Introduction
Butter is produced traditionally and industrially, and is consumed worldwide (Mallia et al., 2008; Akgül et al., 2021). It is most commonly used in breakfast, foods, cookies, and many kitchen products (Fındık and Andiç 2017; Akgül et al., 2020; Tamura et al., 2021). Due to its high fat, vitamin, and mineral content, butter has high energy and nutritional value (Mallia et al., 2008; Méndez-Cid et al., 2017).
Butter can be produced using yoghurt or cream (sweet or sour) (Mallia et al., 2008; Fındık and Andiç 2017), whereby yoghurt is typically used in traditional, and cream in industrial production methods (Mallia et al., 2008; Akgül et al., 2021). Industrial butter is manufactured either by using sour cream fermented by a starter culture or by using a non-fermented sweet cream (Mallia et al., 2008). The starter culture used in butter production contains lactic acid bacteria (LAB) such as Lactococcus lactis subsp . cremoris ( Lc. cremoris) , Leuconostoc subsp . (Leu.), Lactococcus lactis subsp. lactis biovar diacetylactis ( Lc. diacetylactis) , Lc. lactis subsp . lactis ( Lc. lactis). In addition, many probiotic bacteria have been used in butter production to improve the properties of butter or to investigate its effects on butter (Erkaya et al., 2015; Hadef et al., 2022). L. rhamnosus is often used in various dairy products as a probiotic strain (Kamal et al., 2018; Liu et al., 2018; Ningtyas et al., 2019; Sezer et al., 2023).
Lactic acid is the main end-product of the lactose metabolism by the LAB. Additionally, other organic compounds, known as aroma compounds, such as diacetyl, acetoin, acetaldehyde, etc., also occur (Gezginc et al., 2022). The aroma profile of products has important effects on their sensory properties (Demirkol et al., 2016; Bertuzzi et al., 2018). Butter with added starter culture is one aromatic dairy product, containing about 230 aromatic compounds (Tamura et al., 2021). The aroma of the butter is affected by numerous factors such as animal feeding, seasons, production process, storage, and starter culture (Mallia et al., 2008; Akgül et al., 2021). Some researchers investigated the volatile components of commercial butter (Demirkol et al., 2016), and the effects of storage and packing on aroma compounds (Lozano et al., 2007). However, research that used starter cultures and investigated aroma components is scarce (Schieberle et al., 1993). Starter cultures improve flavour and control sensory properties in butter, which can make butter production significantly more economical and reduce health problems (Sulejmani et al., 2023).
The purpose of this study was to examine the effects on the aroma profile of production without starter culture, using commercial culture, prepared culture, and addition probiotic bacteria ( L. rhamnosus B19) in butter samples, and to compare the aroma compounds in butter samples produced using different starter cultures.
Materials and methods
Materials
For the current study, pasteurized cream (fat content 40 %) and a commercial starter culture were provided by Bayburt Ova Dairy Products Factory (Bayburt, Turkey) and Danisco Choozit Probat 322 (Choozit Probat 322 LYO 100 DCU, England) respectively. Butter samples were collected from Bayburt, Gumushane and Erzurum provinces in Turkey and MRS and M17 agar were used to isolate microorganisms from these samples. In order to select the non-commercial culture, gram staining, molecular and biochemical analyses were performed on the isolates.
Butter production
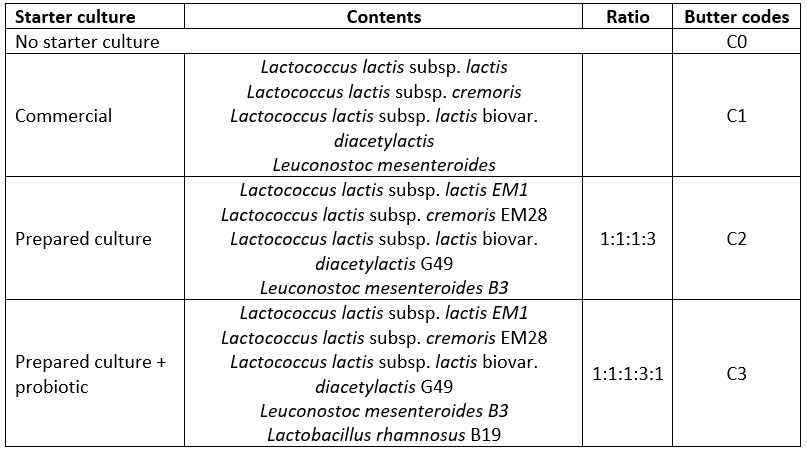
The butter samples were produced in the Bayburt University Food Engineering Dairy and Food Technology Laboratory. The butter samples were produced using three different starter cultures, as well as without starter culture. A commercial starter culture was used for one group of samples, while for the other two groups, bacteria isolated from traditional butter samples were used. The isolates selected from traditional butter samples were activated and incubated in broth medium. Then, the broth was centrifuged (at 5000 rpm for 10 min) and the obtained supernatant was removed. The pellet was used as a non-commercial culture for butter production. The bacterial strains and their ratios were chosen as the most suitable resulting from the pre-experimental trials. These bacteria were combined to prepare a starter culture. The starter culture composition and butter sample codes are shown in Table 1. The cream was pasteurized (pasteurizer, Milkoscan, İstanbul, Turkey) at 85 °C for 10 min and then cooled to 25 °C (cooling tank, Peymak, Sakarya, Turkey). The cream was divided into 4 equal 2 kg parts. The creams produced without starter culture were physically ripened at 8 °C for 24 h, churned (churning machine, Orjinal Krom, Sakarya, Turkey) and then washed. Other creams were supplemented by adding the starter culture (2%). The other creams were biologically ripened until pH 4.8. Afterwards, they were physically ripened at 8 °C for 24 h. The maturated creams were then churned and washed. Finally, the kneaded creams were packed and stored at +4 °C until analysis.
Table 1. Starter culture types used the butter production and sample codes
Methods
Analysis of aroma components
According to Bulat and Topcu (2020), with a few modifications, the aroma components were identified using a solid-phase microextraction (SPME) fibre with the GC-MS system (Thermo Scientific TRACE 1310 GC with ISQ-QD single quadrupole mass detector, Thermo Fisher Scientific, Waltham, MA, USA). A layer of 0.5 cm was removed from the surface of the butter and 2 g of sample was taken into a 20 mL crimp-neck headspace amber vial, and then, a mixture of 80 μL of 2-methyl-3-heptanone (20 ppm in water, prepared from a stock solution of 5 mLL-1 in methanol, was added to the vials as the internal standard (IS). Vials were quickly sealed with a bi-metal magnetic crimp-cap seal with PTFE/Silicone septa and equilibrated at 45 °C for 30 min with pulsed agitation for 4 s at 250 rpm. The headspace of the vial was exposed to SPME fibre (1 cm, 50/30 μm, Stable Flex divinylbenzene/carboxen/polydimethylsiloxane, Supelco, Bellefonte, PA) for 30 min at 45 °C. The equilibration, extraction and injection were all carried out by using the Thermo Scientific Tri Plus RSH autosampler (CTC Analytics AG, Zwingen, Switzerland). The volatile compounds were desorbed from the fibre at 260 °C for 3 min in the splitless mode. Separation was achieved by TR-WaxMS column (60 m × 0.25 mm I.D. × 0.25 μm film thickness, Thermo Fisher Scientific, Bellefonte, PA). Helium was used as carrier gas, at a constant flow of 1.0 mL min-1. The GC oven’s temperature was initially set at 40 °C for 10 min before being raised to 250 °C at a rate of 5 °C/min for 10 min. The temperature of the GC-MS transfer line and ion source was 260 °C. Mass spectral data was collected in the scan mode within a mass range of m/z 35-350 amu. Thermo Xcalibur program (Ver. 3.1, Thermo Fisher Scientific Inc.) was used for data acquisition. The peaks were identified using the Wiley Registry (WILEY), National Institute of Standards and Technology (NIST) mass spectral libraries, and external standards. The ratio of compound peak area/IS peak area was used as arbitrary units to calculate quantities for each compound (Bulat and Topcu, 2020). The concentrations were calculated by comparing the peak areas of the internal standard containing unknown compounds. Each compound was then expressed in micrograms per kg butter (µg kg-1 butter).
Statistical analysis
The replicates were not independent. The experimental design was made up of four different starter cultures and two replicates. The replicates were produced on different days. The statistical effect of the use of different starter cultures was determined utilizing the one way-ANOVA using SPSS 17 packet program on the aroma compounds in butter samples. The differences among samples were found using Duncan’s multiple range tests at level p<0.05.
Results and discussion
Numerous microorganisms are involved in the formation of aroma compounds (Bertuzzi et al., 2018). A key factor in the fermentation and aroma components of fermented dairy products is LAB (Chen et al., 2017). Glycolysis, proteolysis, and lipolysis, all of which are needed for the formation of aroma components, are performed by the LAB (Chen et al., 2017; Bertuzzi et al., 2018). In the study, 93 aroma compounds in the butter samples were detected, and the distribution of these aroma compounds was indicated in Table 2. The sample using the prepared starter culture mix (C2) had the most aroma components, with the other samples following in order as the samples C1 (which used the commercial starter culture), C3 (which used the prepared starter culture + L. rhamnosus B19) and C0 (which did not use starter culture) (Table 1). Similarly, amount of aroma compounds of the butter samples was ordered as C1 (12987.33 µg kg-1 butter) >C2 (12271.65 µg kg-1 butter) >C3 (7516.70 µg kg-1 butter) >C0 (3766.48 µg kg-1 butter).
Table 2. Aroma compound numbers of the produced butter samples using the different starter culture
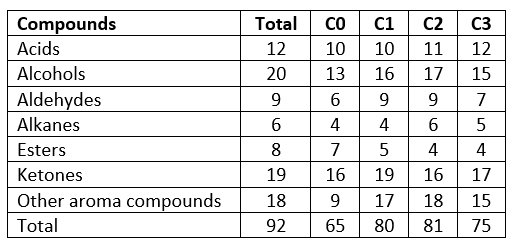
C0: butter content without starter culture, C1: butter contents commercial starter culture, C2: butter produced from prepared starter culture mix, C3: butter produced from prepared starter culture mix and Lactobacillus rhamnosus B19
Table 3. Acid contents of the produced butter samples using the different starter culture (µg kg-1 butter)
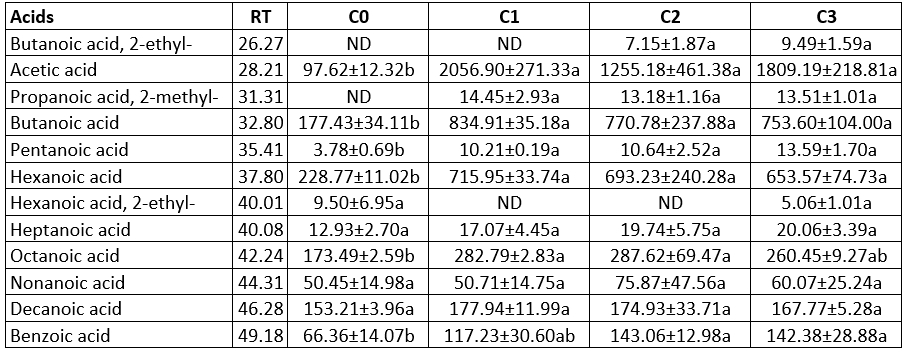
a, b, c: Statistically significant at level p<0.05 was shown with lower letters in the row. RT: Retention time, ND: Not detected, The means and standard values were obtained from two replicates (n=2). C0: butter content without starter culture, C1: butter contents commercial starter culture, C2: butter produced from prepared starter culture mix, C3: butter produced from prepared starter culture mix and Lactobacillus rhamnosus B19
Acids
Acids are derived from carbohydrates and lipids via the LAB metabolism (Sarhir et al., 2021). Acetic acid is the predominant aroma compound among acids, with butanoic, hexanoic, octanoic acids following close (Table 3). The least amount of butanoic, pentanoic, hexanoic, octanoic, and benzoic acid contents were found in butter produced without starter culture. Only samples C2 and C3 contained butanoic acid, whereas only sample C0 did not have propanoic acid, 2-methyl-acetic acid is formed as a result of lactose (by heterofermentative LAB) or citric acid metabolism (by homo- or heterofermentative LAB) (Demirkol et al., 2016). The acetic acid concentrations in the butter samples were between 97.62 µg kg-1 and 2056.90 µg kg-1, but the differences in samples were not statistically significant (p>0.05). Tamura et al. (2021) found the acetic acid content in the sweet cream butter samples as 11.630 ppm. Acetic acid is characterized by a sour or pungent odour (Schieberle et al., 1993; Tamura et al., 2021). The benzoic acid could be produced by LAB (Sieber et al., 1995). The benzoic acid concentration of the butter sample produced without a starter culture was lower in comparison to the other samples. The samples using the prepared starter culture mix (C2) and (C3) had the highest benzoic acid concentrations (Table 2).
The butanoic and hexanoic acid contents are directly correlated to lipolysis in butter (Iradukunda et al., 2018). The butanoic (buttery) and hexanoic (pungent, musty) acids are odour-activity components (Schieberle et al., 1993; Peterson and Reineccius 2003). Threshold values for the butanoic and hexanoic acids are 0.090-0.180 mg kg-1 and 3.6-72 mg kg-1, respectively (Schieberle et al., 1993). Butanoic contents of butter samples ranged between 177.43 µg kg-1 and 834.91 µg kg-1, whereas the hexanoic acid amounts of samples ranged between 228.77 µg kg-1 and 715.95 µg kg-1. The sample produced without stater culture had the lowest butanoic and hexanoic acid concentrations (Table 3). In the present study, the observed butanoic acid values were higher than the threshold value, while hexanoic acid values were lower. Butanoic and hexanoic acid is among the primary aroma components for butter flavour (Schieberle et al., 1993; Peterson and Reineccius 2003). Peterson and Reineccius (2003) determined that the butanoic and hexanoic acid content of sweet cream salted butter were 192 µg kg-1 and 732 µg kg-1, respectively. Sarhir et al. (2021) found that the butanoic acid concentration of fermented Smen (traditional fermented butter in Morocco) samples were 161-193 mg kg-1, and the hexanoic acid amount was 25.6-70.3 mg kg-1.
Alcohols
Alcohols may result from a variety of metabolic activities such as glycolysis, proteolysis, and lipolysis (Chen et al., 2017; Sarhir et al., 2021). While ethanol is created through fermentation (lactose or citrate) and the catabolism of alanine, methyl alcohols are derived from branched-chain amino acids. Only the sample made with the commercial starter culture (C1) contained 2-pentanol, 2-heptanol, 2-hexanol, 5-methyl- and ethanol, 2-phenoxy-, whilst 3-pentanol, 1-decanol and phenol, 2-methyl-5- were not found (Table 4). The ethanol and isoamyl alcohol amounts were between 292.72 - 395.77 µg kg-1, and 82.09-102.69 µg kg-1, respectively. Ethanol and isoamyl alcohol contents were statistically similar in all the samples (p>0.05). Sarhir et al. (2021) determined the ethanol concentration in fermented Smen (traditional butter) samples as 8.40-16.0 mg kg-1. According to Tahmas-Kahyaoğlu et al. (2022), ethanol content predominated in all butter samples produced from different animal milks. They found that ethanol and isoamyl alcohol amounts were between 15.55-34.89 µg 100 g-1, and 1.84-3.41 µg 100 g-1, respectively.
Table 4. Alcohol contents of the produced butter samples using the different starter culture (µg kg-1 butter)
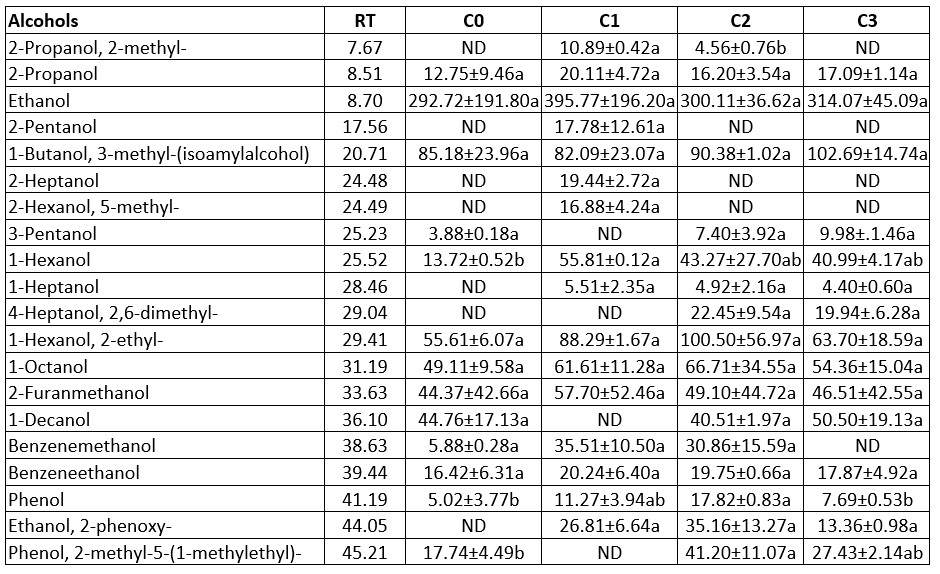
a, b, c: Statistically significant at level p<0.05 was shown with lower letters in the row. RT: Retention time, ND: Not detected, The means and standard values were obtained from two replicates (n=2). C0: butter content without starter culture, C1: butter contents commercial starter culture, C2: butter produced from prepared starter culture mix, C3: butter produced from prepared starter culture mix and Lactobacillus rhamnosus B19
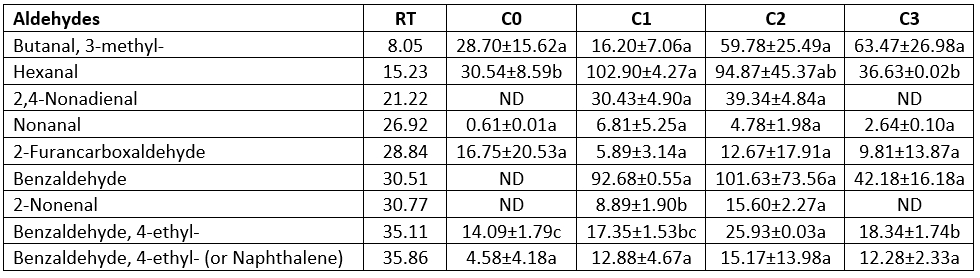
Table 5. Aldehyde contents of the produced butter samples using the different starter culture (µg kg-1 butter)
a, b, c: Statistically significant at level p<0.05 was shown with lower letters in the row. RT: Retention time, ND: Not detected, The means and standard values were obtained from two replicates (n=2). C0: butter content without starter culture, C1: butter contents commercial starter culture, C2: butter produced from prepared starter culture mix, C3: butter produced from prepared starter culture mix and Lactobacillus rhamnosus B19
Aldehydes
Aldehydes can be derived from amino acids and unsaturated fatty acids such as hexanal and nonanal, and can result in an off-flavour in dairy products (Demirkol et al., 2016; Chen et al., 2017). Aldehyde contents of the butter samples are shown in Table 5. Hexanal and benzaldehyde were the two main aldehydes in the butter samples. It was determined that samples C1 and C2 contain all the aldehydes. No significant difference was found among samples in terms of all aldehyde components except for hexanal and benzaldehyde, 4-ethyl- (p>0.05). Hexanal contents of samples C0 and C3 were found to be lower than the other samples (p<0.05), but there were no significant differences (p>0.05). The sample produced without starter culture (C0) had the lowest benzaldehyde, 4-ethyl- concentration (14.09 µg kg-1), whereas the sample using the prepared starter culture mix (C2) had the highest value (25.93 µg kg-1). Some aldehydes such as hexanal and 2-nonenal were detected by Schieberle et al. (1993) in Irish sour cream butter, and benzaldehyde was detected by Sarhir et al. (2021) in fermented Smen samples. Nonanal contents of butter samples ranged between 0.61 µg kg-1 and 6.81 µg kg-1, and statistical significance was not determined among samples (p>0.05). Demirkol et al. (2016) determined nonanal contents of commercially sold butter samples between 10 and 139 µg kg-1.
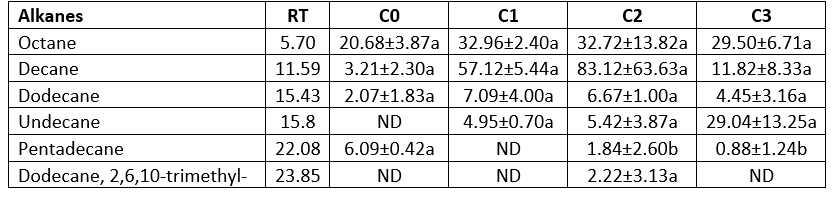
Table 6. Alkane contents of the produced butter samples using the different starter culture (µg kg-1 butter)
a, b, c: Statistically significant at level p<0.05 was shown with lower letters in the row. RT: Retention time, ND: Not detected, The means and standard values were obtained from two replicates (n=2). C0: butter content without starter culture, C1: butter contents commercial starter culture, C2: butter produced from prepared starter culture mix, C3: butter produced from prepared starter culture mix and Lactobacillus rhamnosus B19
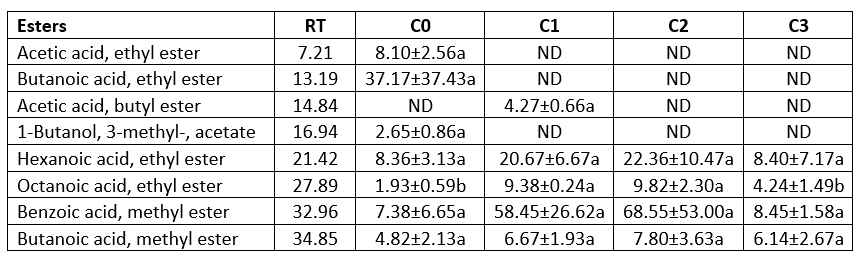
Table 7. Ester contents of the produced butter samples using the different starter culture (µg kg-1 butter)
a, b, c: Statistically significant at level p<0.05 was shown with lower letters in the row. RT: Retention time, ND: Not detected, The means and standard values were obtained from two replicates (n=2). C0: butter content without starter culture, C1: butter contents commercial starter culture, C2: butter produced from prepared starter culture mix, C3: butter produced from prepared starter culture mix and Lactobacillus rhamnosus B19
Alkanes
For samples C0 and C3 octane, and for samples C1 and C2 decane were predominant components (Table 6). Undecane and pentadecane were not detected only in samples C0 and C1, respectively, while dodecane, 2,6,10-trimethyl- was determined only in sample C2. Octane, decane, and dodecane contents of the samples showed changes, but these changes were not observed to have any statistical significance (p>0.05), as seen in Table 5. Sample C2 had all alkane concentrations. The sample produced using mix starter culture+ L. rhamnosus B19 did not only have dodecane, but 2,6,10-trimethyl as well. Tamura et al. (2021) determined that the decane concentration of sweet cream butter was 0.145 ppm. Pentadecane has a high aromatic threshold and little contribution to the aroma (Fan et al., 2014).
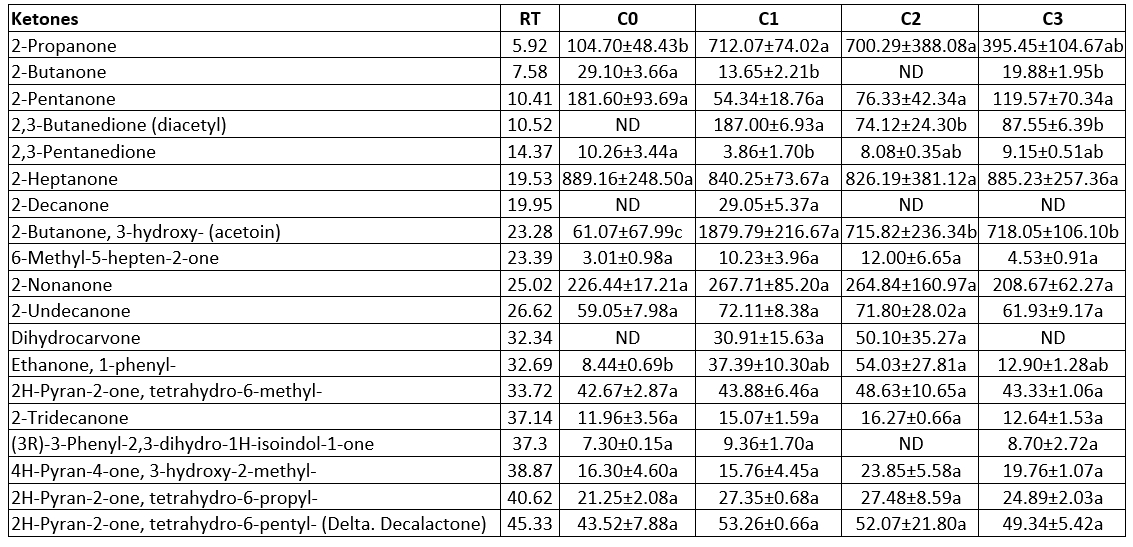
Table 8. Ketone contents of the produced butter samples using the different starter culture (µg kg-1 butter)
a, b, c: Statistically significant at level p<0.05 was shown with lower letters in the row. RT: Retention time, ND: Not detected, The means and standard values were obtained from two replicates (n=2). C0: butter content without starter culture, C1: butter contents commercial starter culture, C2: butter produced from prepared starter culture mix, C3: butter produced from prepared starter culture mix and Lactobacillus rhamnosus B19
Table 9. Other aroma compound contents of the produced butter samples using the different starter culture (µg kg-1 butter)
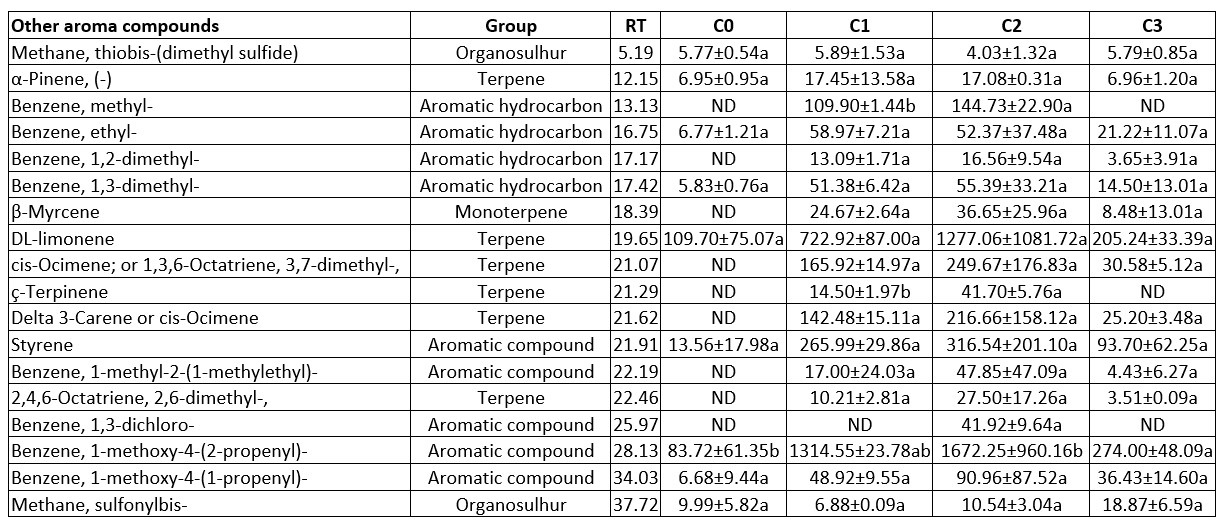
a, b, c: Statistically significant at level p<0.05 was shown with lower letters in the row. RT: Retention time, ND: Not detected, The means and standard values were obtained from two replicates (n=2). C0: butter content without starter culture, C1: butter contents commercial starter culture, C2: butter produced from prepared starter culture mix, C3: butter produced from prepared starter culture mix and Lactobacillus rhamnosus B19
Esters
Particularly the ethyl esters of short-chain fatty acids could be produced as a result of microbial activity. The ester compounds have an impact on odour (Liu et al., 2004). Esters in fermented dairy products at low concentrations contribute to the aroma, while high concentrations can result in a fruity aroma (Gallois and Langlois 1990; Tahmas-Kahyaoğlu et al., 2022). The formation of the ester derive could be accepted as an indicator of a decreasing sharpness and bitterness caused by the fatty acids and amino acids (Gallois and Langlois 1990). Acetic acid ethyl ester, butanoic acid ethyl ester, and 1-butanol 3-methyl- acetate were detected only in sample C0, whereas acetic acid butyl ester was only detected in sample C1 (Table 7). Hexanoic acid ethyl ester, octanoic acid ethyl ester, benzoic acid methyl ester, and butanoic acid methyl ester were detected in all butter samples. Only the octanoic acid ethyl ester in the samples indicated significant changes (p<0.05). Octanoic acid ethyl ester amounts in samples C0 and C3 were greater than in samples C1 and C2 (p<0.05). Tahmas-Kahyaoğlu et al. (2022) investigated the aroma profile of butter produced from different animal milk and determined 22 ester compounds in butter samples.
Ketones
Typically, ketones are identified as essential components for the aroma compounds found in dairy products (Sarhir et al., 2021). Among the analysed ketones 2-heptanone was detected in the highest concentration (Table 8). 2-butanone and (3R)-3-phenyl-2,3-dihydro-1H-isoindol-1-one were only not detected in sample C2, and 2-decanone was detected only in sample C1. 2-propanone and ethanone 1-phenyl- amounts were the lowest in sample C0. There were no significant changes among the butter samples in terms of 2-pentanone, 2-heptanone, 2-nonanone, 2-undecanone, as seen in Table 8 (p>0.05). Key ingredients for fermented dairy products like butter and yoghurt include diacetyl and acetoin, which are responsible for the buttery flavour of these samples (Mallia et al., 2008; Chen et al., 2017). Diacetyl is a particularly unique compound for aroma due to its low threshold (Schieberle et al., 1993; Chen et al., 2017). 2,3-Butanedione (diacetyl) was only not found in sample C0, while it's contents in all the other samples were between 74.12 µg kg-1 and 187.00 µg kg-1. 2,3-Butanedione (diacetyl) contents of samples C2 and C3 were lower compared to the sample C1 (p<0.05). Diacetyl was determined in butter samples in some previous studies as well (Lozano et al., 2007; Demirkol et al., 2016). Tamura et al. (2021) did not detect diacetyl in the sweet cream butter samples. Similarly, in the present study, diacetyl was not detected in samples produced without a starter culture, since diacetyl could not be produced as a result of fermentation by LAB (Lozano et al., 2007; Chen et al., 2017).
Similar to the production of diacetyl, acetoin is generated by LAB as a by-product of lactose or citrate metabolism (Demirkol et al., 2016; Chen et al., 2017). In odour identifications, acetoin is identified as buttery-creamy (Sarhir et al., 2021). 2-butanone 3-hydroxy- (acetoin) was detected in all butter samples and its content ranged from 61.07 µg kg-1 to 1879.79 µg kg-1 (Table 8). 2-Butanone 3-hydroxy- (acetoin) contents of the samples which contained the starter culture mix prepared by ourselves were lower than the sample containing commercial starter culture, while it was still higher than the sample without starter culture (p<0.05). Demirkol et al. (2016) found that the acetoin contents of the commercially sold butter samples were 785 µg kg-1 and 3880 µg kg-1. Sarhir et al. (2021) found that the acetoin amounts of fermented butter samples (Smen) were 6.08-8.48 mg kg-1. The low acetoin contents of sample C0 could be a result of absence of the starter culture.
Other compounds
Table 9 shows that the predominant compound found inside the other components was DL-limonene. Sample C2 contained all the other aroma compounds, while sample C0 had the lowest number of other aroma compounds. Six terpenes, 5 aromatic compounds, 4 aromatic hydrocarbons, 2 organosulphur, 1 monoterpene, and 1 organic solvent were detected in the butter samples as other aromatic. Aromatic hydrocarbons can arise from amino acid breakdown.
Lactic acid bacteria typically have an important effect on formation of aroma compounds (Guarrasi et al., 2017). Odour descriptions of benzene ethyl, and benzene 1,3-dimethyl are as phenol and spice, and plastic, respectively (Chen et al., 2017). Benzene methyl, and benzene 1,2-dimethyl were not identified in sample C0 (Table 9). Aromatic hydrocarbons did not indicate significant changes in samples (p>0.05). Terpenes play an important role in the quality of dairy products. However, there is not enough information in the literature regarding their effects on sensory properties (Nogueira et al., 2005; Tahmas-Kahyaoğlu et al., 2022). Terpenes are secondary metabolites in plants and pass into dairy products through feed (Demirkol et al., 2016; Iradukunda et al., 2018). The butter samples had α-pinene, DL-limonene, cis-ocimene, ç-terpinene, delta 3-carene and 2,4,6-octatriene, 2,6-dimethyl- as terpenes (Table 9). α-pinene contents of the samples varied between 6.95 µg kg-1 and 17.45 µg kg-1, and was detected in all the samples (Table 9). The differences among the samples were not statistically significant (p>0.05). Demirkol et al. (2016) determined pinene concentrations of butter samples in the range between 54.4 µg kg-1 and 1136 µg kg-1. Sarhir et al. (2021) detected 13 terpene compounds in butter samples. They determined α-pinene between 27.71 µg 100 g-1 and 38.28 µg 100 g-1. Limonene is an odour component that is associated with citrusy or lemon-like notes (Sarhir et al., 2021; Ürkek et al., 2022). DL-limonene contents of the butter samples ranged between 109.70 µg kg-1 and 1277.06 µg kg-1. There were no significant changes in the DL-limonene contents of the samples (p>0.05). Sarhir et al. (2021) determined that the DL-limonene concentration of fermented Smen samples was 1.82 mg kg-1. Demirkol et al. (2016) found that the limonene contents of commercial butter samples were between 61.03 µg kg-1 and 3851 µg kg-1.
Conclusion
The aroma compounds were detected in the butter samples which were produced using three different starter cultures and contained no starter culture. The butter samples produced without a starter culture (C0) had the least amount of aroma components. The number of different aroma compounds in butter samples were ordered as follows: C2>C1>C3>C0. The aroma compounds of the samples produced by a starter culture had higher concentrations of aroma compounds than those produced without a starter culture. The predominant aroma compounds for the C0, C1, C2, and C3 were 2-heptanone (889.16 µg kg-1), acetic acid (2056.90 µg kg-1), benzene, 1-methoxy-4-(2-propenyl) (1672.25 µg kg-1), and acetic acid (1809.19 µg kg-1), respectively. Adding L. rhamnosus B19 into the mix of starter culture did not negatively affect the aroma components according to the main butter starter culture mix.
References
Erkaya, T., Öztekin, A., Özdemir, H., Şengül, M. (2015): Changes in some quality properties of kefir during storage and inhibition effect of water soluble extracts on angiotensin-I converting enzyme purified by human plasma. International Journal of Food Engineering 11, 659-665. https://doi.org/10.1515/ijfe-2015-0057
