Introduction
Lactation is a very demanding period for the animal, which requires a significant effort of the organism to meet all needs, but also the effort of farmers to satisfy those needs. Sheep successfully maintain a homeostatic balance system. However, during lactation, significant metabolic disorders may occur, which is manifested in metabolic diseases and losses in animal production. Piccione et al. (2009) observed that during lactation, the secretory cells in mammary glands utilized 80 % of the blood circulating metabolites aimed for milk synthesis, depending on the speed of infiltration of milk compounds (i.e. free amino acids, glucose, fatty acids).
Blood analysis is often used for monitoring animals, especially those with high milk potential, along with monitoring their production status and the quality of the obtained products (Antunović et al., 2017; Djoković et al., 2019). Thus, monitoring the mammary gland and the quantity and quality of milk as well as liver function of the animal can be included as a quality criterion to eliminate possible diseases. This contributes to better financial performance for the producer due to the reduced treatment costs and the reduced removal of animals from breeding (Antunović et al., 2002; Djoković et al., 2013). Blood parameters are often monitored at the beginning of lactation during its transition and in the middle of lactation (Djoković et al., 2017).
Biochemical parameters and activities of enzymes provide an information on the ewes’ health status reflecting its responsiveness to internal and external environments (Aksoy et al., 2018). There is only a limited number of studies on their follow-up throughout lactation. Liver in animals is an important organ that contribute to the production of milk. It has an impact on the level of many blood parameters and on various systems in the body (Djoković et al., 2019). Studying the liver enzymes (ALT-alanine aminotransferase, AST-aspartate aminotransferase and GGT-γ-glutamyl transferase) and blood indicators (bilirubin, albumin, glucose, cholesterol, triglycerides) in the serum, and their relation to the quantity and quality of milk, can be considered as parameters of liver function and production potential of animal (Jozwik et al., 2012; Hrković-Porobija et al., 2017; Djoković et al., 2019; Kuczyńska et al., 2021). Possible alternations in liver function may have deleterious effects on the metabolism, milk production or reproduction of animals (González et al., 2011). Therefore, the aim of the present study was to research correlation between milk composition and selected blood indicators of liver function in ewes during lactation.
Material and methods
The Bioethics Committee for Research on Animals of the Faculty of Agrobiotechnical Sciences, University of Osijek, confirmed that the present research was carried out by obeying all legal provisions according to the Animal Protection Act (NN 133/06, NN 37/13 and NN 125/13). The research was conducted on 99 samples of sheep milk and blood from Travnik Pramenka collected during lactation in the area of western Slavonia in Croatia from sheep grazing on natural pastures. These ewes were selected from the herd of 1000 animals. The selected ewes were healthy and in a good physical condition. The present study was conducted in 2019 when ewes were in the third lactation and had a single lamb in the litter. The lactation lasted in average for 120 ± 5 days. The family farm was located in Velika Peratovica, 10 km from Grubišno polje (Croatia, 45°45'25"N, 17°14'51"E, ~211 m above sea level). The monthly mean temperature for this area from May to July 2019 was 19 °C, while the mean monthly rainfall was 113 mm.
Milk sample collection and indicators analysed
Milk yield (MY) of each ewe was recorded with a measuring cylinder. Milk samples were collected (200 mL), transferred into mobile coolers and cooled down to 4 °C. Within 24 hours from collection, milk samples were subjected to laboratory analysis. Dry matter without fat (DMNF), milk fat, protein, lactose, somatic cells count (SCC) and the total number of microorganisms (CFU) were analysed in sheep milk. Determination of the non-fat dry matter was calculated by subtracting the milk fat from the dry matter (DM), which was determined by drying samples to constant mass. Milk fat, protein and lactose contents in milk were determined by infrared spectrometry (HRN EN ISO 9622: 2001) using the MilkoScan FT 6000 analyser within the Comby system. The SCC was determined by fluoro-opto-electronic method (HRN EN ISO 13366-2 / Correction. 1. 2007) with the Fossomatic 5000 analyser, while the number of CFU was carried out with the epifluorescent method of flow cytometry (IDF 161A: 1995). The SCC and CFU values were expressed as logarithms, in order to obtain normal distribution.
Blood samples and preparation for analyses
Blood samples were collected from the jugular vein (10 mL) into two sterile vacuum tubes Venoject ® (Sterile Terumo Europe, Leuven, Belgium). From each ewe during lactation and before grazing, blood samples were centrifuged at 1609.92 g for 10 min. The obtained serum samples were placed into the Olympus AU400 and were subjected to determination of biochemical indicators (glucose-GUK, albumin-ALB, cholesterol-CHOL, triglycerides-TGC, bilirubin-BIL), and liver enzymes activities (alanine aminotransferase-ALT, aspartate aminotransferase-AST and γ-glutamyl transferase-GGT) by using Olympus System reagents (Olympus Diagnostic GmbH, Lismeehan, Ireland).
Statistical analyses
The distribution of data from blood was verified by the Shapiro-Wilk test (PROC UNIVARIATE). Results were obtained by MEANS procedure. Values of SCC and CFU were logarithmically converted to a linear score with the aim to approximate normal distribution. Correlations between indicators of ewes´ milk and blood, as well as within these parameters, were evaluated by Pearsons´ correlation with CORR procedure. The correlations were declared significant if P<0.05. Statistical analysis was performed using the statistical software SAS 9.4 ® (2002-2012).
Results and discussion
The analysis of chemical composition of sheep milk (Table 1) was performed in accordance with the provisions of the Official Gazette of the Republic of Croatia (NN 27/17), which define that milk contains at least 3 % and at the most 12 % of milk fat, and at least 3.8 % and at the most 8 % of proteins. Milk production or milk components were in a similar or even in a higher amount in confrontation with phylogenetically related animals breed under extensive conditions (Macuhova et al., 2017; Kusza et al., 2018). Antunović et al. (2017) determined lower fat and protein content in sheep milk in southern Croatia, being 5.32 % and 5.66 %, respectively, and similar lactose content (4.47 %), SCC (4.99) and CFU (4.11) in comparison with present investigation (Table 1). Sajko-Matutinović et al. (2012) determined a slightly lower milk fat content (6.96 %) in Dalmatian Pramenka. Antunović et al. (2001) determined a similar fat (7.22 %), protein (5.27 %) and lactose content (4.78 %) in Merinoladschaf milk in Slavonia. Oravcová et al. (2015) reported a fat content of 5.55 - 8.85 % and 5.28 - 7.42 % proteins, and 6.96 - 9.37 % fats and 5.12 - 6.39 % proteins respectively. The MY of Tsigai sheep in this research was 0.414 - 1.066 kg day -1, and in improved Wallachian ewes from 0.379 to 0.874 kg day -1. Significant positive correlations were also found between MY: lactose (r = 0.37), protein : fat (r = 0.51), protein : DM (r = 0.75), protein : DMNF (r = 0.87), protein : SCC (r = 0.28), protein : CFU (r = 0.33), fat : DM (r = 0.93), fat : SCC (r = 0.31), fat : DMNF (r = 0.48), DM : DMNF (r = 0.77), SCC : CFU (r = 0.57). Significantly negative correlations were as following, MY : protein (r = -0.35), MY : fat (r = -0.37), MY : DM (r = -0.36), protein : lactose (r = -0.52), lactose : SCC (r = -0.50) and lactose : CFU (r = -0.33) (Fig 1.). Oravcová et al. (2007) also found similar correlations in sheep milk of the Tsigai breed as in the present study. Similar results for the correlations of SCC : fat, SCC : protein and SCC : lactose were obtained by Oravcová et al. (2018) in sheep milk in Slovakia, while Riggio et al. (2007) found similar in sheep milk in Italy. This is in agreement with significant positive correlation in cows during late lactation, which were found by Mordak et al. (2020) between CFU : fat, CFU : DM, SCC : protein, fat : DM, protein : DMNF as well as ALB : protein, ALB : DMNF, and significant negative correlation between CFU : lactose, fat : lactose, fat : DMNF, lactose : DM and DM : DMNF. It is well known that increase of CFU and SCC is expected in milk of sheep during infection of the mammary glands or clinical mastitis (Raynal-Ljutovac et al., 2007). However, this research was conducted on healthy ewes which indicates a positive correlation between CFU : fat. The aforementioned may also be confirmed by activities of ALT, AST, which were within the reference values. Significant positive correlation between MY : protein, fat : DM, and fat : DMNF could be explained by a strong effect of fat and proteins on the content of DM in ewes milk. The determined negative correlation between lactose : fat, although not significant, indicated their metabolic competitiveness in milk. A relatively high significant negative correlation was found for lactose : DM, indicating that lactose does not play a significant role in DM content in ewes´ milk as it was in fat and protein content. Similar conclusions in late lactating cows were observed by Mordak et al. (2020). Compared to the reference values, blood indicators and enzyme activities in lactating sheep were adequate since their concentrations were within the reference range (Table 2). In the present research the concentration of BIL was determined in blood, which was within reference values indicating that ewes were clinically healthy and that milk production was not high (Table 1). The determined average concentrations of CHOL and TGC were on the upper limit of the reference values. This may indicate a minor feeding imbalance in energy content. However, it is well known that contribution in TGC transport mobilized by adipose tissue is expected during early lactation in cows (Ling et al., 2003). Determination of liver enzymes (AST, ALT, GGT) and blood metabolites (bilirubin, albumin, glucose, cholesterol) are important parameters regarding liver function (Aschenbach et al., 2010). It is evident that the average activity of liver enzymes in the blood of lactating sheep were within the reference values (Kaneko i sur., 2008). The determined higher maximum values for the activity of AST and GGT in the blood indicate increased metabolic changes in the body of lactating sheep. However, the increasing AST concentration in the blood serum in cows could indicate a growing intensity of metabolic changes, mainly of proteins, especially in the late lactation (Sakowski et al., 2012).
Table 1. Milk yield and chemical composition of sheep milk
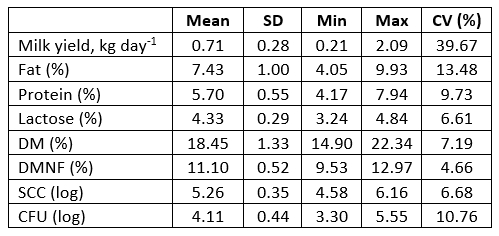
SD - standard deviation; CV - coefficient of variation; MY - milk yield; DM - dry matter; DMNF - dry matter non-fat; SCC - somatic cells count; CFU - total number of microorganisms
Table 2. Blood indicators in sheep
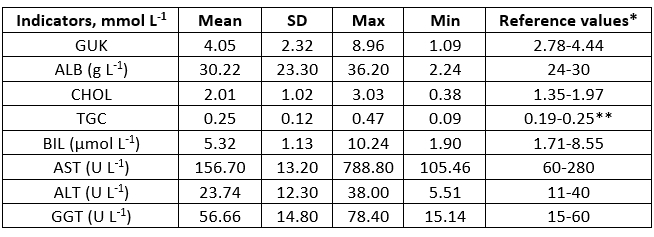
SD - standard deviation; GUK - glucose; ALB - albumin; CHOL - cholesterol; TGC - triglycerides; BIL - bilirubin; ALT - alanine aminotransferase; AST - aspartate aminotransferase; GGT - γ-glutamyl transferase; * Kaneko et al. (2008); **Antunović et al. (2011)
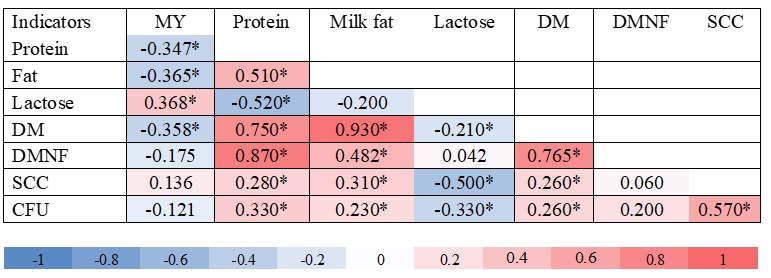
Figure 1. Heatmap correlation (* means P<0.05) among milk yield, chemical composition, somatic cells count (SCC) and number of bacteria (CFU) in milk of sheep, where: MY = milk yield, DM = dry matter, DMNF = dry matter non-fat, SCC = somatic cells count and CFU = total number of microorganisms.
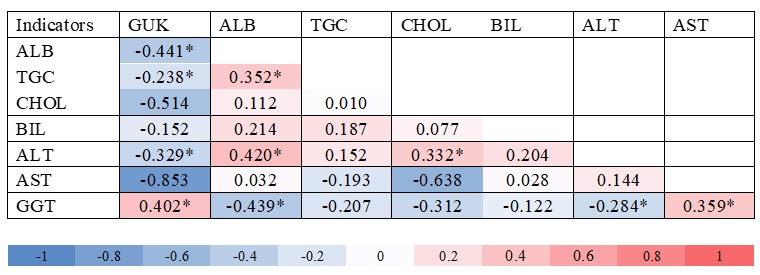
Figure 2. Heatmap correlation (* means P<0.05) among metabolites and activities of enzymes in blood of sheep, where: GUK = glucose, ALB = albumin, CHOL = cholesterol, TGC = triglycerides, BIL = bilirubin, ALT = alanine aminotransferase, AST = aspartate aminotransferase and GGT = γ-glutamyl transferase
By analysing data presented in Fig 2. it is evident that a significant positive correlation between ALB : TGC (r = 0.35), ALB : ALT (r = 0.42), GUK : GGT (r = 0.40), CHOL : ALT (r = 0.33) and AST : GGT (r = 0.36) could be determined, while a significant negative correlation was determined between GUK : ALB (r = -0.44), GUK : TGC (r = -0.24), GUK : ALT (r = -0.33), ALB : GGT (r = -0.44) as well as ALT : GGT (r = -0.28). Similar correlations in the blood of late lactating cows between ALT : CHOL, ALT : ALB, GUK : GGT were found by Mordak et al. (2020). These could be related to the higher rate of liver metabolism in dairy cows. Positive correlation between GUK : GGT in serum indicated reliability of these indicators in monitoring the metabolic health of animals. However, Bertoni and Trevisi (2013) have pointed out that GGT enzymes in various tissues and especially in the liver can be taken as a good diagnostic marker in the assessment of metabolic health of dairy cows. Similar conclusions in cows were observed by Mordak et al. (2020). Jozwik et al. (2012) determined a significant positive correlation between AST : GGT and a negative correlation between CHOL : ALT in the blood of cows during lactation. In the blood of Tsigai sheep in the first third of the lactation period, Antunović et al. (2011) determined a significant correlation between TGC : ALB.
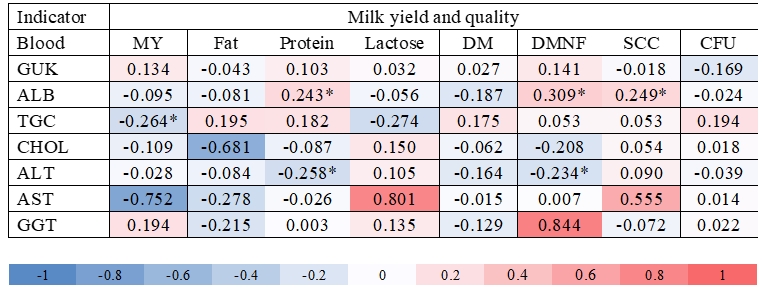
Figure 3. Heatmap correlation (* means P<0.05) among milk composition and blood indicators of sheep during lactation, where: MY = milk yield, DM = dry matter, DMNF = dry matter non-fat, SCC = somatic cells count, CFU = total number of microorganisms, GUK = glucose, ALB = albumin, CHOL = cholesterol, TGC = triglycerides, ALT = alanine aminotransferase, AST = aspartate aminotransferase and GGT = γ-glutamyl transferase
A significant positive correlation was determined between ALB : protein (r = 0.24), ALB : DMNF (r = 0.31) and ALB : SCC (r = 0.25), as well as negative correlation between TGC : MY (r = -0.26), ALT : protein (r = -0.26), lactose : TGC (r = -0.27) and ALT : DMNF (r = -0.23) (Fig. 3). The determined positive correlation between ALB : protein and ALB : DMNF indicated that serum albumin can be naturally translated into protein content in milk because serum albumin is a common constituent of body fluids, both plasma and milk in various mammals (Lieske et al., 2005). A significant positive correlation between ALB : SCC can be caused by the natural stimulation of microorganisms in the mammary gland which increases the number of leukocytes and epithelial in milk, and also increases all proteins in the serum as found in studies on cows by Mordak et al. (2021). A negative correlation between ALT : DMNF pointed out the importance of biochemical pathways related to liver function (Rezaei et al., 2016). Djoković et al. (2019) determined a significant negative correlation between the concentration of CHOL and the milk fat content in cows. In the present study, a significant negative correlation was observed between these two indicators, although without significant differences. The reason could be a different lactation period and different status of ewes. Cimen et al. (2007) determined a negative correlation between plasma CHOL and milk fat (r = -0.56; P < 0.05) in early lactating non-dairy sheep. These authors concluded that such findings were probably due to high starch intakes by sheep in the studied lactation period, since barley was the main component of the sheep diet (53 %). El-Tarabany et al. (2018) determined a negative correlation between the daily MY and TGC (r = -0.55, P < 0.01) as well as for the daily MY and CHOL (r = -0.33, P <0.05) in Baladi goats during lactation. In the present study, a negative correlation between daily MY and AST in blood was found, although not significant. Sakowski et al. (2012) also determined a negative correlation between the average daily MY and blood AST activity in organically reared dairy cows, although correlations were not significant.
Conclusion
The relations between milk composition, milk production and sheep blood indicators as well as their mutual connections supported the practice of using the analysis of liver status indicators (liver enzymes: AST, ALT, GGT and metabolites: bilirubin and cholesterol). The obtained results indicated that in this way the metabolic pathways of liver status indicators could be better monitored, which is important regarding the prevention of possible feeding errors and major production losses while preserving the health of lactating sheep.
Acknowledgements
The study was carried out within the research team Innovative breeding and technological processes in animal production (No. 1126) at Faculty of Agrobiotechnical Sciences Osijek.
Korelacija između kemijskog sastava mlijeka i određenih pokazatelja funkcije jetre u ovaca tijekom laktacije
Sažetak
Cilj istraživanja bio je utvrditi korelacije između kemijskog sastava mlijeka i pojedinih pokazatelja funkcije jetre u krvi ovaca tijekom laktacije. Istraživanje je provedeno na uzorcima mlijeka i krvi travničke pramenke (n = 99). Uzorci su prikupljeni na području zapadne Slavonije (Hrvatska) od ovaca u laktaciji koje su pasle na prirodnim pašnjacima. Određen je kemijski sastav ovčjeg mlijeka te pojedini biokemijski pokazatelji funkcije jetre u serumu. Utvrđena je pozitivna korelacija između ALB : TGC, ALB : ALT, GUK : GGT, CHOL : ALT te AST : GGT kao i negativna korelacija između GUK : ALB, GUK : TGC, GUK : ALT, ALB : GGT i ALT : GGT. Također je utvrđena pozitivna korelacija između pokazatelja statusa krvi i sastava mlijeka, točnije između ALB : protein (r = 0.243), ALB : DMNF (r = 0.309) te ALB : SCC (r = 0.249). Negativna korelacija utvrđena je između TGC : MY (r = -0.264), ALT : protein (r = -0.258), laktoza : TGC (r = -0.274) i ALT : DMNF (r = -0.234). Povezanost između kemijskog sastava mlijeka i količine mlijeka te pokazatelja statusa krvi ovaca, kao i njihove međusobne povezanosti ukazuju na opravdanost analize pokazatelja statusa jetre. Na taj se način mogu kvalitetnije pratiti metabolički procesi koji se odvijaju u jetri što je važno u pogledu sprječavanja mogućih grešaka u hranidbi i velikih gubitaka u proizvodnji uz istodobno očuvanje zdravlja ovaca u laktaciji.
Ključne riječi: ovce; mlijeko; krv; laktacija; jetra
