Introduction
The functional potential of plants is related to their phytochemical component, including prebiotic polysaccharides, phenolic compounds, flavonoids, antioxidants, tannins, and other oxidative stress gene response metabolites (Alongi and Anese, 2022). Aloe barbadensis Miller, also known as Aloe vera (belonging to the Liliaceae family), is a succulent plant native to Africa, the Arabian Peninsula, and the Indian Ocean Islands. Carbohydrates (sugars and polysaccharides) are the most abundant component in the inner leaf part containing the gel with a ratio of 72 %, followed by minerals (16 %), lipids (4 %), protein (7 %), and phenolic compounds (1 %). Polysaccharides found in Aloe vera gel, especially acetylated polysaccharides known as "acemannan", have been associated with many health disorders and are known to exhibit biological activity alone or separately from other gel components (Hamman, 2008). Aloe vera mucilaginous gel has nutritional and therapeutic properties with its high bioactive contents. Aloe vera pulp and leaf gel have wound healing, anti-fungal, anti-diabetic, anti-inflammatory, anti-cancer, immunomodulatory and gastric protective properties (Im et al., 2010; Budai et al., 2013). In addition to its health benefits, this gel is used as a functional food source in beverages, ice creams and confectionery due to its flavouring, texture improving and protective properties (Boudreau et al., 2006). Most of the medicinal effects of Aloe vera gel polysaccharides i.e. glucomannan may be due to the synergistic effects of other components and may have potential prebiotic properties (Valverde et al., 2005).
Prebiotics are selectively fermentable ingredients that stimulate the activity of the gastrointestinal (GIS) system microbiota, providing benefits to host health (Brownawell et al., 2012). In addition, they promote the growth of bifidobacteria and lactobacilli in the colon without hydrolysis in the small intestine (Patel and Goyal, 2011). Aloe vera mucilaginous gel is rich in a polysaccharide acemannan (β-(1,4)-acetylated soluble polymannose) and could possibly be a source of alternative prebiotics for GUT microbiota (Rodriguez et al., 2010; Campestrini et al., 2013). The prebiotic potential of Aloe vera mucilage was evaluated by in vitro fermentation by Gullón et al. (2015). Prebiotic effects of similar polysaccharides with Aloe vera gels were previously reported by Hamman (2008), Simoes et al. (2012) and Zapata et al. (2013). Konjac glucomannan hydrolysate promotes the growth of probiotic strains Lactobacillus sp. and Bifidobacteria sp. Al-Ghazzewi et al. (2007) and Connolly et al. (2010) determined the in vitro prebiotic potential of konjac glucomannan hydrolyzate. Rivas et al. (2012) determined the bifidogenic properties of mannan from the Pinus pinaster tree.
Obesity occurs as a result of excess fat accumulation in the body and can be indicated by a Body Mass Index values above 30 kg/m2 (Jagielski et al., 2023). Stevia attracts attention as a new and promising natural, calorie-free herb in the world market. Stevia is 250-300 times sweeter than sugar and with this feature, it finds application as an alternative to sucrose/sugar substitutes or artificial sweeteners (Anton et al., 2010; Zou et al., 2020).
Prebiotic substrates are selectively fermentable bioactive compounds that benefit host health by creating specific changes in the composition of the GIS microbiota (Dorna et al., 2019; Usta-Gorgun and Yilmaz-Ersan, 2020). Polysaccharides contained in the Aloe vera extract can exhibit prebiotic functions. However, the prebiotic characteristics of Aloe vera gels have not been studied in detail yet. In order to better understand the beneficial effects of plant bioactive compounds on the general health of the host and to plan effective nutritional models, it is very important to examine the interactions between food components and intestinal populations (Luca et al., 2020; Molinari et al., 2022).
In this study, the effect of Aloe vera plant mucilaginous gel polysaccharides on the gel fermentation, firmness, functional properties and correlation with stevia in functional yoghurts were investigated by examining bacterial growth, bioactive and textural properties.
Material and methods
Materials
Cow milk used in the production was obtained from a dairy plant in the Bursa region. Stevia was obtained from PCSB (PureCircle Sdn Bhd, Malaysia). The Aloe vera plant was purchased from regional producers. In the study, a mixed probiotic yoghurt culture containing S. thermophilus, L. delbrueckii subsp. bulgaricus, L. acidophilus and B. animalis subsp. lactis (Yo-mix 205) was obtained from Danisco (France).
Preparation of Aloe vera gel
The large-leaved Aloe vera plant was obtained from the producers and used in the preparation of the gel. After the leaves were separated from the stem, the cut pieces were filtered for 1 hour in an upright position to remove the yellow-coloured latex liquid. Then, the outer shell layer was separated from the inner part with the help of a knife and the gel in the mucilage structure was taken out. The ontained gel was mixed for 15 minutes by a mixer at 23±1 °C and passed through a fine sieve. Then, the gel was heat treated at 65 °C for 15 minutes. At the end of the period, rapid cooling was carried out with ice.
Yoghurt production
Cow milk (11.4 % dry matter, 3 % fat, 3 % protein, 4.7 % carbohydrate and 120 mg Ca/100 mL) used in yoghurt production was divided into three parts (I, II, III) according to the study design given in Table 1. Then the milk was heat treated at 95 °C for 5 minutes and cooled to 45±1 °C. Subsequently, stevia (0.025 %) and Aloe vera gel (3 %) were added to the milk samples and mixed for 1 minute with an Ultra-Turrax blender until they became homogeneous. The starter culture used in production was prepared according to the method stated Karaman and Ozcan (2021). 3 % lyophilized mixed probiotic yoghurt culture was added to the cooled milk (40 °C) and incubated at 40±1 °C until pH reached 4.70. At the end of the incubation, the yoghurts were cooled to +4 °C for 24 hours to determine the fermentation profile and milk gel properties. This study was carried out in 2 replications and all analyses were carried out in a parallel.
Table 1. Experimental design of production
Analysis
Lactic acid bacteria (LAB) was determined by the plate count method on MRS (Man Rogosa Sharpe) agar (Fonteles et al., 2013). The plates were incubated at 37 °C for 72 h under anaerobic conditions and total number of LAB was expressed in log10 cfu/g of yoghurt.
pH values in the yoghurt fermentation stage (1 h, 3 h and 6 h) and the final product (overnight cold storage) were measured with Hanna pH 2211 (Hanna Instrument-USA) brand pH meter (AOAC, 2012). Colour determination was determined using the MSEZ-4500L HunterLab (Virginia, USA) instrument. Readings were made on black and white plates to standardize the device. Brightness (L* 0 black, 100 white), a* (+red, -green) and b* (+yellow, -blue) values were determined. Textural properties were measured by a TA.XT plus (Stable Micro Systems, Godalming, UK) using back extrusion test. The parameters evaluated as a result of the measurement are consistency (gs) and cohesiveness (g) (Karaman and Ozcan, 2021).
The total phenolic constituents of the sample extracts were determined as equivalent to gallic acid using the Folin-Ciocalteu reagent (Velioglu et al., 1998). The extract solution (0.1 mL) of samples was put in a volumetric flask. After adding 0.75 mL of Folin-Ciocalteu reagent to 100 μL of extract the mixture was incubated for 5 minutes at 25±2 °C. Then 0.75 mL of 6 % sodium carbonate solution was added and incubated for 90 minutes at 25±2 °C. Absorbance was measured at 725 nm by using an Ultraviolet-visible spectrophotometer (UV-1800 Shimadzu, Japan). It was determined using the gallic acid standard curve (linear range: 10-400 ppm, R2= 0.998) and the results were expressed as mg gallic acid equivalent (GAE) per 100 mL of sample. In the CUPRAC assay, cupric reducing the antioxidant capacity of yoghurt samples was determined using the method by Apak et al. (2004). 1 mL of 10 mM copper (II) chloride, 1 mL of 7.5 mM Neocuproin, 1 mL of 1 M ammonium acetate and 1 mL of distilled water were added to 100 μL of extract, respectively. After 30 minutes of incubation at 25±2 °C, absorbance was read at 450 nm at a spectrophotometer (UV-1800 Shimadzu, Japan). The results were recorded as mg trolox equivalent (TE) per 100 mL of sample.
The sensory analysis was performed by using a 9-point structured hedonic scale. Samples were served at 4±1 °C in a randomized with transparent 50 mL plastic cups with a three-digit codes. The overall acceptability of samples was evaluated by nine consumers using the aferomentioned scales with 1 standing for extremely dislike and 9 standing for extremely liked (Drake, 2007).
ANOVA and Fisher’s least significant difference (LSDs) method of Minitab 17, USA, software was used for statistical analysis (p<0.05 and p<0.01). In addition, principal component analysis (PCA) was performed using a Statistica software.
Results and discussion
Fermentation and bacterial viability
Lactose as the main energy and carbon source in milk is fermented by LAB. The fermentation profile, the total number of lactic acid and bacterial growth in probiotic yoghurt samples containing Aloe vera gel and stevia are given in Figure 1. The pH (1 h, 3 h and 6 h of fermentation) of the yoghurt samples containing Aloe vera (II) decreased faster and the formation of yoghurt gel occurred earlier during the fermentation. It is thought that Aloe vera polysaccharides and phenolic compounds increase acidity in yoghurts and promote the growth of microorganisms, and as a result of increased micro activity, they cause a faster decrease in pH. In general, the pH profiles of the samples were similar.
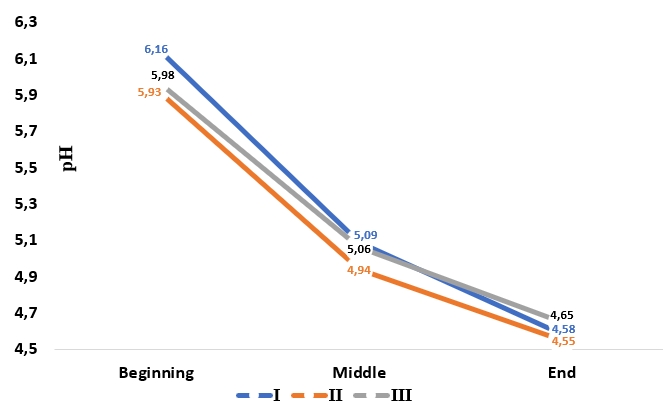
Figure 1. Fermentation process of yoghurt samples (at 1 h, 3 h and 6 h) without additives (I), with Aleo vera mucilaginous gel (II) and with Aleo vera mucilaginous gel and stevia (III)
The acidity that develops at the end of fermentation and during cold storage plays an important role in the stabilization of the yoghurt gels. During cold storage for 24 h, the lowest pH value was determined in the sample containing Aloe vera gel and stevia (III) (Figure 2a). The effect of stevia on potential bacterial growth and pH profile has been explained by Ozdemir and Ozcan (2020). When the total lactic acid bacteria count of yoghurt samples was examined, the highest value was found at 9.05 log cfu/g in the sample containing stevia and Aloe vera gel correlated with Figure 2 b (p<0.01). Plant extracts, juices and cell polysaccharides with phenolic compounds and fermentable carbon sources increase the activity of LAB and probiotic strains in the milk matrix (Kling, 2016; Barat and Ozcan, 2018; Karaman and Ozcan, 2021). L. delbrueckii subsp. bulgaricus and S. thermophilus are also known as yoghurt bacteria, important in the formation of yoghurt gels by biochemical ways such as glycolysis, proteolysis and lipolysis. When yoghurt bacteria form a synbiotic system with probiotic bacteria such as L. acidophilus and B.animalis subsp. lactis, a different substrate fermentation and metabolic degradation (Ruiz-Aceituno et al., 2020).
In order to increase beneficial microorganisms in the intestine, foods containing probiotics, prebiotics and symbiotics should be consumed. It has been stated that the minimum amount that should be taken with food in order to see the therapeutic effects of probiotics is 10⁶-10⁷ cfu/g (Yahfoufi et al., 2018). In the study, it was determined that the addition of Aloe vera gel and stevia promoted the growth of probiotic bacteria in probiotic yoghurts and the microorganism viability level was at the biotherapeutic level (>7 log cfu/g). Aloe vera mucilage polysaccharides and stevia appear to stimulate the total number of lactic acid bacteria (Figure 2b). Various researchers already examined in vitro and in situ the potential prebiotic effect of Aloe vera mucilage (Basannavar et al., 2014; Gullón et al., 2015) and steviol glycosidase (Koyama, 2003; Ozcan et al., 2021; Ozcan and Eroglu, 2022; Ozcan and Eroglu, 2023) on the growth of probiotic bacteria in order to improve GIS health.
Short-chain fatty acids (SCFAs) are formed as a result of the fermentation of carbohydrates that reach the colon without being digested by bacteria. It has been determined by researchers that Aloe vera gel and stevia increase SCFA production in in vitro environment and milk matrix (Gullón et al., 2015; Ozcan and Eroglu, 2022; Ozcan and Eroglu, 2023). Studies show that Aloe vera is used in the production of functional milk and dairy products (Verma et al., 2018). Panesar and Shinde (2012) stated that the numbers of L. acidophilus and B. bifidum bacteria were sufficient in probiotic yoghurts fortified with Aloe vera. Bassannavar et al. (2014) also stated that the addition of Aloe vera gel to fermented milk caused an increase in the number of L. casei
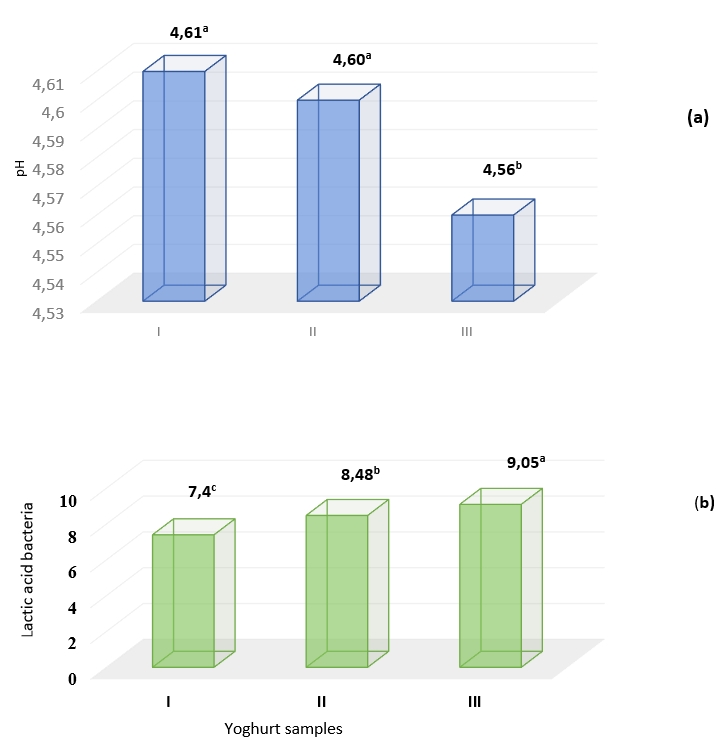
Figure 2. a) Acidity/pH and b) the number of viable lactic acid bacteria (log cfu/g) of yoghurt samples without additives (I), with Aleo vera mucilaginous gel (II) and with Aleo vera mucilaginous gel and stevia (III) +
+Significance level; at p<0.01, different letters indicate significant differences of treatments
Various non-starch carbohydrates such as phenolic compounds, dietary fibers, and polysaccharides contained in plant cell mucilages are hydrolyzed by probiotic bacteria and used as a source of prebiotics (Saad et al., 2013; Karaman and Ozcan, 2021). It can be said that Aloe vera polysaccharides and the phytochemicals will have stimulated the growth of probiotic bacteria and fermentation.
Bioactive properties
Plants have antioxidant effects with their high phenolic compounds (Ignat et al., 2011). It has been determined in many studies that foods enriched with phenolic components and antioxidants can help prevent various diseases such as cancer, diabetes, neurodegenerative and cardiovascular diseases (Shebis et al., 2013; Akbarirad et al., 2016). To measure antioxidant capacity numerous methods have been developed. In method CUPRAC, the basic reaction is the reduction of Cu (II) to Cu (I) by the sample antioxidants (reductants). In the study, phenolic component biotransformation occurred in the yoghurt samples containing Aloe vera gel and stevia. In samples containing stevia and Aloe vera mucilaginous gel (III), the use of phenolic compounds as substrates due to the development of bacteria and fermentation caused (Figure 1, 2) the total pheneolic content to be lower. Antioxidant activity decreased in correlation with the amount of phenolic components (p<0.01) (Figure 3a,b).
Probiotic LAB loses its viability during industrial processing and when exposed to unfavourable factors such as high or low temperature, low pH, bile salts, oxygen or limited nutrition that cause stress in the GIS tract. The selection of strains with high viability and functionality, high-stress resistance, presence of fermentable polysaccharides, and an antioxidant system that supports the growth of anaerobic strains is critical to guarantee a sufficient number of viable bacteria in the final product and effective host health-promoting action. Especially among the stress factors above, the protection of strains against oxidative stress is necessary for vitality and bioactivity as it greatly affects viability and product quality (Cardona et al., 2013).
Phenolic compounds and antioxidants positively modulate the gut microbiota and protect probiotic bacteria from the stress conditions during GIS passage and in different food matrices (Zhang and Tsao, 2016).
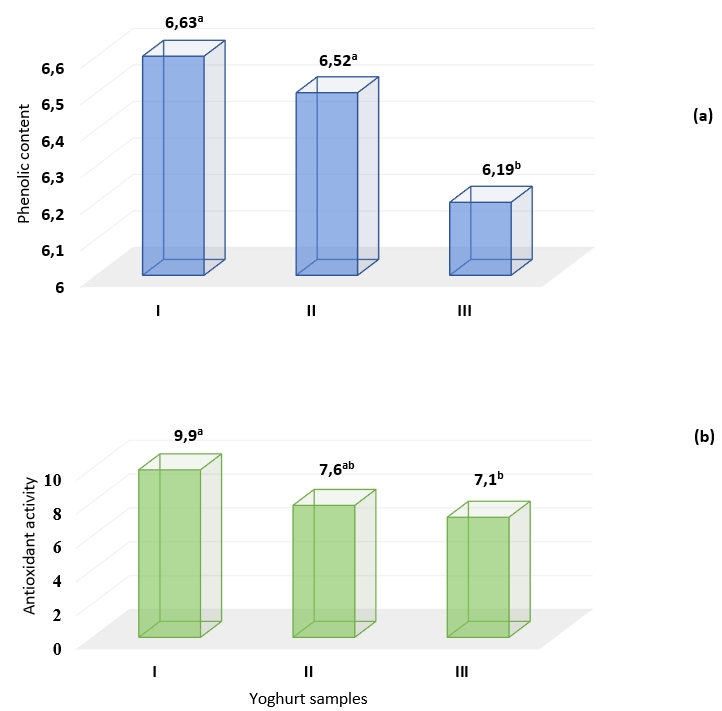
Figure 3. Bioactive properties a) phenolic content (mg GAE/100 mL) and b) antioxidant activity - CUPRAC (mg TE/100 mL) of yoghurt samples without additives (I), with Aleo vera mucilaginous gel (II) and with Aleo vera mucilaginous gel and stevia (III) +
+Significance level; at p<0.01, different letters indicate significant differences of treatments
Milk gel firmness
Aloe vera is used as a water binder, texture improver, gelling agent and stabilizer in the food industry (Femenia et al., 2003; Kiran and Rao, 2014; Minjares-Fuentes et al., 2017).Cohesiveness is defined as the strength required to resist the attraction between the food surface and the tooth and palate surface, and in a textural sense, it expresses a strong connective structure ( Ozdemir and Ozcan, 2020). The consistency value, which gives information about the density and consistency of the product, indicates that the yoghurt gel and texture develop well at the end of fermentation (Prajapati et al., 2016). In terms of the textural stability of the samples, the consistency and cohesiveness values were higher in the samples containing Aloe vera (II) (Figure 4 a, b). It turns out that in the sample containing Aloe vera, the addition of plant mucilage may have increased the hardness and viscosity by binding the water in the yoghurt or by strengthening the protein-polysaccharide interaction in the protein network and also increased the internal stickiness. Mudgil et al. (2016) reported that the addition of Aloe vera juice to fermented milk reduces phase separation and increases viscosity. It has also been emphasized that nutritional, physiochemical and sensory properties have improved.
Colour and sensory properties
Colour in foods is one of the important parameters that affect consumer liking and preferences. It was determined that the brightness (L*) decreased, the green value (-a*) increased and the yellowness value (+b*) decreased with the addition of Aloe vera and stevia in the samples. The yellowness value decreased with the colour change due to the degradation of colour with the progression of lactic acid fermentation (Figure 5 a, b, c). It is stated that the stability of the colour components changes at different pH levels (Alves et al., 2008).
LAB can not only prevent many disorders caused by biological oxidation in the host but also contribute to improving the technological quality and flavour of the food, each by different enzymatic pathways. For measuring consumer liking and preference, hedonic scale sensory evaluation is probably the most useful sensory method.
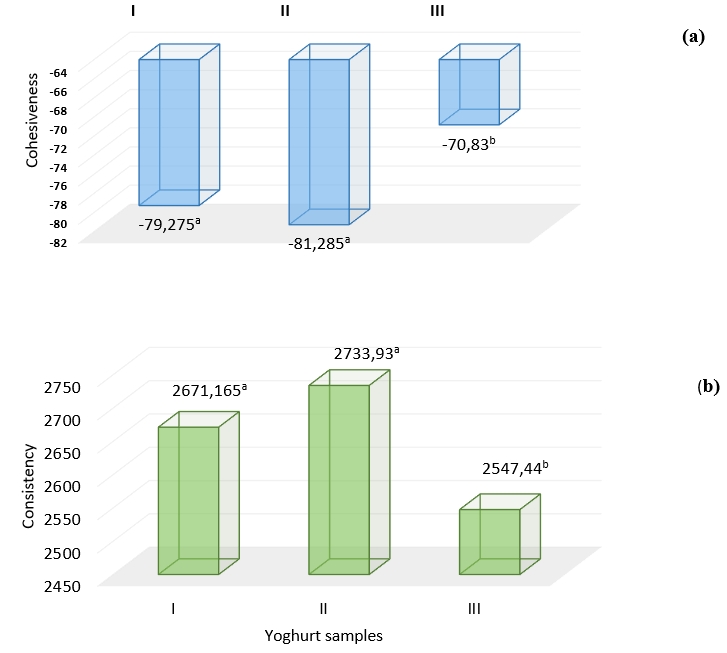
Figure 4. Textural properties a) cohesiveness and b) consistency of yoghurt samples without additives (I), with Aleo vera mucilaginous gel (II) and with Aleo vera mucilaginous gel and stevia (III) +
+Significance level; at p<0.01, different letters indicate significant differences of treatments
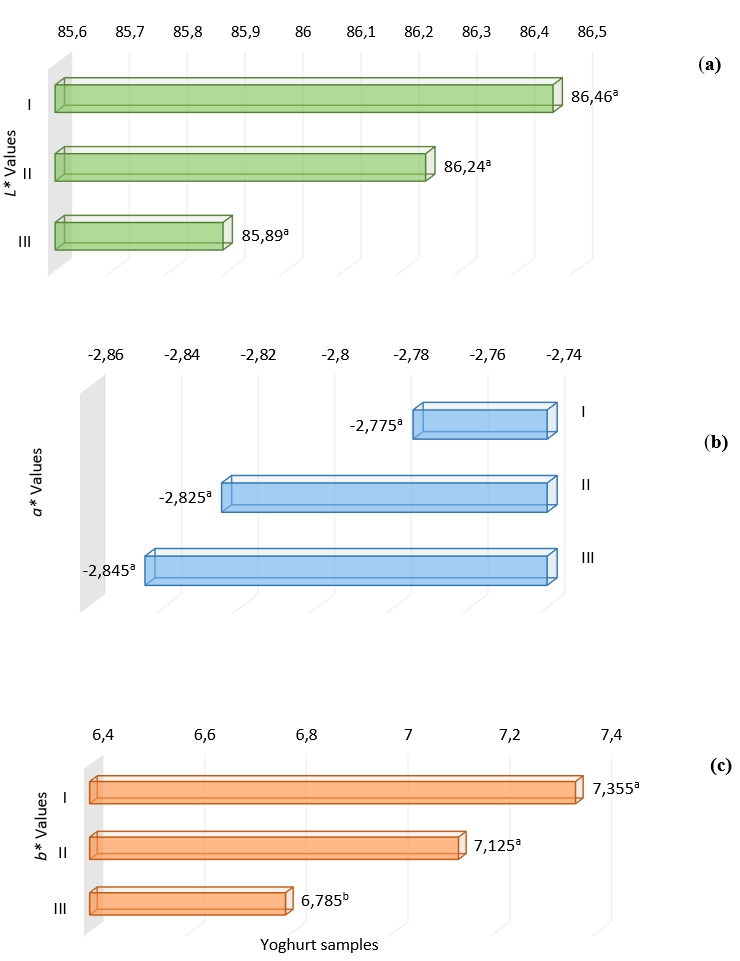
Figure 5. Colour properties a) L* values, b) a* values and c) b* values of yoghurt samples without additives (I), with Aleo vera mucilaginous gel (II) and with Aleo vera mucilaginous gel and stevia (III) +
+Significance level; at p<0.01, different letters indicate significant differences of treatments
Sensory values of probiotic yoghurt samples are given in Figure 6. As a result of evaluations, probiotic yoghurt samples were examined in terms of colour, brightness, smoothness, acidity, sweetness, fermented, acetaldehyde, flavour, odour, homogeneity, hardness, consistency and creamy properties. In terms of colour and brightness, samples containing stevia and Aloe vera were more appreciated, while samples with stevia were perceived as sweeter but less fermented taste. In these samples, acetaldehyde aroma was also evaluated at a lower intensity. Hardness and consistency values were lower in samples containing stevia. In general, no significant differences were detected in the sensory properties of the samples. General sensory acceptability was scored by similar and high values for all samples (p<0.01).
Principal component analysis (PCA) of the samples
PCA showed the clustering of analyzed properties and the samples based on their similarity (Figure 7). While PC1 described 77.5% of the total variance, PC2 accounted for 22.5 %. Some sensorial properties (creamy, flavour, consistency and preference) and textural parameters (consistency and cohesiveness) were separated on the positive side of PC1 and PC2. The sample YII (yoghurt with Aloe vera mucilaginous gel) was generally characterized by these attributes. The sweetness and smoothness attributes on both axes’ negative side of were related to the sample YIII (yoghurt with Aloe vera mucilaginous gel and stevia). The YI sample (yoghurt without additives) was characterized by physicochemical properties (acidity and pH), sensorial attributes (odour, acetaldehyde, hardness, fermented), b*, antioxidant activity and phenolic content loaded on the negative side of PC2. These attributes were opposite by brightness, colour, LAB, a* and homogeneity.
The ADI limit of stevia varying between 0 and 4 mg/kg body weight expressed as steviol equivalents was concurred by JECFA (The Joint FAO/WHO Expert Committee on Food Additives). EFSA (European Food Safety Authority) mentioning that these levels do not pose a risk of genetic damage following consumption of steviol glycosides (EFSA, 2019; JECFA, 2019).
Conclusions
Obesity, which has become widespread in recent years, is caused by insufficient physical activity, hormonal effects, sedentary life and malnutrition. The main reason for the increase in the use of non-nutritive sweeteners is the fact that sugar in diets in relation to obesity causes many health problems. The addition of bioactive plant mucilages and carbohydrate-based ingredients ensures that probiotic functional dairy products contain therapeutic levels of probiotic bacteria at the consumption stage. The results of this study showed that Aloe vera mucilage is suitable for use in functional dairy products, as it increases the nutraceutical substance content in dietetic yoghurts containing stevia, improves the textural properties, keeps the bacterial viability level at the therapeutic level by showing a prebiotic effect, and increases consumer appreciation. However, thithe observed effects may vary depending on the species. Examining the fermentation abilities of different species and in the different prebiotic mediums will be beneficial in terms of both, the production of tribiotic products and increasing metabolic b ioaccessibility studies.
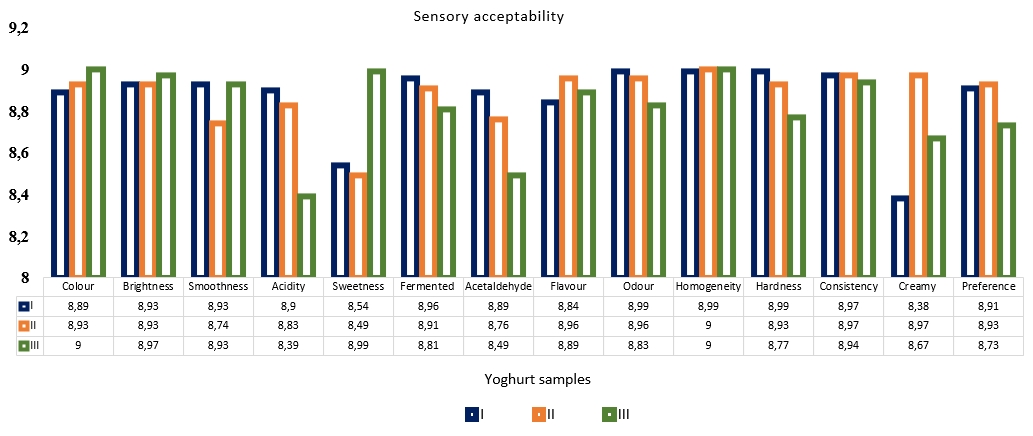
Figure 6. Sensory properties of yoghurt samples without additives (I), with Aleo vera mucilaginous gel (II) and with Aleo vera mucilaginous gel and stevia (III)
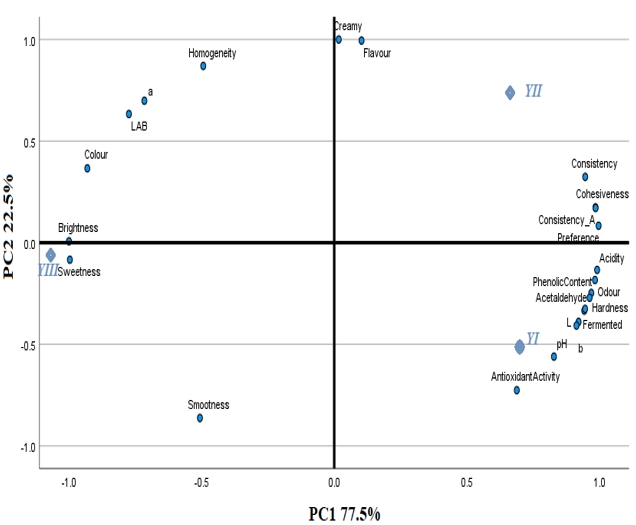
Figure 7. PCA biplot of the samples (YI; yoghurt without additives, YII; yoghurt with Aloe vera mucilaginous gel; YIII: yoghurt with Aloe vera mucilaginous gel and stevia)
Funding
This research has been funded by the scientific research of Bursa Uludag University (Project number: FHIZ-2022-1094).
Učinak polisaharida biljnog mucilaginoznog gela na oksidativnu stabilnost i fermentaciju dijetetskih jogurta
Sažetak
Cilj ovog istraživanja bio je utvrditi učinke polisaharida biljnog gela ekstrahiranog iz lista aloe vere na fermentaciju i terapijska svojstva funkcionalnih jogurta. Gel aloe vere je u ovom istraživanju korišten kao potencijalni prebiotik, izvor dijetalnih vlakana i stabilizator, stevija kao prirodni zaslađivač, a mješovita kultura sačinjena od jogurtne kulture (Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus) i probiotičkih sojeva ( Bifidobacterium animalis ssp. lactis, Lactobacillus acidophilus) kao starter kultura. Kako bi se procijenila funkcionalna i bioaktivna svojstva jogurta, određivani su preživljavanje sojeva probiotičkih bakterija, udio ukupnih fenolnih spojeva i antioksidacijski kapacitet izražen preko sposobnosti redukcije iona bakra. Stabilnost gela jogurta određena je analizom parametara teksture, a senzorksa svojstva pomoću testa preferencije potrošača primjenom hedonističkih skala. Određivanjem fitokemijskog učinka pokazalo se da polisaharidi i fenolni spojevi prisutni u gelu aloe vere podržavaju rast bakterija. Osim toga, polisaharidni gel utjecao je na formiranje čvršćeg koaguluma. Dodatak stevije u jogurt ubrzao je fermentaciju što je vjerojatno rezultat potencijalno stimulativnog djelovanja steviol glikozida na rast sojeva bakterija u sastavu starter kulture. Uzimajući sve dobivene rezultate u obzir, aloe vera gel i stevija djeluju sinergistički s bioaktivnim polisaharidima u proizvodnji hrane za posebne prehrambene namjene, poboljšavajući svojstva krajnjeg proizvoda i modulirajući crijevnu mikrobiotu.
Ključne riječi: biljni mucilaginozni gel; aloe vera; stevija; jogurt
