate, and 1 ml of dansyl chloride were placed on it. The mixture was incubated at 40 ºC for 45 minutes. It was left at room temperature for 10 minutes. 50 µL of 25 % NH3 was added to it. It was kept at room temperature for another 30 minutes. Five mL ammonium acetate: acetonitrile mixture was added to it. It was passed through a 0.45 µm filter and injected into the HPLC system. Shimadzu Prominence Brand HPLC, CBM: 20ACBM, Detector: DAD (SPD-M20A), Column Oven: CTO-10ASVp, Pump: LC20 AT, Autosampler: SIL 20ACHT, Computer Program: LC Solution, Mobile Phase: A: 0.1 M Ammonium acetate B: Acetonitrile. Evaluation of chromatographic data of amino acids is presented in Table 1.
Microbiological analyses
For the enumeration of bacteria and yeasts in kefir samples, serial dilutions of each sample were prepared by suspending 10 g of kefir in 90 g of peptone water and homogenizing with a stomacher (Laboratory Blender Stomacher 400, Seward, London, UK) for 5 min, and then plated in triplicate on agar plates (Vanderzant and Splittstoesser, 1992).
Table 1. Evaluation of chromatographic data of amino acids
The results were expressed as log cfu/g (log colony-forming unit/g). For the enumeration of total mesophilic aerobic bacteria, Plate Count Agar (PCA; Merck, Darmstadt, Germany) was used, and the plates were incubated at 30 °C for 2 days. Counts of lactococcus and lactobacillus were performed on M17 agar (Difco) (pH 7.2) and on MRS agar (Difco) (pH 6.5), respectively and the plates were incubated at 30 °C for two days under anaerobic conditions in a CO2 incubator (5 %). For the count of L. acidophilus, MRS agar, including 10 % sorbitol, was used. Enumeration of Bifidobacterium spp. was performed on MRS Agar including NNLP (20 %); neomycin sulphate (100 mg/L), nalidixic acid (50 mg/L), lithium chloride (3000 mg/L), and paramycin sulphate (200 mg/L) (Kök Taş et al., 2012) by incubating plates at 37 °C for 2 days. Yeasts were counted on Potato Dextrose Agar (Merck) with the addition of 1.4 mL tartaric acid to 100 mL of the medium, and the plates were incubated at 25 °C for five days.
Evaluation of sensory properties
Sensory analysis was performed by seven experienced panellists from members and students of the Food Processing Department at the Milk and Milk Products Application and Research Center Sensorial Test Room under fluorescence light. Each kefir sample was filled into 50 mL in the glass cups after being equilibrated to 20 °C from storage temperature (+4 °C). The scoring test was used in the study evaluated for overall acceptance (score 0-10), appearance (score 0-10), structure (score 0-10), taste (score 0-5), odour (score 0-5). After each sample tasting, the panelists cleaned their mouths by rinsing them with water (Ertekin and Guzel-Seydim, 2010; Temiz and Kezer, 2014).
Statistical analysis
The statistical analysis was performed using SPSS Ver. 23:0 (IBM, Armonk, NY, USA). The values obtained were presented as the mean ± standard deviation (SD). The significance level at p<0.05 and p<0.01 was determined using analysis of variance followed by Tukey’s multiple range tests. Three replications were carried out for production, and each measurement was taken in duplicate for each analysis and averaged in all samples.
Results and discussion
Properties of milk samples
The composition of milk varies according to the animal species and depends on many factors such as nutrition, care, and diseases. Furthermore, such factors as climate and geographical structure, and vegetation in different countries influence the composition of milk from the same breeds (Kebede, 2018). Chemical composition and some physical properties of donkey and cow milk are given in Table 2. The total solids, fat, protein, and ash were lower, while the lactose content was higher in donkey milk than in cow milk (p˂0.05). The pH value was also higher in donkey milk than in cow milk. In other words, donkey milk is slightly alkaline while cow milk is slightly acidic. According to Öztürkoğlu Budak and Gürsel (2012), fat content is in the range of 0.38-1.82 % in donkey milk, it is between 3.4-3.8 % in cow milk and 3.64-3.80 % in human milk.
Table 2. Composition and some properties of donkey and cow milk 1 (mean±SD) 2
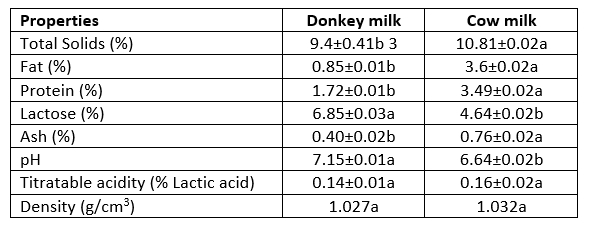
1Data are the averages of triplicates
2Standard deviation
3 a, b: Means within a row with different lowercase letters show significant differences between milk type, p<0,05
Although the fat content of donkey’s milk is relatively low compared to cow's milk, it is crucial for health that unsaturated fatty acids, especially polyunsaturated fatty acids such as omega 3 and omega 6, show a level close to that in breast milk (Öztürkoğlu Budak and Gürsel, 2012). Additionally, the biological value of albumin milk is different from that of casein milk. The protein content in donkey milk is 1.63 g/100 mL, as reported by Martini et al. (2018), and is between 1.5-1.8 g/100 mL (Polidori and Vincenzetti, 2012). Malissiova et al. (2016) stated that pH value was 6.68-7.60 in donkey milk collected from different regions of Greece and Cyprus. These authors attributed these results to additional feeding and breeding regimes in the other regions.
Scan electron microscopy
In this study, first of all, directly kefir samples were investigated for SEM. However, densely populated microorganisms cannot be observed in the images of SEM. For this reason, kefir samples were centrifuged, and serum parts of them were used for SEM investigation. In Figure 1.a and 1.b, images are the outer surface of kefir serum samples. Leaf-like structures are present in donkey milk kefir serum (Figure 1.a). On the other hand, porous structures are present in cow milk kefir serum sample (Figure 1.b). Also, fibrous-like structure can be seen in cow milk kefir serum (Figure 1.b). In Figure 1.c, while it was observed that Lactococcus sp. was widely distributed, it was determined that some yeast groups were observed between the lactic acid bacteria. Bacteria and yeast mixture were shrinkaged and covered by exopolysaccharide structure (Figure 1.c). Also, above the microorganisms, an enormous structure can be seen. It is probably lactose because, it is similar to Hassan et al. (2008) images. This photo is a closer photo of Figure 1.d. On the other hand, microorganisms were embedded like a table-cloth structure. This table-cloth-like structure is the exopolysaccharide structure of cow milk kefir serum. Similar to donkey milk kefir serum, a few yeasts are present among the lactic acid bacteria that mainly consist of lactococci. Many studies have shown that lactic acid bacteria, yeast, and acetic acid bacteria in the kefir microbiota are located in the exopolysaccharide matrix. It is stated that the polymer matrix supports and protects cells (Pihurov et al., 2021). In the examinations made with SEM, it is stated that a complex packaged biofilm surrounds these microorganisms in the outer part of the kefir grain and consists of unstructured material in the inner part (Schwan et al., 2015). According to the SEM inspection obtained in the study, it is thought that the microbiota is present in kefiran, which is intensely present in the serum.
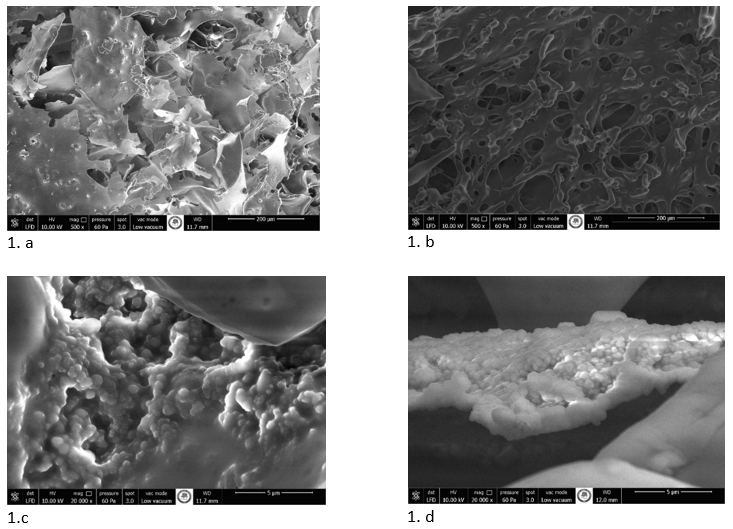
Figure 1. Scanning electron microscope images of donkey milk kefir serum and cow milk kefir serum (1.a and 1.b is 500 x magnified image of donkey milk and cow milk kefir serum; 1.c and 1.d is 20000 x magnified images of donkey milk kefir and cow milk kefir serum, respectively).
Chemical, physical and biochemical properties of kefir samples
Chemical and physical properties
DMK had lower values for total solids, fat, protein, and ash than CMK (p˂0.05). Total solids and fat contents of kefir samples differed depending on the characteristics of the milk used in the production (Table 3). However, the effect of storage time on the total solid content was not statistically significant (p>0.05). In addition, the protein content of CMK was higher due to the relatively high casein content naturally found in its structure. However, the compositional values of DMK or CMK remained almost unchanged (p>0.05) during the storage period (Table 3). Yirmibeşoğlu and Tefon Öztürk (2020) also found that the total fat and protein content of DMK was low (0.55 % and 1.87 %, respectively). Wzolek et al. (2001) reported that kefir samples made using goat or sheep milk and different starter cultures showed significant differences in total solids and protein contents.
Table 3. Some chemical and physical properties of kefir samples 1 (mean±SD) 2
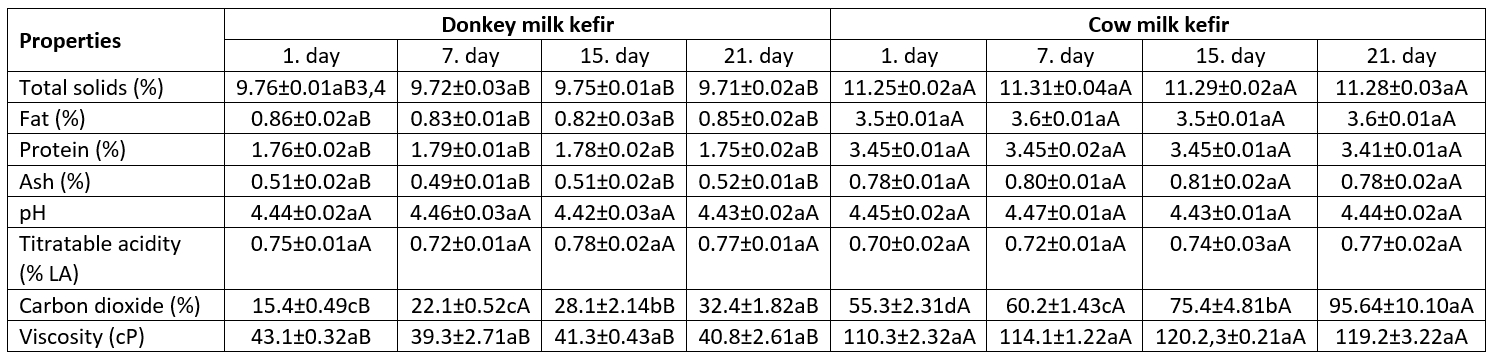
1Data are the averages of triplicates
2Standard deviation
3a, b, cMeans within a row with different lowercase letters show significant differences between storage days, p<0.05
4A, BMeans within a row with different uppercase letters show significant differences between milk type, p<0.05
According to the findings of the aforementioned authors, total solids content was at the level of 11.02 % in kefir from goat milk and 14.85 % in kefir from sheep milk; while protein content was found at the level of 2.85 % and 6.45 % in kefir from goat milk and kefir from sheep milk, respectively. Tsakali et al. (2017) found that the fat content of yogurt from donkey milk was at the level of 0.59 % and protein content at the level of 1.69 %. The reason for the high-fat value in this research has been attributed to species of animals and type of diet. Despite the low content of fat, kefir made from donkey milk can be considered as a healthy beverage for older people and infants due to easy digestibility of fat, However, its energy value is low due to low-fat content (Martemucci and D’Alessandro, 2012). The low protein content in DMK is due to the low ratio of casein in the milk protein fraction of donkey milk, as mentioned previously.
The pH value showed slight differences between kefir samples, and no significant changes in pH values were observed during the storage (p>0.05). The values for titratable acidity in donkey’s milk kefir were close (p˃0.05) to that of the cow’s milk kefir. Changes in titratable acidity were also similar between days of storage (p˃0.05). The pH value of kefir can vary depending on the type of milk used for manufacturing, kefir flora, amount of inoculum, incubation temperature, temperature, and duration of storage. For example, Gürsel et al. (1990) observed an increase in pH value of kefir samples during the storage for seven days, while Beshkova et al. (2002) observed no changes after 7 days of storage in pH values of kefir made by using pure culture. Meanwhile, the titratable acidity of donkey milk kefir was found to be 0.8 % by Yirmibeşoğlu and Tefon Öztürk (2020).
Carbon dioxide and alcohol are produced due to the activities of yeasts found in kefir flora (Grønnevik et al., 2011). In the present study, donkey’s milk kefir had lower (p˂0.05) levels of carbon dioxide compared to the cow’s milk kefir (Table 2). Carbon dioxide concentration increased almost 2-fold at the end of storage. However, Beshkova et al. (2002) observed lower amounts of alcohol in kefir produced by using grain (105 mg/100 mL) than those produced by using commercial starter culture (175-198 mg/100 mL). The authors also observed that when 4.5 g/L sucrose was added to milk, the carbon dioxide content reached higher levels (183 mg/100 mL) after 16 h. In a study on the effect of different starter cultures on some properties of kefir, Yıldız (2009) manufactured kefir by using grain (5 %) with thermophilic bacteria (1 %), probiotic bacteria (5 %) or yeast cultures (5 %). In this study, the highest amount of carbon dioxide (122.73-183.60 mg/100 mL) was observed in kefir made using grain mixed with yeast culture.
The consistency of fermented dairy products such as kefir, yogurt and ayran (drink yogurt) depends on the balance among total solids, fat, and protein in a product. Kefir is included in the group of non-Newtonian fluids, and its viscosity varies depending on such factors as product composition, heat treatment, agitation, and cooling temperature (Dimitreli et al., 2013). In milk with high casein content, the viscosity of a product changes depending on the high fat and protein contents and the interaction between β-lactoglobulin and K-casein through thiol-disulfide interchange reactions. In other words, the disulfide bridge of K-casein reacts with whey proteins to form a complex, and the ability of whey proteins to bind water increases, affecting the viscosity and rheological properties of fermented milk products such as kefir, yoghurt and ayran (Dissanayake et al., 2013). In the present study, DMK had a lower (P˂0.05) viscosity compared to CMK. The viscosity value was in the range of 39.3-43.1 cP in DMK while it was in the range of 110.3-119.2 cP in CMK (Table 3). Cais-Sokolińska et al. (2016) reported that the viscosity index of kefir from donkey milk is close to that of kefir made from mare's milk which is at the level of 81 g.s. According to these authors, such low viscosity fermented dairy beverages contain lower casein levels like fermented beverages from mare’s milk. Considering the compositional values for donkey milk, it can be said that the consistency of DMK is close to that of ayran and koumiss, which are traditional products.
Free amino acids composition
The biological benefits of amino acids, as building blocks of proteins and milk constituents are significant. Some of the amino acids released from proteins during proteolysis in fermented dairy products are called essential and non-essential amino acids (Perna et al., 2019). Totally 16 essential and non-essential amino acids were detected in CMK, whereas 12 amino acids were determined in DMK (Table 4). Thus, using cow milk resulted in the richer composition of amino acids than using donkey milk. Glutamic acid, leucine, proline, tyrosine and aspartic acid were the critical amino acids in CMK. Serine, isoleucine, phenylalanin, and threonin were other amino acids found at higher levels after significant amino acids. The lowest level was found for glycine (p˂0.05). The DMK had the highest content of tyrosine, whereas the lowest (p˂0.05) level was found for methionine. Levels of valine and arginine were also high in DMK. Histidine, lysine, aspartic acid, and glutamic acid were not detected in DMK. Compared to other fermented milk products, amino acid formation in kefir depends not only on the activity of lactic acid bacteria but also on the degradation of proteins by yeasts and acetic acid bacteria. Guzel-Seydim et al. (2003) emphasized that threonine, serine, alanine and lysine were the principal amino acids detected in kefir. In another study, Bensmira and Jiang (2012) showed that the concentration of lysine was at the highest level in kefir made by using cow milk. Glutamic acid, proline, and leucine were the other amino acids found in the amino acid composition of kefir, as reported by Bensmira and Jiang (2012). Since there is no research on amino acid composition of kefir from donkey milk, the present study results could not be compared.
Table 4. Amino acids composition of kefir samples (g/ 100 g) 1 (mean±SD) 2
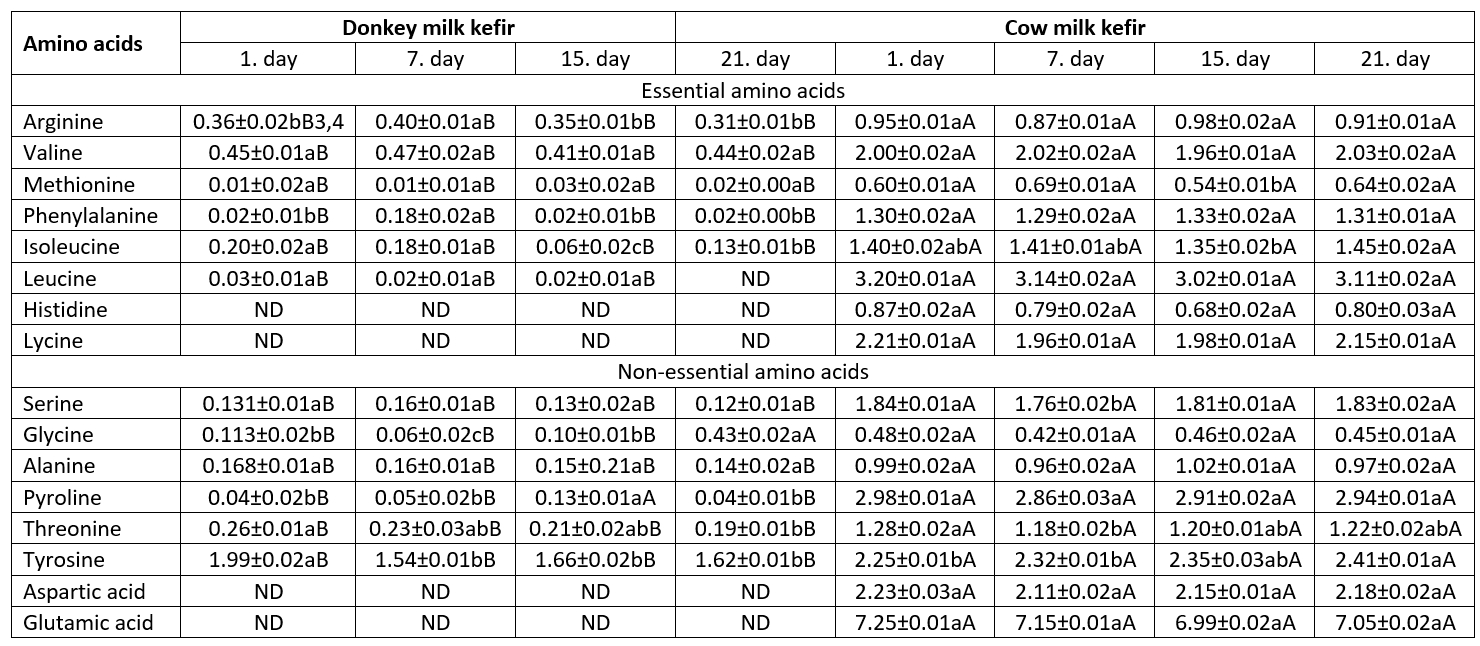
ND: Not detected
1Data are the averages of triplicates
2Standard deviation
3 a, b, c Means within a row with different lowercase letters show significant differences between storage days, p<0.05
4 A, B Means within a row with different uppercase letters show significant differences between milk type, p<0.05
However, Liu et al. (2019) reported that aspartic acid, glutamic acid, valine, isoleucine, leucine, lysine, arginine, and proline were higher concentration. Still, threonine, serine, cysteine, tyrosine, phenylalanine, and histidine were very low amounts in koumiss made from mare’s milk which shows similarities to kefir from donkey milk.
As reported previously by Öztürkoğlu Budak and Gürsel (2012), donkey milk is notably richer in whey proteins than cow milk. These proteins have an essential role in antioxidant defence due to the high level of sulphur-containing amino acids. Especially, cysteine and methionine have a higher biological activity than other amino acids in whey proteins (Marshall, 2004). The antioxidant capacity of kefir is related to the presence of many bioactive peptides released from milk proteins that vary according to animal type milk during the fermentation period by the proteolytic activity of lactic acid bacteria and yeasts (Perna et al., 2019). Thus, it can be said that concentrations of bioactive peptides in DMK are related to the amino acid content of milk. Last decades, many researchers were increased their interest in this issue. According to research findings, donkey milk kefir seems to affect health depending upon amino acid content positively.
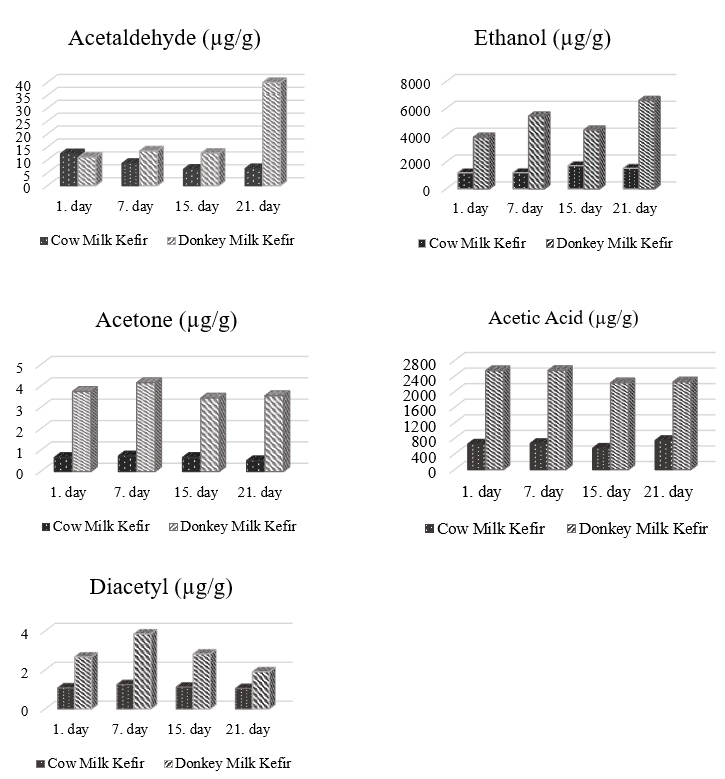
Figure 2. Key volatile aroma compounds of kefir samples
Volatile aroma compounds
Aroma compounds, whether inbound or free form, are essential for the taste and odour of foods (Bayrak, 2006). These properties are affected by many factors like milk fermentation. Figure 1 shows key volatile aroma compounds found in kefirs made by using donkey and cow milk. The acetaldehyde content of DMK was significantly (p<0.05) higher than that of CMK. It was in the range of 11.06-41.95 µg/g in DMK and the content of 6.63-12.35 µg/g in CMK. The concentration of acetaldehyde increased considerably in the kefir made using donkey milk but decreased gradually in the sample produced by using cow milk during the storage. It seems that a higher ratio of whey proteins in milk protein fraction of donkey milk led to a higher concentration of acetaldehyde in the kefir sample. Gursel et al. (2012) reported that goat milk yogurt fortified with whey protein concentrate or whey protein isolate had a higher acetaldehyde concentration than the yogurt fortified with sodium caseinate or yogurt texture improver. According to Guzel-Seydim et al. (2005), oxidation of acetaldehyde to acetate depending upon the changes in pH may decrease acetaldehyde concentration during storage. Another reason for this decrease is the conversion of acetaldehyde to ethanol by the alcohol dehydrogenase enzyme found in starter culture (Ozer et al., 2007). An increase in ethanol content observed in this study during the storage confirms such considerations. Ethanol, a product of carbohydrate metabolism, was 3-4 times higher in DMK than CMK because of a higher lactose content. Ethanol content was found in the range of 3859.6-6597.7 µg/g in DMK and the range of 1209.8-1739.7 µg/g in CMK. Yeasts have an important place in kefir microbiota, and ethanol produced by yeasts is very effective on the aroma of kefir. Yeasts such as Kluyveromyces spp. can synthesize complex B vitamins by hydrolysing milk proteins, producing thereby CO2 and ethanol by using the available oxygen (Lopitz-Otsoa et al., 2006). In addition, such homofermentative bacteria as Lactobacillus kefir can produce ethanol (Magalhães et al., 2011). While yeasts support bacteria with nutrients such as amino acids and vitamins necessary for their growth, bacteria create the energy required for the activities of yeasts (Viljoen, 2001). Due to the symbiotic relationship between yeasts and bacteria, the incubation temperature and the variety of kefir microbiota, and its ratio in kefir are critical in the formation of aroma substances. In one of the studies on kefir production using grain collected from different provinces, ethanol content was found in the range of 23.19 %, 27.9 %, 9.27 %, and 16.13 % in 4 kefir samples by Dertli and Çon (2017). Similarly, the level of acetic acid, which may be another product of carbohydrate metabolism, was four times higher in DMK than in CMK in this study. Acetic acid concentration remained almost unchanged during the storage (P˃0.05). Diacetyl, an important ketone, is formed by the breakdown of the pyruvate, a product of lactose or citrate metabolism in fermented milk products. However, it is rapidly transformed into acetone employing a diacetyl reductase enzyme (Walsh et al., 2017). In the present study, diacetyl concentration was 1.91-3.85 µg/g in DMK and 1.05-1.25 µg/g in CMK. Diacetyl and acetone concentrations showed irregular changes during storage. The differences in aroma profiles between kefir samples are due to differences in milk constituents from donkey or cow. Additionally, some lactic acid bacteria such as Lactobacillus rhamnosus and Lactobacillus casei grow better in donkey milk and affect the aroma, as stated by Chiavari et al., (2005). The amount of valeric acid was lower compared to other aroma compounds in CMK and could not be detected in DMK. Amounts of butyric and isovaleric acids were 12.25-14.74 µg/g and 3.85-5.42 µg/g in CMK. Butyric acid was not detected in DMK. The higher level of butyric acid in CMK could be attributed to the high content of fat in this sample.
Microbiological results
Observations on microbial counts revealed similarities (P>0.05) between donkey and cow milk kefirs. The number of microorganisms generally increased in both samples as the storage period prolonged. The changes in microbial counts of kefir samples were as follows during the storage for 21 d; total mesophilic aerobic bacteria 7.20-9.50 log cfu/mL, Lactococcus sp. 7.62-10.37 log cfu/mL, Lactobacillus sp. 7.45-7.76 log cfu/mL, L. acidophilus 7.40-7.85 log cfu/mL, Bifidobacterium sp. 5.78-6.91 log cfu/mL and yeasts 7.02-8.74 log cfu/mL (Table 5). Recently, Yirmibeşoğlu and Tefon Öztürk (2020) found that the numbers of total mesophilic aerobic bacteria, yeasts, Lactobacillus sp. and Lactococcus sp. in DMK were as follows 7.87, 6.99, 8.38 and 8.13 log cfu/ml, respectively. Bifidobacterium sp. count maintained the therapeutic level (>6 log cfu/mL) after 21 d of storage in both kefirs. Thus, the examined kefir samples keep their probiotic properties during the storage and donkey’s milk kefir can be considered as a valuable product for health. Similar results have been obtained in many studies on the microbiota of kefir (Guzel-Seydim et al., 2005; Ertekin and Guzel-Seydim, 2010).
Sensory properties
Kefir is a refreshing drink that contains alcohol and CO2 due to acid and alcohol fermentation of milk. It has foamy and thick (yogurt consistency) body characteristics and a slightly sour taste. Table 6 shows the results of the sensory evaluation of the experimental samples. The kefir made using cow milk had a more viscous and foamy structure and preserved these structural characteristics until the end of storage. As a result of these properties, it had a higher point for this attribute. On the other hand, the kefir made using donkey milk had lower scores for the body because of a weaker structure resulting from low contents of fat and protein; however, it points for overall acceptance close to that of the kefir from cow milk. In addition, the donkey’s milk kefir does not have an animal-like taste and odour as intense as koumiss produced from mare’s milk, so the points for taste and aroma were close to the points given for CMK. Yirmibeşoğlu and Tefon Öztürk (2020) stated that panelists preferred DMK less than CMK. In one of the studies conducted on the use of donkey milk for production of fermented products, it was reported that the probiotic fermented donkey milk product containing Lactobacillus rhamnosus and Lactobacillus casei had slightly acidic taste and odour and is appreciated for its sensory properties (Chiavari et al., 2005).
Table 5. Microbiological properties of kefir samples (log cfu/mL) 1 (mean±SD) 2
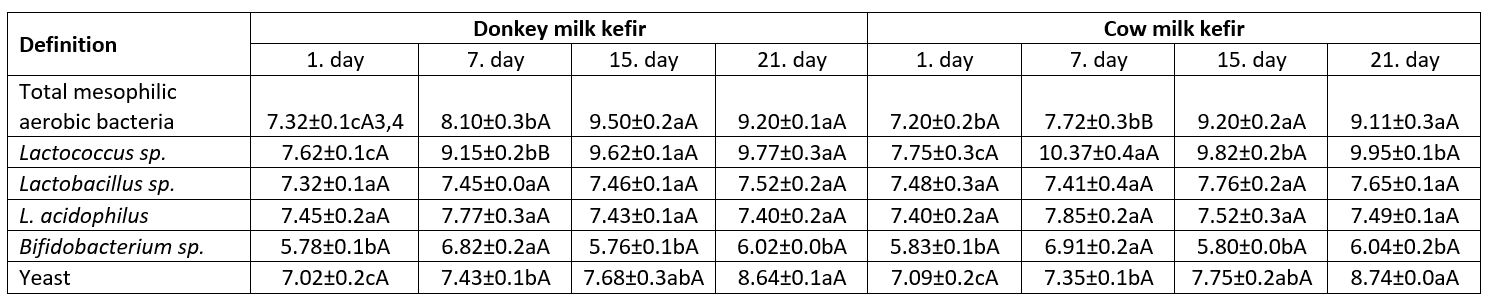
1Data are the averages of triplicates
2Standard deviation
3 a, b, c Means within a row with different lowercase letters show significant differences between storage days, p<0.05
4 A, B Means within a row with different uppercase letters show significant differences between milk type, p<0.05
Table 6. Sensory properties of kefir samples 1 (mean±SD) 2
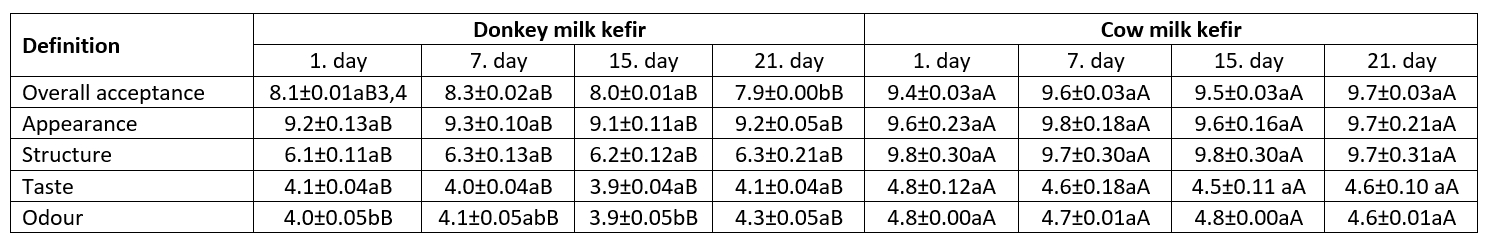
1Data are the averages of triplicates
2Standard deviation
3 a, b, cMeans within a row with different lowercase letters show significant differences between storage days, p<0.05
4 A, BMeans within a row with different uppercase letters show significant differences between milk type, p<0.05
Conclusion
The results showed that donkey milk could be a suitable raw material for manufacturing kefir. The kefir sample made by using donkey milk had a weak structure and low viscosity than that of the kefir sample by using cow milk. However, kefir was preferred with respect to its appearance and taste. With donkey milk, concentrations of volatile aroma compounds increased more in DMK. The kefir sample made by using cow milk was richer in amino acid composition than DMK. It is essential for health that microbiota of DMK with probiotic properties remained at high numbers during storage for 21 days as in kefir produced from cow milk.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
I grateful to Prof. Dr. Asuman Gürsel, Prof. Dr. Zeynep Güzel Seydim and Dr. Çağlar Gökırmaklı for support in the project.
Usporedba sastava, senzorskih svojstava i aromatskih spojeva kefira od mlijeka magarice i od kravljeg mlijeka
Sažetak
U ovom je istraživanju korišteno mlijeko magarice i kravlje mlijeko za proizvodnju kefira. Proizvedenim uzorcima kefira je tijekom 21 dan skladištenja pri 5 °C određivan sastav, mikrobiološka i senzorska svojstva, koncentracija hlapljivih spojeva arome, te sastav aminokiselina. U usporedbi s kefirom od kravljeg mlijeka (CMK), kefir od mlijeka magarice (DMK) imao je niže udjele ukupne suhe tvari, masti i proteina. Korištenje mlijeka magarice kao sirovine rezultiralo je slabijom konzistencijom i punoćom okusa uslijed niskog udjela ukupne suhe tvari i niskog udjela kazeina. S druge strane, u uzorcima DMK utvrđene su izrazito visoke koncentracije acetaldehida i octene kiseline. Osim toga, visok udjel laktoze u mlijeku magarice uzrokovao je veću proizvodnju etanola u kefiru. Najzastupljenije aminokiseline u uzorcima DMK bile su tirozin, valin i arginin. Kefir od mlijeka magarice pokazao se prihvatljivim i prilikom ocjene senzorskih svojstava, ponajviše okusa i mirisa. Na temelju dosadašnjih rezultata može se zaključiti da se od mlijeka magarice može proizvesti probiotički fermentirani napitak.
Ključne riječi: spojevi arome; mliječni napitak; mlijeko magarice; kefir; fermentacija; senzorska procjena
References
Karatepe, P., Yalcin, H. (2014): Health with Kefir. Journal of the Institute of Science and Technology 4 (2), 23-30.
Lopitz-Otsoa, F., Rementeria, A., Elguezabal, N., Garaizar, J. (2006): Kefir: a symbiotic yeastsbacteria community with alleged healthy capabilities. Revista Iberoamericana de Micología 23, 67-74.
Yıldız, G., Hafızoğlu, M., Gün, İ., Balkan, B.M. (2016): The effect of Donkey milk and kefir on serum biochemical parameters in Ehrlich ascites solid tumor in mice, (pp:62-63). 18th International Veterinary Medicine Students Scientific Research Congress, Istanbul, Turkey.
