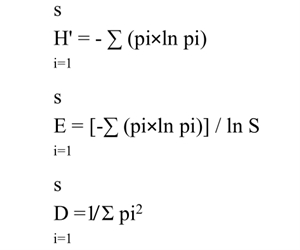Introduction
Allelopathy is a process that occurs when a donor plant releases chemical compounds into the environment that exert an adverse or positive effect on associated species (Rice1984). These compounds can leach from leaf litter, be exuded from roots, or arise from the decomposition of plant residues. Their release is modulated by environmental factors such as temperature, soil moisture, microorganisms and nutrients (El-Khawas and Shehata 2005). Depending on their concentrations, these substances can hinder the germination and growth of plant species, and their effect varies according to species (Quddus et al. 2014). Leaf litter can be found in abundance under the canopies of some trees. In urban ecosystems, leaf litter may arise naturally from deciduous trees (Hassan 2018) or be generated when evergreen trees undergo pruning. Several toxic compounds may be released from this leaf litter into the surrounding environment, adversely affecting the cover, diversity, species richness and species composition of the understory species (Chou 1999,Hassan 2018). For example, toxic compounds released from the leaf litter of Eucalyptus globulus Labill. and Acacia melanoxylon R.Br. have been shown to suppress some understory species (Souto et al. 1994). Under precipitation conditions, Acacia dealbata Link canopy released phytotoxic compounds that inhibited the net photosynthetic rate and consequently affected the distribution of understory species (Lorenzo et al. 2011). In this article, we will highlight such phenomena.
Soil is a complex medium that influences the availability of phytotoxic compounds released from plant residues, thereby affecting plant growth (El-Khatib et al. 2004). When these compounds are released into the soil, retention, transformation and transport processes may occur. These processes can be influenced by soil properties, chemical compound nature, and the physical environment (Cheng 1992,Kobayashi 2004). Substantial amounts of toxic substances that leach from litter or are released by its decay accumulate on the soil surface and interfere with plant growth (Facelli and Pickett 1991). In general, allelochemicals released from plant residues can influence the germination and growth of tested species when they are present in sufficient concentrations in the associated soil (Inderjit et al. 1996,El-Khatib 2000,El-Khatib et al. 2004). Therefore to assess the allelopathic effect of a particular plant, it is necessary to study its associated soil. Several studies have been undertaken to assess the potential release of allelochemicals and their effects on the understory vegetation (Molina et al. 1991,Espinosa-Garcia et al. 2008). However, knowledge of the allelopathic potential of the leaf litter of some trees is still lacking.
Ficus is a genus of about 750 woody species belonging to the Moraceae family (Semwal et al. 2013). Ficus species are highly distributed all over the world, widely used in medicinal purposes and are native to India, Southwest China and Nepal (Rawat et al. 2012). Ficus retusa is an evergreen tree that can grow to a height of 15 m with a wide-spread canopy (Semwal et al. 2013,Khan 2017). It was introduced to Egypt for ornamental purposes. Trees dominate the urban ecosystem of the new city of Beni-Suef governorate, and most of them are F. retusa trees. They are widely distributed in gardens, streets and along roadsides. Previous studies revealed the presence of a variety of phenolic compounds and flavonoids in the stem and leaves of F. retusa (Takahashi et al. 2002,Khan et al. 2011,Rawat et al. 2012,Aly et al. 2013,Singhal et al. 2017). Additionally, several compounds such as luteolin, ß-sitosterol acetate, ß-amyrin, friedelenol and new polyphenolic compounds called retusaphenol and (+)-retusa afzelechin were first isolated from the aerial parts of F. retusa (Sarg et al. 2011). Many of these compounds were shown to be allelochemicals (Rice 1984). Nevertheless, the allelopathic activity of F. retusa leaves has not been investigated. The goal of this study was to fill this gap in scientific knowledge.
The annual pruning of ornamental trees, such as F. retusa, and the residents’ lack of interest in removing the falling leaves cause the accumulation of substantial amounts of leaf litter underneath their canopies. There is a lower cover of some species under these canopies than in adjacent regions. In the light of litter effects, two main hypotheses were tested. The first hypothesis was that potential allelochemicals can be released from Ficus litter, accumulate in the soil, and affect the cover, richness and diversity of the understory vegetation. To test this hypothesis, a field study was conducted to measure the cover, richness and diversity indices of species under F. retusa canopies and compared them with those in neighboring areas away from the canopies. Additional testing in a greenhouse assessed the effects of litter-affected soils on the germination and growth of selected co-occurring species detected in the field study by comparing them with the effects of soils unaffected by litter. To confirm the allelopathic effect of field soil, HPLC analysis was used to investigate the putative allelochemicals (phenolics and flavonoids) present in litter-affected and unaffected soils. This analysis reflected the presence of allelochemicals released from leaf litter into the soil under natural conditions. The second hypothesis stated that the litter altered the chemical properties of the soil in a way that led to adverse effects on the cover and diversity of understory plants. In other words, the litter may interfere with soil pH, electrical conductivity (EC), organic matter (OM) and nutrient availability. To test this hypothesis, soil samples collected from under and outside the tree canopy were analyzed to study the potential changes in these properties. Since changes in the EC of field soil may exert an osmotic effect on the understory vegetation, a polyethylene glycol (PEG) 6000-based bioassay was used to assess this possibility. The primary goal of the present study was to assess the allelopathic effects of F. retusa leaf litter on the cover, composition and floristic diversity of the understory vegetation.
Material and methods
Study area
This study was conducted in the second district of the city New Beni-Suef (29°01.50′ to 29°02.50′ N, 31°06′ to 31°07.30′ E). This region is considered one of the common new urban areas in Egypt. It is found about 124 km south of Cairo and located east of the Nile Valley at an elevation of 32 to 42 m above sea level. It has been completely built since 1990 and has an area of about 1.42 km2. The climate of this city is characterized by mild winters with low precipitation and hot, dry summers (Hassan and Hassan 2019). The average annual precipitation, which falls from November through April, is about 11.98 mm. The texture of the soil in this region is sandy, sandy loam or sandy clay loam. Other properties of the soil in the gardens of this area, such as pH, EC, organic matter and some available nutrients, were measured byHassan (2018). Many tree species have been introduced into this area for ornamental purposes. F. retusa trees are among the most common trees in this territory.
Field study
To assess the effect of F. retusa tree litter on the understory vegetation, a total of 62 plots, each of 3 × 0.1 m2, were set randomly under and just outside the tree canopies. Thirty-one plots located under tree canopies were designated as treatment areas and the remaining plots outside those canopies (about 2 m away) were set as controls. These plots were selected in different seasons (mid-winter and mid-summer of 2018) to include the total species richness present in this area through the entire year. In each plot, the vegetative parameters measured in this study were the cover of each species, total plant cover of all species, species richness, bare length (measured by the area not occupied by plant cover), relative cover of species [(cover of species i/cover of all species) × 100] and diversity indices (Simpson’s index (D), Shannon-Weaver index (H') and Evenness index (E)) as shown byHassan (2018). The species detected were identified usingBoulos (2000,2002,2005):
where pi is the relative cover of species i.
Soil samples taken from 0-20 cm depth of each plot were air-dried, passed through a 2 mm sieve and stored in plastic bags prior to analysis. The measured parameters in soil samples were pH, EC, available nutrients (N, P, K, Cu and Zn) and organic carbon.
Soil pH was measured in a soil-water extract (1:2.5, w/v) using a pH meter (AD 3000), while soil EC was measured in a soil-water extract (1:5, w/v) using a conductivity meter (Jenway 3305). Available nitrogen was determined as described byAllen (1989). Briefly, 5 g of soil was added to 50 mL of 2N KCl, shaken for 30 min and then filtered. Ammonium nitrogen and nitrate nitrogen in the filtrate were measured with a Technician Auto Analyzer. Total available nitrogen is expressed as the sum of ammonium and nitrate nitrogen. Available P, K, Cu and Zn were determined as described bySoltanpour (1991). Briefly, 20 g of soil sample was added to 40 mL of a solution containing diethylenetriaminepentaacetic acid (DTPA, 97%) and ammonium bicarbonate at pH 7.6 and then mixed well. After 15 minutes of shaking, the extract was filtered and the filtrate used for further analyses. P, Cu and Zn were measured using inductively coupled plasma (ICP) spectrometry (Ultima 2 JY Plasma), while K was measured using a flame photometer. Soil organic carbon was determined using the method described byWalkley and Black (1934).
Allelopathic potential of litter-affected soils under Ficus canopy
To assess the allelopathic effect of F. retusa leaf litter, its residual toxicity in field soil collected under tree canopies was determined using selected understory species, Melilotus indicus (L.) All., Trifolium resupinatum L. and Amaranthus viridis L. These species showed appreciably suppressed cover under canopies in field conditions.
Litter-affected soil samples were collected under F. retusa canopies and mixed well to form a composite treatment soil, while litter-free soil samples collected outside those canopies were used as a control. These samples were used for germination and growth of the three tested species. Specifically, the soil samples were shade-dried, passed through a 2-mm sieve, and then put in plastic pots (11 cm diameter × 11 cm deep) that received about 0.5 kg soil each. In each pot, ten seeds of each tested species were sown at a depth of 0.2 cm. Regular irrigation was carried out by spraying when needed. This experiment was kept with four replicates in a protected area for 30 days under prevailing environmental conditions (12 h light and 12 h dark photoperiod, 24 to 34 °C daytime temperature, 14 to 22 °C nighttime temperature, and 27 to 28% relative humidity). The germination rate (in %) was calculated after the emergence of the tested species ceased. Three growth criteria were measured at harvest: shoot length, root length and biomass. Individuals from each target species were dried in an oven at 70 °C for 72 h then weighed to determine the dry mass.
To assess the osmotic potential of the litter-affected soil and separate its osmotic effect from its allelopathic effect, another experiment was conducted using PEG 6000. In this experiment, solutions with osmotic potentials equivalent to those of the field soils (litter-affected and litter-free) were prepared. From soil analysis, EC values of the field soil were converted to osmotic potentials (Osm·kg-1 H2O) by using the following equation (Gomaa et al. 2014):
The osmotic potentials of litter-affected and unaffected soil were -0.0097 and -0.0039 Osm·kg-1 H2O, respectively. To obtain a PEG solution with osmotic potential equal to that of litter-affected soil, 32.3 g of PEG 6000 was dissolved in 1 liter of H2O at room temperature (28 °C) (Michel and Kaufmann 1973). By diluting the previous PEG solution, we prepared a solution of osmotic potential value equivalent to that of the unaffected soil.
To show the effect of the osmotic potential of field soil on the target species, a germination test was performed using the prepared PEG solutions. Due to the hard seed coat and dormancy characteristics of the legume and Amaranthus species, scarification treatments were done (Ates 2011,Assad et al. 2017). Seeds of the legume species were soaked in concentrated H2SO4 (98%, v/v) for 10 min while Amaranthus seeds were soaked for 2 min. After soaking, the seeds were washed with distilled H2O, sterilized with 5% (w/v) sodium hypochlorite for 2 min, and rinsed three times with distilled H2O. Twenty-five seeds of each tested species were put on filter paper in sterile petri dishes (9 cm diameter) and then supplied with 5 mL of the prepared PEG solutions. All the experimental samples were kept in a dark chamber at 23 ± 2 °C in a completely randomized design with four replicates. After seven days, germination (in %) was assessed by counting the number of germinated seeds. Root lengths, shoot lengths and seedling biomasses were determined seven days after seeding by measuring similar seedlings in each dish. Root and shoot lengths were estimated as described byHussain et al. (2011).
Determination of phenols and flavonoids in litter-affected and unaffected soils
Polyphenols were extracted from the field soil samples with aqueous methanol (80%) in a 250 mL Erlenmeyer flask using an ultrasound-assisted method (Kim and Lee 2002). Briefly, aqueous methanol (80%, 100 mL) was added to soil (10 g) and the mixture was subjected to continuous sonication for 60 min. After filtration, the filtrate was evaporated in a vacuum evaporator at 40 °C. The residue was dissolved in 50 mL methanol then diluted to a final volume of 100 mL with distilled H2O. The resulting solution was centrifuged for 15 min at 12,000 rpm and stored at −20 °C until analysis.
The total phenolic content was determined according toKim et al. (2003) protocol. One mL of the stock extract, 9 mL of distilled H2O and then 1 mL of Folin-Ciocalteu phenol reagent were added to a 25-mL volumetric flask and mixed well. After 5 min, 10 mL of 7% Na2CO3 solution were added to the mixture with shaking. Finally, distilled H2O was added to reach 25 mL and then the solution was left standing for 90 min. At this point, the absorbance of the solution at 750 nm was measured versus a prepared blank. Gallic acid was used to prepare a calibration curve. Total phenolic content was expressed as mg gallic acid equivalent (GAE) per gram of soil sample (mg·g-1 soil sample).
The total flavonoid content was performed using the method described byChun et al. (2003). One mL of the stock extract and 4 mL of distilled H2O were added to a 10-mL volumetric flask. After 5 min, 300 μL of 5% NaNO2 followed by 300 μL 10% AlCl3 were added to the mixture. This mixture was allowed to stand for 6 min, and then 2 mL of 1M NaOH was added and the final volume was adjusted to 10 mL using distilled H2O. The absorbance at 510 nm was measured versus a prepared blank. Rutin was used to prepare a calibration curve. Total flavonoids in the soil sample were expressed as mg of rutin equivalents (RE) g-1 soil (mg·g-1 soil sample).
HPLC analysis
Phenolics and flavonoids were analyzed according toHassan (2018). This analysis was carried out using a Shimadzu HPLC system equipped with an LC 1110 pump, a Kromasil C8 column (4.6 mm × 250 mm; particle size, 4.6 μm; pore size, 100 Å) and a diode-array UV detector and run using WinCrome Chromatography software (version 1.3). Phenolic compounds were analyzed using a solvent gradient consisting of acetonitrile: 0.05% H3PO4 (99:1; solvent A) and water: H3PO4 (99:1; solvent B) and a flow rate of 1 mL·min-1. The elution program consisted of 90% A from 0 to 30 min, a linear decline to 50% A from 30.01–40 min, and a further decline to 0% A from 40.01 to 55 min. Flavonoid compounds were analyzed using a solvent gradient composed of methanol:H3PO4 (99:l; solvent A) and water:H3PO4 (99:1; solvent B) and a flow rate of 1 mL·min-1. Flavonoid compounds were detected using UV (250–400 nm) absorbance. Phenolic and flavonoid compounds were identified by comparison of their retention time with those of phenolic standards including trans-cinnamic, vanillic, p-coumaric acids, catechol, sinapic, protocatechuic, syringic, caffeic, p-hydroxybenzoic acids, vanillin and resorcinol; and flavonoid standards including hesperidin, luteolin, rutin, apigenin, catechin, kaempferol and quercetin. The concentrations, expressed as mg g-1, were estimated according to knowledge of the heights and areas under peaks of detected compounds in soil samples. Values reported are the average of three replicates.
Statistical analysis
Kolmogorov–Smirnov and Levene's tests were used to check the normality and homogeneity, respectively, of the data obtained from field, greenhouse and laboratory experiments. When the data were normally distributed, they were analyzed using the independent Samples T test. Data showing abnormality and heterogeneity were analyzed using the nonparametric Mann-Whitney U test. All data in this study were analyzed with the use of the SPSS Statistics software package, version 20.0 (IBM Corporation, USA) at probability levels *P ˂ 0.05 and **P ˂ 0.01.
Results
Field study
A total of 12 species belonging to 11 genera and five families were detected throughout the study area (Tab. 1). Poaceae had the highest number of species (five) followed by Fabaceae (three), Plantaginaceae (two), Amaranthaceae and Euphorbiaceae (in each one). Nine species were detected as annuals and three species were detected as perennials (Tab. 1).
Sites heavily covered with F. retusa leaf litter attained lower cover of some understory species. Among the annuals, the covers of Amaranthus viridis L., Medicago polymorpha L., Melilotus indicus (L.) All. and Trifolium resupinatum L. under tree canopies were significantly lower than those outside the canopies. In addition, Plantago amplexicaulis Cav. was completely absent from the infested sites (Tab. 1). For perennials, only the cover of Cynodon dactylon (L.) Pers. was significantly reduced at the litter-affected sites, whereas the remaining perennial species were not affected.
Significant reductions in vegetation cover, and species richness were also recorded in the plots affected by litter, compared with those free from litter (Tab. 2). Also, there was a significant increase in bare length in litter affected plots. However diversity, as measured using Shannon-Weaver, evenness and Simpson’s indices, was not influenced (Tab. 2).
Most of the soil analysis criteria were unaffected by the presence of litter. However, the litter-affected soils exhibited lower pH and higher EC values (Tab. 3).
Greenhouse experiment
Generally, litter-affected soil collected from under F. retusa canopies drastically reduced germination and some of the growth variables of studied species. Litter-affected soils significantly decreased seed germination of the selected target species (P ˂ 0.01) (Fig. 1A). Litter-affected soil significantly decreased the root lengths of A. viridis and T. resupinatum (Fig. 1B), while the shoot length was significantly reduced for the later (Fig. 1C). Moreover, the biomass of both species was significantly suppressed (Fig. 1D).
The PEG solution with an osmotic potential equivalent to that of the litter-affected soil did not significantly affect the germination (Fig. 2A) or growth parameters (Fig. 2B-D) of the tested species, compared with the control.
Biochemical analysis
The concentrations of the phenolic and flavonoid compounds detected in litter-affected and unaffected soils are summarized inTab. 4. Among the free compounds, quercetin and resorcinol were completely absent in control soils (Tab. 4). Litter-affected soils also contained significantly more quercetin, resorcinol, caffeic acid, coumaric acid and ellagic acid than unaffected soils. Furthermore, the total phenolics and flavonoids were significantly higher (by 65.84% and 47.54%, respectively) in litter-affected than in control soils (Tab. 4).
Discussion
The results of this study clearly demonstrate that F. retusa leaf litter has an inhibitory effect on the selected understory species. Significant reductions in the cover of many species, total plant cover and species richness were observed in plots under tree canopies. These observations are similar to those of previous studies, which illustrated considerable inhibition of plant cover beneath the tree canopy, compared with areas outside the canopy, due to the presence of leaf litter (Ahmed et al. 2008,Souza et al. 2010,Hassan 2018). Many tree species have been shown to negatively affect the cover, diversity and composition of some understory herbaceous species (Barbier et al. 2008). Moreover,Loydi et al. (2013) indicated that a high amount of oak tree litter reduced the cover, composition, species richness and biomass of some associated species. This effect may be due to toxic compounds leaching from litter residues (Batish et al. 2007).
The soil analysis revealed that the differences in organic matter and available nutrients between litter-affected and unaffected soils were not significant. This result suggests that the litter does not interfere with these soil criteria. It also indicates that Ficus trees do not have a competitive effect on associated weed species. Therefore, the reduction in plant cover and species richness could not be attributed to decreased soil organic matter or nutrient availability. These results are similar to those obtained byHassan (2018). In contrast, there was a considerable reduction in pH and a significant increase in the EC of litter-affected soils. These results may be due to phenolic compounds and minerals liberated from litter residue, as mentioned byHassan et al. (2014). In this study, the pH value decreased from 7.9 to 7.63. These values seem to be in the normal range for the germination and growth of plants (Xuan et al. 2005). On the other hand, the EC value of litter-affected soil in this study was 0.664 mS·cm-1.Xuan et al. (2005) reported that EC values < 1 mS·cm-1 did not affect the growth of tested species. Similarly,Hassan et al. (2014) confirmed that EC had a significant role in the inhibition of target species growth when it was >1.5 mS·cm-1. Therefore, since the EC value observed in this study was within a range appropriate for plants, the decline in vegetation cover and richness is not attributable to a high EC value in the field soil.
Under greenhouse conditions, the litter-affected soil collected under the canopies of F. retusa trees substantially reduced the emergence and growth of the tested species. Moreover, HPLC and spectroscopic analyses showed that the litter-affected soil contained greater amounts of phenols and flavonoids. These findings suggest that the litter-affected soil collected under the Ficus canopy had an inhibitory effect on the understory species. This inhibition could be due to the presence of phenolic and flavonoid compounds released into the soil during Ficus leaf litter decay or leached from Ficus leaf litter during irrigation or rainfall. Among different classes of secondary metabolites, phenolic compounds are the major group that inhibits plant growth (Appel 1993). The compounds detected by HPLC are potent allelochemicals that frequently inhibit the seed germination, growth and productivity of some species (Qasem and Foy 2001). For example,Golisz et al. (2007) indicated that some phenolics, such as ferulic acid, gallic acid and chlorogenic acid, reduced the germination and seedling growth of lettuce to differing degrees. Likewise, quercetin, apigenin, rutin and kaempferol are considered inhibitory substances that suppress the emergence and performance of many species (Basile et al. 2000,Sadeghi and Bazdar 2018). These compounds harmfully affect cell division, the ability to absorb nutrients, membrane permeability, protein creation and the activities of enzymes, eventually reducing the growth of target species (Li et al. 2010). Additional studies have demonstrated that the cover and diversity of plant species could be changed by allelopathic compounds such as those detected during the HPLC analysis conducted in this study (Chou 1999,Hassan 2018,Hassan and Mohamed 2020). The inhibitions observed under field conditions and in greenhouse experiments may be equivalent.
In this study, litter-affected soils attained substantially higher values of EC than those of litter-free soils. Although the values observed were normal for plant growth, the effect of the osmotic potential of soil solutes on seed germination and seedling growth had to be assessed to exclude potential osmotic interference with the allelopathic effect in soil. The results of our work showed that PEG solutions with osmotic potentials equivalent to those of litter-affected soils did not significantly reduce the germination and growth of the target species. Therefore, osmotic potential did not play a significant role in the inhibition of target species under greenhouse conditions or in the understory vegetation. Accordingly, the inhibitory impact of field soil collected under the tree canopy on the germination and growth of tested species under greenhouse conditions was due to the allelochemicals present in the field soil.
Conclusion
The field study indicated that the cover of selected understory species and species richness were reduced under the canopy of F. retusa trees. The greenhouse experiments showed that litter-affected soil suppressed the germination and growth parameters of the selected species. The biochemical analysis of the litter-affected soils confirmed the presence of elevated levels of many phenolic and flavonoid compounds, as compared with unaffected soils. These compounds have been shown to be phytotoxins that negatively affect the germination and growth of some understory species. The results of the soil analysis indicated that there were no statistically significant differences between soils obtained underneath and outside the Ficus tree canopy; thus, soil chemical characteristics did not contribute to the reduction in plant cover. Consequently, the allelopathic potential of the F. retusa leaf litter was the main cause of the observed reduction in plant cover. Due to its allelopathic potential, F. retusa leaf litter should be continuously removed to maintain the general landscape of vegetation within the new urban ecosystem.