Introduction
Due to the nutritional and health effects of goat’s milk, there has been an increase in fermented dairy products produced from goat milk in recent years. The outstanding properties of goat milk such as the hypoallergenic character and smaller fat globules provide a basis for higher digestibility and higher levels of short-chain fatty acids compared to cow milk might be further enhanced by using probiotics in the manufacture of fermented dairy products (Ranadheera et al., 2012). Indeed, many researchers highlighted that small-molecule fatty acids in fermented goat milk products significantly affect the formation of the characteristic taste and aroma (Park, 2007).
Numerous studies showed that regular consumption of products containing probiotic bacteria promotes the human immune system, might reduce cancer risk, prevents digestive tract infections, reduce cholesterol level and improves digestive difficulties. The health effect of probiotics is not only originating from the microorganism cells but also from the metabolites that was excreted by the cell. Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium longum and Bifidobacterium breve are the most commonly used bacterial species in the production of probiotic products (Yazıhan and Ozer, 2021).
On the other hand, one of the most emphasized criteria for the production of high-quality milk is SCC which is considered as an indirect indicator of the raw milk’s hygienic quality and udder health. Somatic cells consisting of blood and tissue cells have an important role in the defence mechanism of the udder. Therefore, SCC is used as a criterion in the determination of udder health and diagnosis of subclinical mastitis (Darbaz et al., 2023). Mastitis is the most effective factor in the increase of SCC in mammary glands. Depending on the severity of mastitis, the increase in SCC becomes substantial in both, individual and bulk milk, and causes significant changes in specific milk components like protein, fat, lactose and mineral contents. Increased SCC also generates an increase in proteolytic and lipolytic enzyme levels and the presence of these enzymes is a potential factor for off-flavours in milk products (Kesenkaş et al., 2018). Although SCC above a typical physiological range indicates microbial inflammation, it is not true for goat milk that naturally contains higher amounts of epithelium cells and their fragments (Jimenez-Granado et al., 2014). In other words, somatic cells are more difficult to associate with possible infections in goat milk by comparison with cow and ewe milk. Because many non-infectious factors can cause substantial changes in the number of somatic cells in goat milk (Chen et al., 2010). Therefore, goat milk may contains a high SCC but product-based research reflecting the relationship between SCC in goat milk and product quality is especially limited. On the other hand, SCC in goat milk is a neglected criterion that can impact dairy processing (Darbaz et al., 2023). The USA Pasteurized Milk Ordinance regulation allows 1.500.000 somatic cells/mL in grade A goat milk (PMO, 2017) similarly, the limit of goat milk SCC has been 1.500.000 cells/mL in Turkish Food Codex (Anonymous, 2017). Podhorecka et al. (2021) reported that even low SCC values may significantly affect the technological properties of goat milk and products. Several research findings reported the characteristics of probiotic goat milk yoghurt (Ranadheera et al., 2012; Lucatto et al., 2020; Mahmoudi et al., 2021), however, to the best of our knowledge, there is no study on the effects of high SCC in goat milk to the product quality. Therefore, the present study aimed to compare the effects of low and high levels of SCC in goat milk on the chemical, textural, microbiological and sensory properties of probiotic fermented milk.
Materials and methods
Goat milk used in this study was collected from a herd available at the farm of the Ege University Faculty of Agriculture Department of Animal Science, Turkey. Saanen goats were selected from amongst animals in the intermediate stages of lactation that were not treated with antibiotics at least 7 days before milk collection. Goat milk was bulked into two groups according to their SCC status: low SCC <500.000 cells/mL (LSCC) and high SCC >1.500.000 cells/mL (HSCC). The probiotic fermented milk culture used in the study was ABT-2 containing L. acidophilus, Bifidobacterium animalis subsp . lactis ( B. lactis) and Streptococcus thermophilus by Chr. Hansen (Hørsholm, Denmark).
Raw milk analyses
For the determination of SCCs per millilitre of goat milk samples, the portable DeLaval Cell Counter (DCC; DeLaval International AB, Tumba, Sweden) was used. Cell counts were determined according to the enumeration principle using somatic cells stained with DNA-specific fluorescent probe propidium iodide. Approximately 60 μL milk sample was drawn into the cassette, the loaded cassette was placed in the measuring chamber of the DeLaval cell counter and somatic cell counts were read from the instrument display in less than a minute. The samples were evaluated within one hour after milking for reliable somatic cell counting in goat milk samples according to Sanchez-Macias et al. (2010). Fat and protein contents were determined according to AOAC (2003).
Probiotic fermented milk production
Both goat milk groups (LSCC and HSCC) were fortified with skim milk powder up to a solid non-fat (SNF) content of 12 % (w/w). They were heated to 90 °C for 10 min, then cooled to 40 °C and inoculated with 3 % (w/w) liquid starter culture activated in sterile skim milk. Then, the inoculated goat milk samples were put into 200-mL plastic containers and incubated at 37 °C until ~pH 4.6 was reached. After fermentation for precooling at room temperature, probiotic fermented milk samples were taken to outside for 15 min and then stored at 4 °C for 21 d for the physicochemical, microbiological, textural and sensorial analyses. Productions were completed in duplicate.
Probiotic fermented milk analyses
Physicochemical analyses
The contents of total solids, fat, protein and lactic acid of the probiotic fermented milk samples were carried out according to AOAC (2003). The pH values were determined using a digital pH meter (Inolab-WTW pH 720). Proteolysis in fermented milk samples was analysed by measuring free amino acids and peptides using the o-phthaldialdehyde (OPA) method (Donkor et al. 2006). Fermented milk lipolysis was estimated by calculating the amount of free fatty acids (FFA) using the Doyle method (Doyle et al. 1994)
Textural analyses
The hardness and consistency of samples were determined with Brookfield CT-3 model texture analyser according to Akalın et al. (2012). The viscosity values of probiotic fermented milk samples were analysed using a Brookfield DV-II viscometer and performed with spindle 63 at 40-100 torque at 12 rpm.
Microbiological analyses
For the enumeration of culture bacteria; a 10 g sample was taken from samples aseptically, transferred to a 90 mL Ringer solution and mixed thoroughly. Subsequently, serial dilutions were prepared and inoculations were carried out using the appropriate dilutions. MRS-Sorbitol agar was used for the L. acidophilus enumerations (anaerobically at 37 °C for 72 h) while TOS Propionate Agar (Merck) was used for B. lactis enumerations (anaerobically at 37 °C for 72 h) and M17 agar was used for S. thermophilus enumerations (aerobically at 37 °C for 72 h).
Sensory analyses
The scoring test was applied for the sensory evaluation by a group of six panellists consisting of academic members of the Ege University Faculty of Agriculture Department of Dairy Technology. The samples were evaluated by scoring the product characteristics between one and five (1: dislike extremely; 5: like extremely) in terms of appearance, texture (firmness and smoothness), colour, taste and overall acceptability. The samples were presented to the panellists with different letter codes approximately at +4 °C (Akalın et al. 2012).
Statistical analysis
The effects of goat milk group and storage time on the characteristics of probiotic fermented goat milks were determined by ANOVA and the mean differences were analysed using Duncan's multiple range test by SPSS 25.0 (SPSS Inc., Chicago, IL, USA) package for Windows. The differences were considered to be statistically significant at p≤0.05. All experiments and analyses were completed in triplicate.
Results and discussion
The compositional parameters and the SCC of raw goat milk used for the manufacture of probiotic fermented milks in both SCC categories are presented in Table 1. The differences between fat and protein contents of goat milk with low or high somatic cell counts were found to be statistically significant (p <0.05). This result may be attributable to the lactation stage, nutrition, health and age differences of goats in these two distinct groups. Mean SCC in low and high SCC goat milk samples were 460.500 and 1.709.000 cells/mL respectively. The SCC in goat milk can be affected by different factors such as lactation, stress, estrus, milking, etc. (Haenlein, 2002; Mehdid et al., 2019). It has been reported that the amount of somatic cells in goat milk is generally higher than that of cow's milk, and more than 1.000.000 cells/mL somatic cells were found in healthy goats even in the late lactation period. Thus, the diagnosis of mastitis in the goat udder by SCC is not reliable. As mentioned in various studies, mastitis was not detected in the mammary glands of goats having milk with high SCC counts (Chen et al., 2010). According to the Turkish Food Codex Communiqué on the Supply of Raw Milk (2017/20), it has been obliged to contain less than 1.500.000 SCC per mL in raw goat milk (Anonymous, 2017). As seen in Table 1, goat milk with high SCC had a lower fat content and lower protein content compared to the other sample. Similarly, some studies reported that milk with high SCC level had a lower fat content (Jaeggi et al., 2003) but higher protein content (Ying et al., 2002). However, as reported by Moradi et al. (2021) there are many contradictory findings on the effects of somatic cell content on different milk constituents from different animals.
Table 1. The properties of raw goat milk used in probiotic fermented milk production
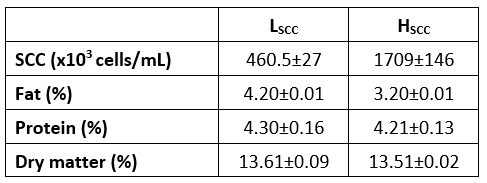
LSCC - milk with low somatic cell count, HSCC - milk with high somatic cell count
Physicochemical properties of fermented milk samples
The contents of SNF, fat and protein in LSCC and HSCC fermented milk samples were found at 13.09-13.84 %, 4.0-3.0 % and 4.48-4.36 %, respectively. The content of SNF (consisting mainly of lactose, protein and mineral matter) in milk for the manufacture of set-type yoghurt is standardized to produce an end product with certain physical properties and flavour (Tamime and Robinson, 2007). The SNF content of fermented dairy products, and therefore the fat and protein contents varies widely. The type of raw milk used in production, the total solid content of the raw milk, standardization methods and the processes applied during the production can cause differences in these contents. The SNF% values of the samples were similar to those of Dave and Shah (1997a, b), Temerbayeva et al. (2018) and Lucatto et al. (2020) where Güler and Şanal (2009) determined the fat in goat yoghurt to be 3.6 %. Oliveira et al. (2002) reported that the compositional characteristics (protein and fat contents) of yoghurt manufactured from goat milk were not influenced by the difference of SCC in goat milk that was divided into three groups <400.000; between 400.000 and 800.000 and >800.000 cells/mL.
pH and acidity
The pH and titratable acidity (LA%) changes of probiotic fermented goat milk during storage are presented in Figure 1. The pH decrease in both probiotic fermented milks during storage (p<0.05) is mainly due to the growth and metabolic activity of starter cultures which are reported to produce lactic acid at refrigerated storage (Shah et al.1995). The goat milk yoghurt containing free bifidobacteria in addition to yoghurt starter bacteria, cells of bifidobacteria are responsible for acidifying the product by producing both, lactic and acetic acids, and they have been reported to maintain this actitvity even at refrigerated storage (Samona et al., 1996). On the other hand, differences between the pH values of LSCC and HSCC fermented milk samples during storage were statistically significant (p<0.05) where HSCC fermented milk showed the lowest pH value (4.16) at the end of storage.
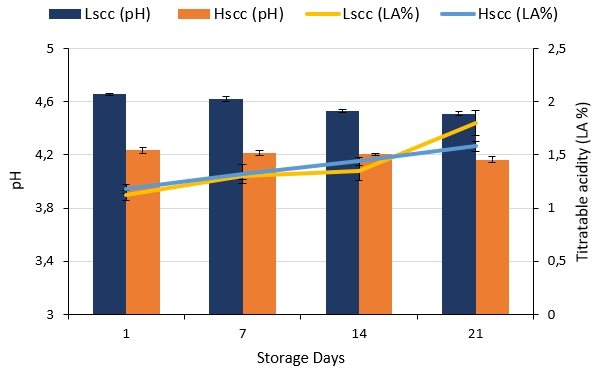
Figure 1. pH and titratable acidity changes during the storage of probiotic fermented milk produced from goat milk
There were no statistically significant differences between the titratable acidity values of the samples during the first 7 days of storage, however, differences were significant on days 14 and 21 (p<0.05). The increase in acidity during storage was also statistically significant for both groups (p<0.05). Similarly, Vivar-Quintana et al. (2006) reported that the pH values of yoghurt produced from ewes’ milk with high SCC were significantly lower than yoghurt produced from low or medium SCC milk after >15 days of storage. In contrast, Oliveira et al. (2002) and Fernandes et al. (2007) reported that the SCC in cow milk did not significantly affect the acidity or the pH of yoghurt during storage.
Proteolytic and lipolytic activity
OPA analysis is important to determine proteolysis in milk and dairy products. OPA values of probiotic fermented milks that show proteolytic activities produced from different groups of goat milk during storage are presented in Figure 2. OPA levels of fermented milk produced from low somatic cell goat milk did not show a statistically significant change during storage whereas OPA levels of fermented milk produced from high somatic cell goat milk increased on the 7th day and then decreased remaining days of storage (p<0.05). However, the differences between the samples were not statistically significant throughout the storage. According to Bulca and Koc (2020) as SCC increase proteolytic activity decreases in yoghurts produced from cow milk. Hachana and Paape (2012) reported that the proteolysis index did not vary significantly during storage for yoghurt produced with low SCC milk but decreased significantly for yoghurts made with intermediate and high SCC milk. Indeed, similar results were obtained especially on the 1st and 21st days of storage in our study.
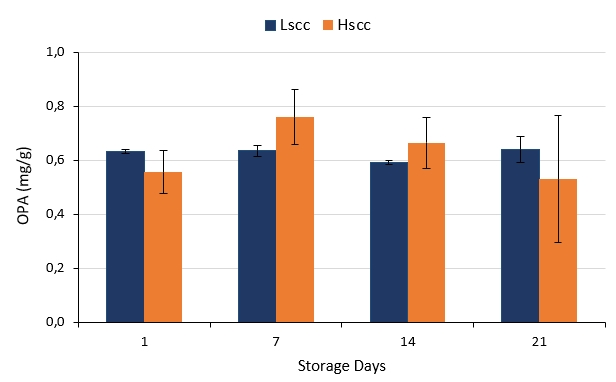
Figure 2. The proteolytic activities of probiotic fermented milk produced from goat milk during the storage
The lipolytic activities of probiotic fermented milks during storage are given in Figure 3 in terms of FFA. As seen, lipolytic activity in LSCC samples was lower than those of the HSCC sample (p<0.05). Ma et al. (2000) reported that the increases in concentrations of FFA in high SCC milk were three times faster than in low SCC milk. In general, FFAs are associated with the triacylglycerol activity of yoghurt starter bacteria. FFAs released by the hydrolysis of triglycerides are considered to be the main components responsible for the characteristic taste, especially in fermented products made from goat's milk. Also, lactose and amino acids (deamination and decarboxylation) are other sources of FFAs.
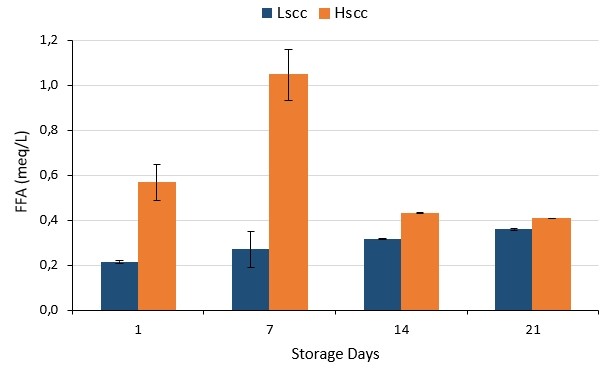
Figure 3. The lipolytic activities of probiotic fermented milk produced from goat milk during storage
Moreover, FFAs have aroma-supporting effects. It has been presented as a common result of many studies that FFAs in fermented products produced from goat milk have significant effects on the characteristic taste-aroma of the products. The sensory quality of fatty acids may be more effective since 12-20 % of the total fatty acids contained in goat milk are short and medium-chain and approximately 46 % of the natural lipase in goat milk is present in the fat phase (this ratio is 6 % in cow milk) (Alichanidis and Polychroniadou, 1995).
Viscosity
The viscosity of yoghurt is an important criterion used to determine the curd structure (Brennan and Cleary, 2005; Domagala et al., 2006). The changes in the apparent viscosity of probiotic fermented milks produced from different groups of goat milk during storage are presented in Figure 4. Compared to the LSCC fermented milk, HSCC fermented milk was characterised by a significantly lower viscosity values on days 7, 14 and 21 of storage (p<0.05). The viscosity of the LSCC sample increased whereas the viscosity of the HSCC sample decreased slightly throughout 21-day storage however, changes occurring during storage were not statistically significant (p>0.05). Probiotic fermented milks produced from Lscc goat milk had higher viscosity values when compared to the other sample contrary to the findings of Fernandes et al. (2007) and Hachana and Paabe (2011) who found higher viscosity values in yoghurts produced with intermediate and high SCC than low SCC yoghurts. According to the latter groups of authors, the occurrence of age-thickening gelation processes associated with the plasmin content in milk could also be a possible reason for the evaluated viscosity in yoghurts produced from milk with high SCC. However, our results are similar to those reported by Rogers and Mitchell (1994), who obtained a negative correlation between yoghurt viscosity and SCC in milk that depends on the proteolytic and lipolytic activity increase in yoghurt.
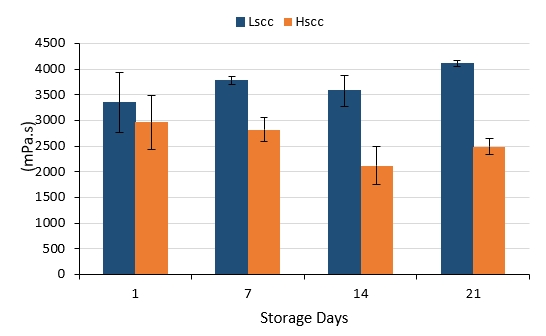
Figure 4. The viscosity changes in probiotic fermented milks produced from goat milk during storage
Hardness and consistency
The average hardness and consistency values of the probiotic fermented milk samples are given in Figure 5. The hardness and consistency values of LSCC fermented milks were higher than that of HSCC fermented milks (p<0.05) whereas the effect of storage on these values was statistically insignificant (p>0.05). In contrast to our results, Najafi et al. (2010) reported that yoghurt from ewe’s milk with lower SCC was softer than the high SCC yoghurt. It must be taken into consideration that factors like the total solid content, the denaturation rate of the serum proteins, heat treatment and acidity could affect the hardness and consistency of fermented milks.
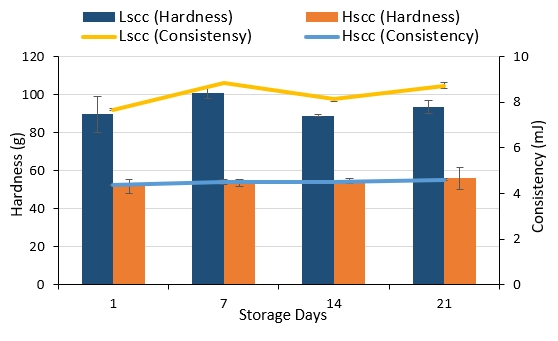
Figure 5. Hardness and consistency values of probiotic fermented milks produced from goat milk during storage
Viability of starter bacteria
The S. thermophilus, L. acidophilus and B. lactis counts of probiotic fermented goat milks are presented in Figure 6. In LSCC probiotic fermented milks, S. thermophilus, L. acidophilus and B. lactis counts did not decrease below 7 log cfu/g. This result showed that the probiotic bacteria counts in these samples were above the 107 cfu/g limit which is valid in regulations of many countries for probiotic products. However, S. thermophilus and L. acidophilus counts were below 7 log cfu/g in HSCC probiotic fermented milks after the 14th day of storage while B. lactis counts did not exceed this limit throughout the storage. These differences were found statistically significant (p<0.05).
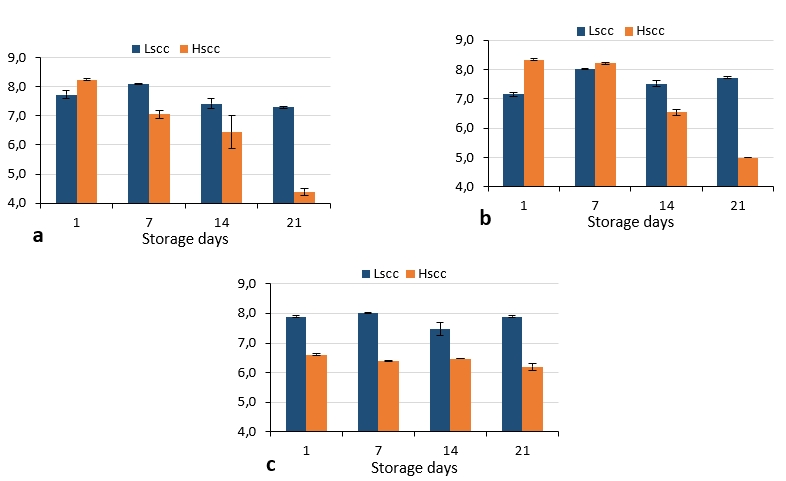
Figure 6. S. thermophilus (a), L. acidophilus (b) and B. lactis (c) counts of probiotic fermented milks produced from goat milk during storage (log cfu/g)
There are several studies on the production of probiotic fermented dairy products with goat milk however, there is no study has been found on the effect of somatic cell count in probiotic fermented milks produced from goat's milk. Bonczar et al. (2002) reported that B. lactis, L. acidophilus and S. thermophilus grew better in goat’s milk compared to cow’s milk. In the study of Ranadheera et al. (2012) probiotic viability was investigated in plain or fruit yoghurts produced from goat milk throughout the 28-day storage. The authors reported that P. jensenii 702 demonstrated the highest viability (108 cfu/g) in all types of yoghurt throughout the storage period and the viability of the bifidobacteria (107 cfu/g) also remained above the minimum therapeutic level while the viability of L. acidophilus LA-5 fell below 106 cfu/g. El Kadi et al. (2007) determined the viable counts of S. thermophilus and L. acidophilus between 3.6-4.1 x 106 cfu/mL and 2.8-3.1 x 106 cfu/mL respectively in yoghurts produced using the ABT culture. Comparing the results obtained in the present study to those reported by Najgebauer-Lejko (2014) for ABT yoghurts, S. thermophilus were lower while L. acidophilus and bifidobacteria counts were similar. Moreover, the counts of L. acidophilus and S. thermophilus and B. lactis were similar to the findings of Lucatto et al. (2019) for goat milk probiotic yoghurts.
Sensory properties
Sensory properties are among the most important properties that determine the appreciation of consumers. The type of milk and culture variety used in the production of fermented milk products are among the factors affecting the sensory quality of the product. The sensory properties of probiotic fermented with goat milks containing low (LSCC) or high (HSSC) somatic cells are given in Table 2.
Table 2. Sensory properties of probiotic fermented milk samples
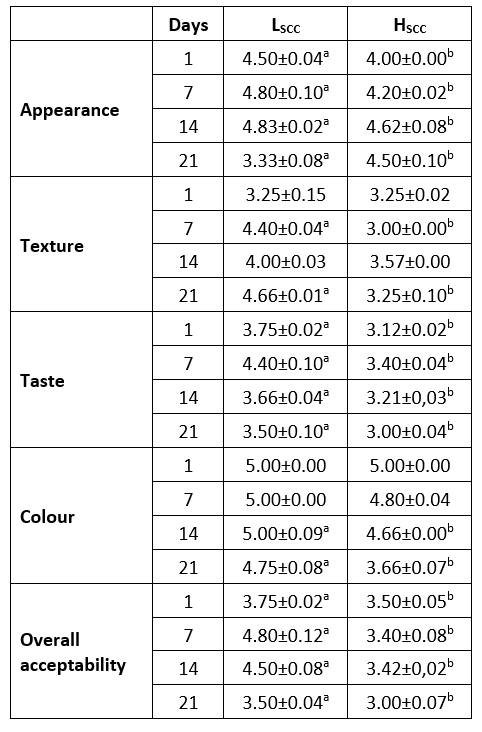
a,b: values in the same row with different superscripts differ significantly at p<0.05.
Appearance, taste and overall acceptability scores revealed significant differences among the samples throughout the storage (p<0.05) and LSCC samples had higher scores similar to the findings of Oliveria et al. (2002) who indicated SCC counts above 400.000 cells/mL in goat milk had a negative effect on the sensory quality of yoghurt. Initially, both samples received lower scores in terms of appearance but the scores increased on the 7th and 14th days and then decreased again at the end of storage (p<0.05). The LSCC sample received the highest texture score on the 21st day of storage, while the HSCC sample had the lowest score on day 7 of storage. The differences between texture scores on day 7 and day 21 were statistically significant (p<0.05). In terms of taste, the panellists gave the highest scores on day 7 for both samples. However, these scores decreased after day 14 possibly related to the increased acidity (Figure 1). Both samples received more stable colour scores whereas the HSCC sample had lower colour scores on days 14 and 21 (p<0.05). In fermented dairy products, mainly fat content or lipolytic activity affect the colour characteristics (Akpınar et al., 2020). So evaluated lipolytic activity in HSCC (Figure 3) may be the reason for lower colour scores for this sample. In line with the other sensory attributes, LSCC samples received more positive feedback from the panellist.
Conclusion
Our results indicate that as the SCC of goat milk increased, the pH of the probiotic fermented goat milks decreased whereas the titratable acidity increased. SCC in goat milk did not affect the extent of proteolysis of the probiotic fermented milk. However, the increased SCC in goat milk led to an increase in the lipolytic activity of probiotic fermented milk during storage for 21 days. The results showed that the low SCC probiotic fermented goat milk was harder and more viscous than fermented milk from milk with higher SCC. In terms of sensory properties, the panellists gave higher scores to probiotic fermented milks produced with low SCC goat milk and the viability of the starter bacteria was more stable in these samples. Notably, B. lactis counts never exceeded 7 log cfu/g in probiotic fermented milk from milk with higher SCC. Considering these results we suggest that there is a great need for further research to underline the threshold of goat milk SCC to guarantee the quality of related dairy products.
