Introduction
The family Orobanchaceae includes the largest number of parasitic species (more than 2000 species from 100 genera) of facultative and obligate hemi- and holo-parasites (Mutuku et al. 2021). The genus Orobanche L. is one of the most harmful weeds in the world, along with the genera Striga Lour. and Phelipanche Pomel from the same family (Mutuku et al. 2021) and the genus Cuscuta L. of the family Convolvulaceae (Mousavi et al. 2018). It is the most species-rich genus in the family with about 150-200 species of obligate root holo-parasites (Piwowarczyk et al. 2019), although only a few of them are capable of infecting crops. The Orobanche species are widespread in the Mediterranean basin, Central and Eastern Europe, Southern and Western Asia, the United States, and Australia (Parker 2009). They were recorded to parasitize more than 87 dicotyledonous host plants (field crops, fruit trees, and forage plants) belonging to 24 families (Pedraja et al. 2007, Qasem and Foy 2007, Qasem 2009, 2011, Akhter et al. 2020). In particular, nine broomrape species ( Orobanche aegyptiaca Pers., O. cernua Loefl., O. crenata Forrsk., O. cumana Wallr., O. foetida Poir., O. minor Sm., O. palaestina Reut., O. ramosa L., and O. schultzii Mutel) are known to threaten a wide array of wild and cultivated plants in the agricultural systems of temperate and semi-arid biogeographical regions (Qasem 2009, 2011, Samejima and Sugimoto 2018).
Orobanche crenata, crenate broomrape is one of the most common and most harmful broomrape species that parasite important legume and oil crops, vegetables, and ornamental plants of Fabaceae, Solanaceae, Apiaceae, and Asteraceae families grown in arable lands (mainly in Mediterranean countries) (Schaffer et al. 1991, Qasem 2009, Rubiales et al. 2009). In Egypt and other Mediterranean countries, O. crenata threatens Vicia faba L., Daucus carota L., and Lupinus albus L. (Korashi et al. 1996, Ghalwash et al. 2014). Furthermore, it attacks several annual weeds such as Ammi majus L., Melilotus indicus (L.) All., and Rhagadiolus stellatus (L.) Gaertn. in Jordan and Matricaria chamomilla L., Polygonum sp., and Sonchus oleraceus L. in Egypt (Korashi et al. 1996, Qasem 2009). Orobanche crenata also parasites on Carthamus tinctorius L., which is cultivated as an important source of a relatively healthy oil, a natural food dye, and fabrics (Qasem 2009, Khalil et al. 2013, Taha and Matthäus 2018). Oliveira-Velloso (1990) reported that Lactuca serriola L. was among 34 non-crop species that were alternative hosts for O. crenata in Spain.
Taxonomically, Orobanche is considered to be one of the most problematic genera. This is mainly due to the slight morphological variations among the species, the phenotypic variability among the individuals of each species, the limited number of potential taxonomically relevant traits (i.e., strongly reduced vegetative parts), and the loss of color during preservation of herbarium specimens (Mohamed and Musselman 2008, Pujadas-Salva and Munoz Garmendia 2010). Therefore, information on the host plant may facilitate, to some extent, the identification of the Orobanche species. The correct identification of the broomrape species is the first step in developing a meaningful control strategy. In this regard and to help to identify O. crenata plants accurately, we record information on its parasitism on seven new host plant species ( Ammi majus, Arctotis fastuosa, Callistephus chinensis, Carthamus tinctorius, Lactuca serriola, Melilotus indicus, and Tropaeolum majus L.), and provide a full description of morphological characteristics diagnostic to the species, supported by vouchers and images for verification.
Plant material
Orobanche crenata samples infecting Ammi majus, Arctotis fastuosa, Callistephus chinensis, Lactuca serriola, Melilotus indicus, and Carthamus tinctorius plants were collected from the experimental farms of the Faculty of Agriculture (27 11 05 N, 31 09 21 E, 52 m a.s.l. and the Faculty of Science (27 11 27 N, 31 10 17 E, 52 m a.s.l.), Assiut University, Assiut, Egypt during February to April 2021 and 2022. Ammi majus, L. serriola, and M. indicus were grown naturally (wild species) while the remaining species were cultivated as scientific samples for students or as ornamentals. Orobanche crenata samples parasitizing Tropaeolum majus plants (grown as ornamentals) were collected from another location (27 11 22 N, 31 10 09 E, 55 m a.s.l.) from Assiut University during the same period.
Samples and techniques
The inflorescence shoots of crenate broomrape and their host plants were photographed in the field before and after boring of the soil. The attachments between the parasitic weed haustoria and infected host roots were observed and documented. Samples of broomrape and host plants were carefully collected from the field and sent for identification to Assiut University Herbarium (ASTU). For the determination of broomrape species, we used the descriptions and taxonomic keys of Rumsey and Jury (1991), Boulos (2002), Joel et al. (2007), Mohamed and Musselman (2008), and Parker (2013). Voucher specimens of O. crenata attached to different hosts were deposited at ASTU Herbarium, Assiut, Egypt.
Morphological measurements and dissection of crenate broomrape flowers were done using an Olympus SZ61 stereo-microscope and photographed with an Olympus SC100 digital camera.
For light microscopy, pollen grains were treated with 10% potassium hydroxide solution, stained with Safranin (1% Safranin solution in 50% ethanol), and mounted in glycerol before observations. Pollen grains and glandular and non-glandular hairs were photographed by an Olympus SC100 digital camera coupled to an Olympus CX41 microscope. For scanning electron microscopy, hairs of different floral parts, seeds, and pollen grains were examined without coating using a TM3000 miniscope SEM (Hitachi High-Tech).
Morphological characteristics of crenate broomrape such as the height of the plant, the length of the inflorescence, and dimensions of the stem, scale, bract, calyx, corolla, androecium, gynoecium, seed, and pollen grains were recorded, and were based on at least 40 observations.
The incidence of O. crenata was estimated in each wild or crop species and calculated using the following formula:
The incidence of the disease was classified into three categories as described by Kroschel (2002) and Mengistu et al. (2017): low incidence (< 20% infestation), medium incidence (20-50% infestation), and high incidence (> 50% infestation).
Results
Orobanche crenata was reported to parasitize certain crops and weeds of the families Fabaceae ( Vica faba, Pisum sativum L., Lupinus albus, and Cicer arietinum L.), Apiaceae ( Daucus carota, Anethum graveolens L., Carum carvi L., and Cuminum cyminum L.), and Asteraceae ( Matricaria chamomilla L. and Sonchus oleraceus) (Tab. 1). In addition, a few members of the families Amaranthaceae ( Beta vulgaris L.) and Polygonaceae ( Polygonum sp.) were attacked by this parasite (Tab. 1). We recorded crenate broomrape parasitism of two ornamentals ( Arctotis fastuosa, and Callistephus chinensis), three wild ( Ammi majus, Lactuca serriola, and Melilotus indicus), and two cultivated ( Carthamus tinctorius and Tropaeolum majus) plant species from Egypt (Fig. 1, Fig. 2, Tab. 1).
Tab. 1. A list of host species infested by Orobanche crenata Forssk. in Egypt including seven new records in this study.

Fig. 1.Orobanche crenata parasitizing Arctotis fastuosa and Callistephus chinensis. A-E – Arctotis fastuosa, F-J – Callistephus chinensis. A, B, F, G – general habit of O. crenata with the host plant in the field. C, H – Orobanche inflorescence. D, I – O. crenata attached to the root of the host plants. E, J – attachment area of O. crenata haustoria on the root of the host plants. Scale bars: 10 cm in ( A, B, C, D, F, G, H, I) and 2 cm in ( E, J).

Fig. 2.Orobanche crenata parasitizing different host species in Egypt. A-D – Ammi majus, E-H – Lactuca serriola, I-L – Melilotus indicus, M-P – Carthamus tinctorius, and Q-T – Tropaeolum majus. A, E, I, M, Q – general habit of O. crenata with the host plant. B, F, J, N, R – Orobanche inflorescence. C, G, K, O, S – O. crenata attached to the root of the host plants. D, H, L, P, T – attachment area of O. crenata haustoria on the root of the host plants. Scale bars: 10 cm in ( A, C, E, G, I, K, M, O, Q, S) and 2 cm in ( B, D, F, H, J, L, N, P, R, T).
A morphological comparison of O. crenata specimens infecting the seven different hosts revealed the main diagnostic morphological characteristics that can be used for easier identification.
In general, morphological observations of whole individuals, inflorescence, scales, and floral parts of specimens indicated that crenate broomrape was erect, stout, unbranched, and 14 to 83 cm in height and 0.4 to 1.5 cm in width (Fig. 1, Fig. 2, Tab. 2). The inflorescence was a dense spike that occupies about 31–72% of the emerged shoot (Fig. 1, Fig. 2, Tab. 2). The scales (leaves) were lanceolate with acute or acuminate tips, 1.1-2.3 cm in length, and 0.3-0.7 cm in width (Fig. 1, Fig. 2, Tab. 2). The flowers were strongly fragrant when fresh and subtended with one bract and no bracteoles (Fig. 1, Fig. 2, Fig. 3). The bracts were narrowly lanceolate, glandular-hairy, yellowish to brownish, 0.8-2 cm in length, and 0.25-0.6 cm in width (Fig. 3, Fig. 4, Tab. 2). The calyx was glandular-hairy, unequally bifid, and deeply divided to more than halfway (50-75%) (Fig. 3, Fig. 4, Tab. 2). The calyx was 0.7-1.5 cm in length and each half of the calyx pair had a width of 0.2-0.6 cm (Tab. 2).
Tab. 2. Morphological characteristics of Orobanche crenata infecting wild ( Ammi majus, Lactuca serriola, and Melilotus indicus) or cultivated ( Arctotis fastuosa, Callistephus chinensis, Carthamus tinctorius, and Tropaeolum majus) plants. Values represent means ± SD of replicates based on 40 measurements per character (N = 40).
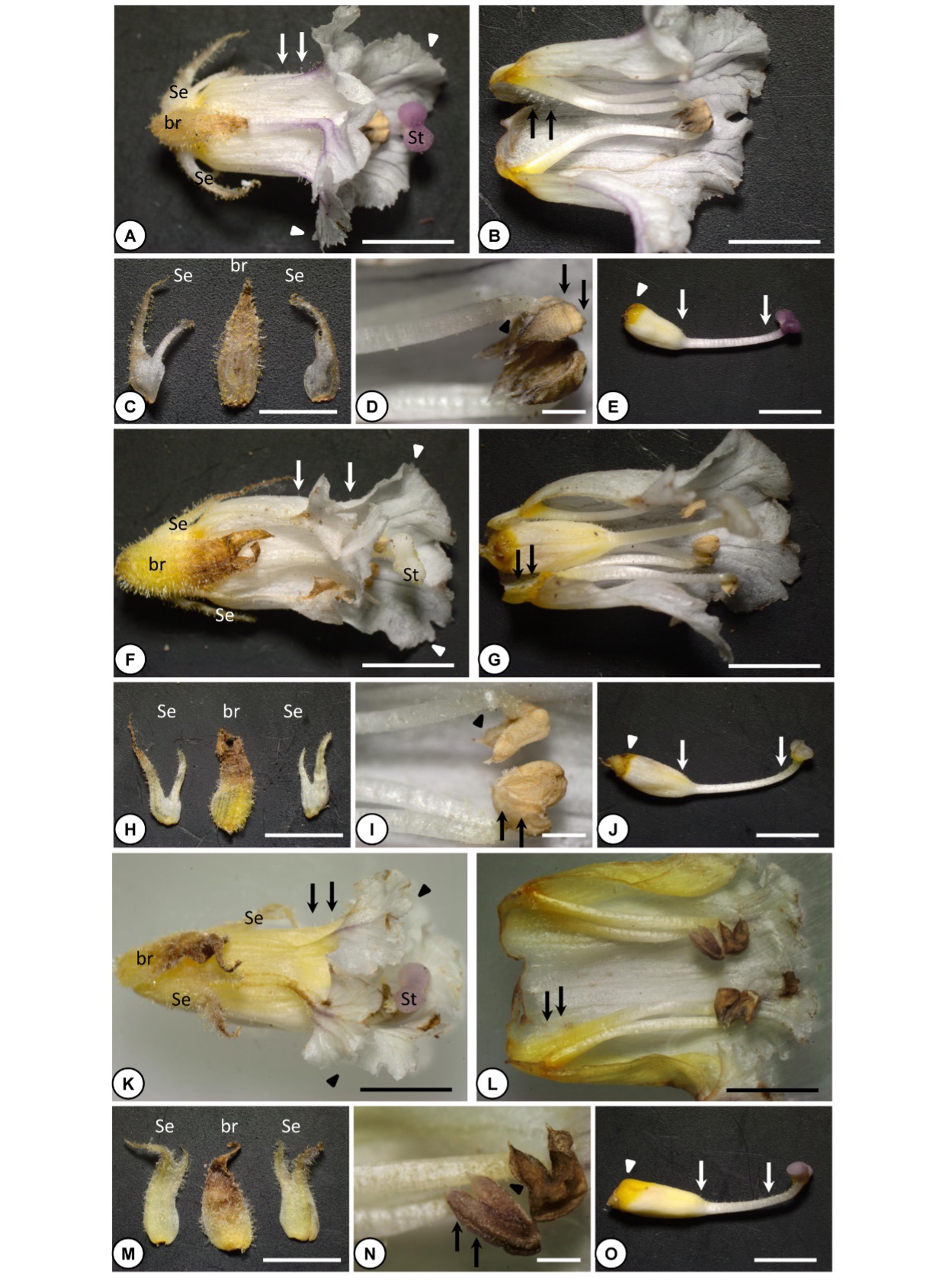
Fig. 3. Morphological characteristics of Orobanche crenata flowers. A-J – O. crenata parasitizing on two individuals of Tropaeolum majus and K-O – O. crenata parasitizing on Ammi majus. A, F, K – a flower at the anthesis stage. Note purplish stigmas and purplish veins of petals in ( A, K), while whitish stigmas and dull whitish veins of petals in ( F). Also, note the glandular hairs visible on the abaxial side of petals (arrows) and the denticulate (irregularly notched) corolla (arrowheads). B, G, L – dissected flower showing the androecium (four stamens). Note non-glandular hairs visible on the lower part of stamens (arrows). C, H, M – a bract (middle) and sepals (right and left) covered by dense glandular hairs. D, I, N – dehiscent anthers. Note the glabrous surface of the anthers (arrows) and the glandular hairs on the upper part of the filaments (arrowheads). E, J, O – gynoecium. Note glandular hairs visible on the style and the upper part of the ovary (arrows) and nectary (arrowheads). br: bract, se: sepals, st: stigma. Scale bars: 0.5 mm, D, I, N: 0.1 mm.
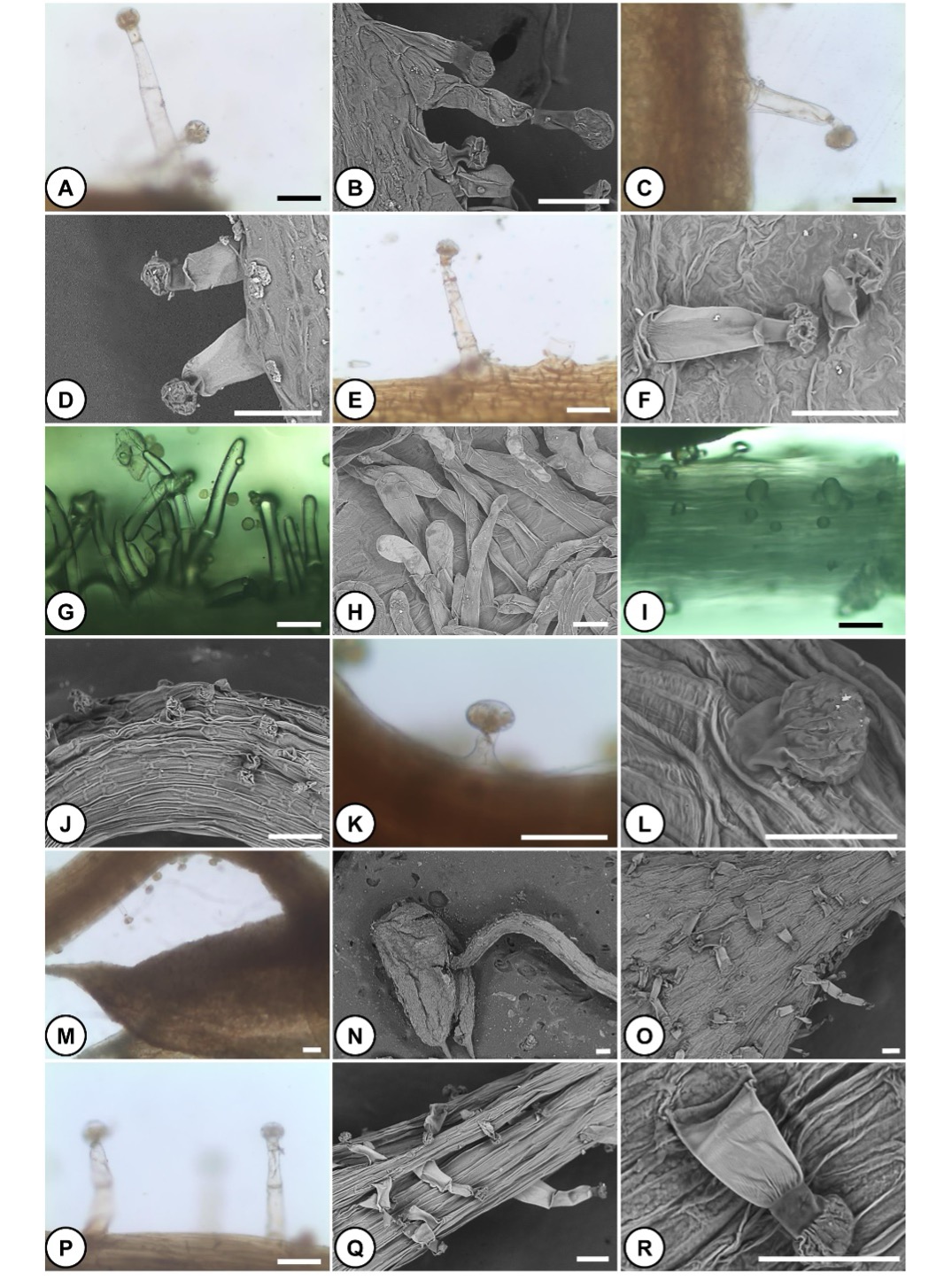
Fig. 4. Hairs of different floral parts of Orobanche crenata parasitizing Tropaeolum majus. A, B – glandular hairs covering the abaxial side of the bract. C, D – glandular hairs covering the abaxial side of the calyx. E, F – glandular hairs covering the abaxial side of the corolla. G, H – non-glandular hairs covering the lower part of the filament. I, J, K, L – glandular hairs covering the upper part of the filament. Note the short length of these hairs. M, N – glabrous surface of anther. Note the density of glandular hairs on filament increased towards the anther. O – glandular hairs covering the ovary. P, Q, R – glandular hairs covering the style. Scale bars: 100 μm in all, except L: 50 μm.
The corolla was whitish or yellowish with purplish veins, especially on the lips (Fig. 3). Few crenate broomrape individuals had flowers with no purplish but usually dull white veins (Fig. 3). The corolla was always yellowish at the base, especially at the position of stamen insertion in the inner side of the corolla (Fig. 3). The corolla was bilabiate with a long tube curved towards the lower lip, opening out to widely divergent lobes (1-2 cm in width), and denticulate (irregularly notched) (arrowheads in Fig. 3). The corolla tube is not constricted, slightly longer than the calyx, and with dispersed glandular hairs at the abaxial side (arrows in Fig. 3, Fig. 4). The length of the corolla was 1.4-2.4 cm (Tab. 2).
The stamens were 0.8-1.3 cm in length, provided with dense multi-cellular non-glandular hairs at the lower 23–40% part of the filaments (arrows in Fig. 3, Fig. 4, Tab. 2) and with dispersed short glandular hairs on the remaining upper part (arrowheads in Fig. 3, Fig. 4). The maximum density of the glandular hairs in the filament was at the region just below the anther. Filaments were inserted 2-4 mm from the base of the corolla tube (Fig. 3, Tab. 2). Anthers were glabrous, brownish-greyish, with a rounded tip and a pointed base at each anther lobe (arrows in Fig. 3, Fig. 4).
The size of pollen grains varies from small to medium (20.9-33.1 μm long and 20.4-31.2 μm wide) (Fig. 5, Tab. 2). Pollen grains were inaperturate with a surface covered by many granules of irregular sizes interrupted with some grooves (Fig. 5).
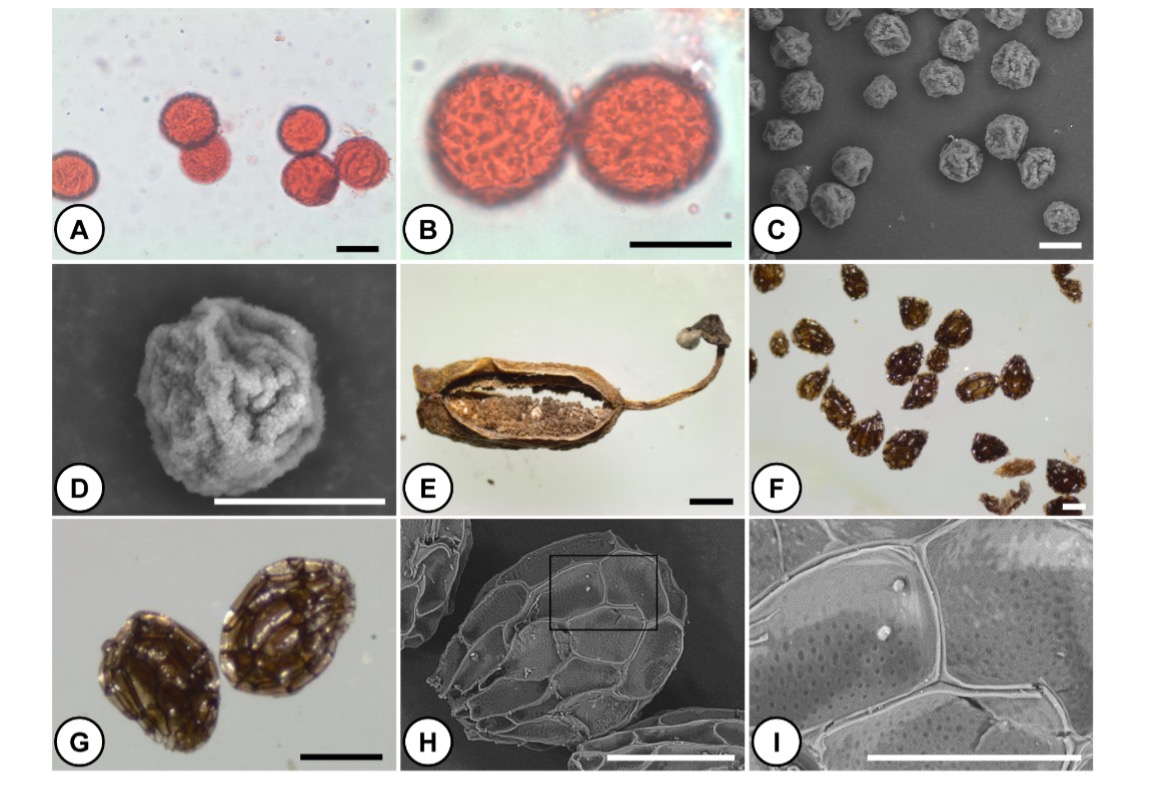
Fig. 5. Pollen grains, capsule, and seeds of Orobanche crenata flowers parasitizing Tropaeolum majus. A, B – light micrographs of inaperturate pollen grains. C, D – SEM micrographs of inaperturate pollen grains. Note the granules on the pollen surface. E – a dehiscent capsule with seeds. F, G – light micrographs of seeds at different magnifications. H – SEM micrograph of a single seed. I – a magnified view of the boxed area of ( H). Scale bars: 20 μm in ( A, B, C, D), 0.5 mm in ( E), 0.2 mm in ( F, G, H), 0.1 mm in ( I).
The ovary was ovoid, 0.5-1.5 cm in length and 0.3-0.8 cm in width, covered with dispersed glandular hairs on its upper part, and lay on a yellow-orange nectary disc (arrowheads in Fig. 3, Fig. 4, Tab. 2). The style was 0.6-1.1 cm in length and covered with dispersed glandular hairs (arrows in Fig. 3, Fig. 4, Tab. 2). The stigma was bi-lobed, 2-4 mm in width, with a pleasant carnation scent, pinkish, pale purplish, or whitish (Fig. 3, Tab. 2).
The fruit was a two-split capsule, with several hundred seeds (Fig. 5). Seeds were very small, 0.26-0.55 mm long and 0.17-0.38 mm wide, with a terminal funicular attachment. Seeds were brown in color, ellipsoid in shape with a reticulated coat made of polygonal cells (Fig. 5).
The incidence of O. crenata varied within the wild and cultivated plants (Fig. 6). Tropaeolum majus and Arctotis fastuosa plants had the highest incidence of O. crenata, 100% and 90%, respectively. Ammi majus was also highly infected (about 75%). Carthamus tinctorius showed medium-level O. crenata parasitism (41.3%). However, the parasitism of O. crenata was also recorded on individuals of the wild plants Lactuca serriola and Melilotus indicus, although with the lowest incidence.
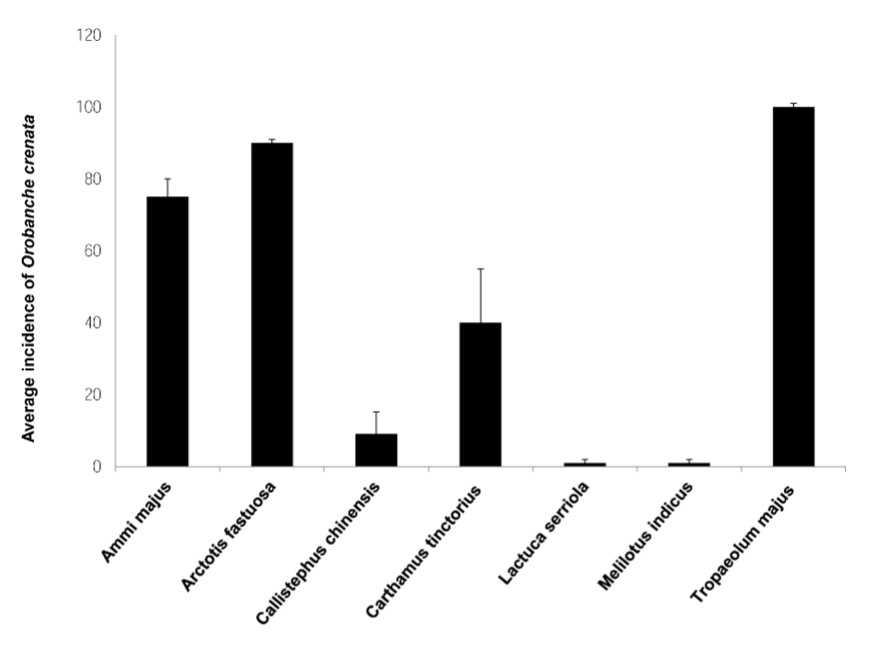
Fig. 6. Incidence of Orobanche crenata in wild ( Ammi majus, Lactuca serriola, and Melilotus indicus) or cultivated ( Arctotis fastuosa, Callistephus chinensis, Carthamus tinctorius and Tropaeolum majus) plants. Error bars indicate the standard error.
Discussion
To the best of our knowledge, this investigation is the first report on parasitism of the crenate broomrape on Arctotis fastuosa and Callistephus chinensis. We also recorded for the first time its parasitism on three wild ( A. majus, L. serriola, and M. indicus) and two cultivated ( C. tinctorius and T. majus) species from Egypt. Results of our study on the vegetative and reproductive parts of O. crenata plants infecting the seven plant species were, in general, consistent with previous reports. Only a few differences in some measurements were recorded; they were mainly due to environmental factors or the characters of host plants.
The crenate broomrape plants exhibited some morphological characteristics that can be used in differentiating it from other broomrape species, and thus, are of great value in the identification and classification of the broomrape species. These diagnostic morphological characteristics include the form of the stem (stout, unbranched); the absence of bracteoles; the shape of the calyx; the size, scent, color, and shape of the corolla; the insertion position of stamens and the distribution of trichomes on stamens; the aperture number and surface ornamentation of pollen grains.
The stems of the crenate broomrape were erect, stout, and unbranched. We showed that the height of O. crenata plants ranged from 14 to 83 cm. Previous studies showed that the height of O. crenata varied from 38-52 cm (Akhter et al. 2020), 30-70 cm (Restuccia et al. 2009), and up to 100 cm (Rubiales et al. 2008). The branching of the stem is an important feature that distinguishes the sections Orobanche and Trionychon, two sections of the genus Orobanche. In contrast, the height of the stems provides no significant value in distinguishing the broomrape species.
Our results showed that the flowers had no bracteoles, the bracts were 0.8-2 cm in length and 0.25-0.6 cm in width, and the calyx was unequally bifid to more than halfway (50-75%), 0.7-1.5 cm in length and each half of the calyx pair had a width of 0.2-0.6 cm. Akhter et al. (2020) reported that the bracts were 0.38-0.72 cm long and 0.25-0.61 cm wide; and the calyx was divided at the base, 1.4-1.8 cm long, and 0.4-0.6 cm wide. Rubiales et al. (2008) described the calyx as bidentate, with segments free, and 1.3-1.8 cm long. The absence of bracteoles in the crenate broomrape and the calyx with two lateral halves is characteristic of species from the section Orobanche. In contrast, the presence of two extra bracteoles besides the bract and the entire campanulate calyx are characteristic of the section Trionychon. The size, shape, and depth of indentation of the calyx halves were previously used as important taxonomic features facilitating the classification of broomrape species (Kreutz 1995).
The color, scent, size, and shape of the corolla are among the most significant traits used for the identification of broomrape species. We showed that the corolla was bilabiate with a long tube curved towards the lower lip, strongly fragrant, whitish or yellowish with purplish veins, especially on the lips; however, few individuals had flowers with no purplish veins. The corolla was with widely divergent lobes, not constricted, denticulate (irregularly notched), and 1.4-2.4 cm long. Rubiales et al. (2008) and Akhter et al. (2020) reported that the corolla of O. crenata had divergent lips often whitish or yellowish with lilac veins, glandular-pubescent, and 1.8 to 2.8 cm long; whereas Restuccia et al. (2009) described the corolla as white, subglabrous, usually 2-3 cm long. Previous studies have reported that the bilabiate corolla with a long tube curved towards the lower lip is characteristic of section Orobanche (Restuccia et al. 2009, Zare and Dönmez 2016). The color of the corolla in broomrape species varied greatly from white to yellow, red, purple, and blue and this diversity allows it to be used in the identification of different species (Boulos 2002, Mohamed and Musselman 2008, Parker 2013). For example, O. cernua can be differentiated from O. crenata and O. minor by the color of the corolla (glossy white with blue limbs in O. cernua) and the constriction of the corolla above the ovary (constricted in O. cernua) (Boulos 2002, Mohamed and Musselman 2008, Parker 2013). Mohamed and Musselman (2008) separated O. crenata from O. cernua by the denticulate (irregularly notched) corolla in O. crenata, in contrast to the entire corolla of O. cernua. The scent, size, and divergence of the corolla are diagnostic of the crenate broomrape and are useful in its differentiation from the closely related O. minor. The corolla of crenate broomrape is larger in size (almost exceeding 1.5 cm), widely divergent, with a pleasant scent. In contrast, the corolla of O. minor rarely exceeds 1.5 cm, is not divergent, and is unscented or has a fetid scent (Rumsey and Jury 1991, Parker 2013).
Our observations confirmed that the stamens were 0.8-1.3 cm long, provided with dense non-glandular hairs at the lower part of the filaments and with dispersed short glandular hairs at the upper part. Anthers were glabrous and brownish-greyish and the filaments were inserted 2-4 mm from the base of the corolla tube. Akhter et al. (2020) showed that the stamens were hairy and 0.5 ± 0.08 cm in length. Rubiales et al. (2008) reported that the anthers were brown, glabrous, or subglabrous, and the filaments were inserted 2-3 mm above the base of the corolla. The insertion position of stamens and the distribution of trichomes on stamens were used by Rumsey and Jury (1991), Joel et al. (2007), and Parker (2013) for the classification of the broomrape species. The crenate broomrape can be differentiated from O. cernua by the insertion position of stamens (in the middle of the corolla in O. cernua vs. in the lower part of the corolla in O. crenata) and the distribution of trichomes on stamens (hairy below in O. cernua vs. hairy throughout the filament in O. crenata).
Pollen grains in our observations had small to medium sizes (20.9-33.1 μm long and 20.4-31.2 μm wide) and were inaperturate and granulate with some grooves. Coutinho et al. (2019) showed that pollen grains of O. crenata were granulate with small to medium sizes (24.7-30.5 μm long and 21.9-26.9 μm wide). Zare et al. (2014) described the surface of O. crenata pollen grains as verrucate, inaperturate, and pollen size as small ranging from 20.4-26.2 μm long and 20.4-26.3 μm wide, whereas Akhter et al. (2020) described much smaller pollen grains of O. crenata (7.8–10.9 μm long and 7.8–10.9 μm wide). Pollen morphological characteristics are of a high value for the classification of the broomrape species (Abu Sbaih et al. 1994, Zare et al. 2014, Coutinho et al. 2019). For example, the aperture number and surface ornamentation of pollen grains suggest the separation of section Orobanche from section Trionychon. Pollen grains of section Orobanche are generally inaperturate with granulate or scabrate-verrucate ornamentation; whereas, those of section Trionychon are, in general, tri-colpate with microreticulate-scabrate ornamentation (Abu Sbaih et al. 1994, Zare et al. 2014).
The fruit in our observations was a two-split capsule, with several hundred very small seeds. Seeds were ellipsoid in shape with a reticulated coat made of polygonal cells, small (0.26-0.55 mm long and 0.17-0.38 mm wide). Zare and Dönmez (2016) showed that the fruit of O. crenata from Türkiye was a loculicidal capsule dehiscing with two slits, and the seeds were small (0.24-0.38 mm long and 0.16-0.24 mm wide) with reticulate ornamentation and ovoid, pear-shaped, to sub-globose shape. Aly et al. (2012) and Akhter et al. (2020) described seeds as small, dust-like, 0.2-0.4 mm in length and 0.1-0.3 mm in width, whereas Plaza et al. (2004) reported that seeds of O. crenata were ellipsoid in shape, 0.28-0.48 mm long, and 0.19-0.3 mm wide. Seed morphological features such as size, shape, and color showed little variation among species and were not useful for the classification of the broomrape species (Zare and Dönmez 2016).
According to Boulos (2002, 2009), there are nine Orobanche species in Egypt, O. aegyptiaca, O. cernua, O. crenata, O. lavandulacea Rchb., O. minor, O. mutelii F.W.Schultz, O. nana Noë ex Reut., O. ramosa L., and O. schultzii. Six of them ( O. aegyptiaca, O. lavandulacea, O. mutelii, O. nana, O. ramosa, and O. schultzii), which represent the section Trionychon, have been transferred to the genus Phelipanche (Schneeweiss et al. 2004, Banfi et al. 2011). O. crenata can be easily distinguished from these six species by the absence of bracteoles, the single stout stem, the calyx with two lateral halves, and the inaperturate pollen grains. The remaining two species, O. cernua and O. minor, in addition to O. crenata, now represent the re-circumscribed Orobanche in Egypt. O. cernua can be distinguished from O. crenata and O. minor by the constriction of the corolla above the ovary, the color of the corolla veins, and the insertion of stamens in the corolla tube. On the other hand, O. crenata can be separated from O. minor by the pleasant scent of the whole plant, the large size of the corolla (> 1.5 cm), and the wide divergence of the corolla (Rumsey and Jury 1991, Parker 2013).
Orobanche crenata is a holo-parasitic species with a high rate of target crop parasitism (Qasem 2009, Restuccia et al. 2009, Qasem 2011). The severity of parasitism hinders the cultivation of many strategic crops in the Mediterranean area including legumes, , and vegetables (Parker 2009, Mutuku et al. 2021). Our results revealed that the severity of incidence of O. crenata reached 90% and 100% in Arctotis fastuosa and Tropaeolum majus plants, respectively, 75% in Ammi majus, and 41.3% in Carthamus tinctorius. However, a low incidence was recorded in Lactuca serriola and Melilotus indicus. Orobanche crenata plants produce a high number of tiny seeds per plant. Seeds remain viable for several years in the soil and they have the ability to spread over long distances to infest new areas. Negligence, improper farming practices, and lack of knowledge about the host range of O. crenata are the main causes of the spread and the increase of infestation. Raising awareness about the biology of Orobanche is a key element in overcoming the spread of infestation.
