1.INTRODUCTION / Uvod
Croatian aquaculture production, both freshwater and marine, exceeded 25,000 tonnes in 2022. The most important species in mariculture, as in other Mediterranean countries, are European seabass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata), whose production accounts for about two thirds of the total Croatian aquaculture production [1]. However, in addition to these two species and bluefin tuna (Thunnus thynnus), new fish species have been introduced into Croatian aquaculture in the last two decades, such as meagre (Argyrosomus regius), common dentex (Dentex dentex) and greater amberjack (Seriola dumerili). Most fish farms are in the middle part of the Eastern Adriatic, in the Zadar region [2].
Aquaculture in general has a diverse environmental impact and hence challenges. In the Mediterranean region, with its great biodiversity and rapidly growing aquaculture, one of the impacts is the wild fish aggregation in the vicinity of farmed cages. Two reasons that are mostly noticed regarding wild fish aggregation are the availability of food in the cages and the farm structures that provide protection from the predators [3,4,5,6]. The food source is mostly pellets that are lost around the cages or particulate organic matter (POM) – a mixture of uneaten food pellets and faeces from farmed fish [3,4].
Bogue Boops boops (Linnaeus, 1758), is an opportunistic fish species that is considered an important food source in the eastern Atlantic, while it also plays an important role in fisheries in the Mediterranean [7,8,9]. It is an omnivore feeder that lives on a variety of bottoms: sand, mud, rock, algae and seagrass beds at depths of up to 350 m [10,4]. In the Mediterranean, bogue is usually caught with bottom trawls, purse seines, longlines and trammels nets (4,7). It is also found around, and in the cages, where it can coexist with farmed fish, and its dominance around off-shore fish cages in the Canary archipelago with regard to other wild fish species, was reported [11]. At the same time, floating cages represent a kind of marine protective areas (MPAs) that provides shelter and food for many marine organisms. They find food in the fouling of the installations and, as already mentioned, in decaying food from the cages (POM) [12,13].
Although it is an abundant species in Croatian fisheries and is also one of the dominant wild fish species around and in the fish floating cages in the Adriatic Sea, data on this species are still scarce and mostly consist of length-weigh relationship [14] and reproductive characteristics [15,16]. Hence, the aim of this study was to examine relationship between condition index, gonadosomatic index and viscerosomatic index of bogue from the middle part of the Eastern Adriatic Sea. This central area of the Adriatic Sea is well known for fish farms, and consequently it is also an area with different trophic/food source locations. Therefore, our goal was to compare the somatic indexes of specimens caught in and around fish cages with bogues belonging to the wild populations, to determine the relationship between body condition and gonadosomatic index.
2. MATERIAL AND METHODES / Materijali i metode
All bogue samples (N=133) were collected in May 2023 from three locations in the middle part of the Eastern Adriatic Sea. Samples were collected in the immediate vicinity of the cages (A), the floating cages (C) and at a location far (>3000m) from the cages (W) (Figure 1). Samples from location W and A were caught with purse seines, while those from floating cages (location C) were sampled during harvesting from commercial fish cages.
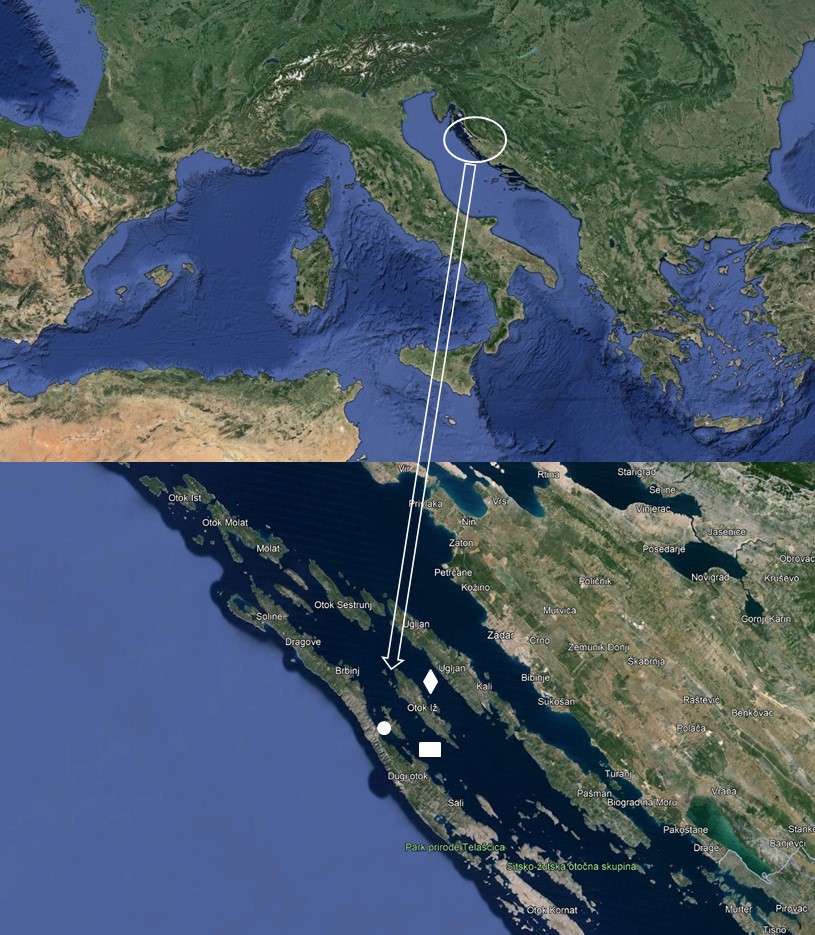
Figure 1 Study area - immediate vicinity of the cages (location A; □), floating cage (location C; ◊), and location far from the cages (location W; ○), middle part of the Eastern Adriatic Sea
Slika 1. Područje istraživanja – neposredna blizina kaveza (lokacija A; □), uzgojni kavez (lokacija C; ◊) i lokacija daleko od kaveza (lokacija W; ○), srednji dio istočnog Jadranskog mora
The total length (TL) of specimens was measured to the nearest 0.1 cm and the total weight (TW) to the nearest 0.1 g. The sex was then determined and the gonadal and visceral weights were measured. Sex ratio was M/F=0.94 (location A), M/F=0.39 (location C) and M/F=1.31 (Location W). From the data obtained, the gonadosomatic index, the viscerosomatic index, the condition index, the gonadal condition index and the viscera condition index without gonads were calculated for each sex.
The Fulton condition index (CI) and gonadosomatic index (GSI) were calculated as follows:
CI= 100 x (TW/TL3); GSI =100 x (GW/TW) (1)
where CI represents the condition factor, TW for the total weight of the fish in grammes and TL for the total length of the fish in centimetres. The gonadosomatic index (% GSI) was calculated as the ratio of the weight of the reproductive organs (gonad weight GW g) to the total body weight of the fish (TW g).
The viscerosomatic index (% VSI) was calculated as the ratio of the weight of the internal organs, i.e., the visceral weight (Wv g), to the total body weight of the fish (TW g):
VSI =100 x (Wv/TW) (2)
The influence of body condition on the gonadosomatic index was determined by decomposition of the condition index. The body weight can be divided into the weight of gonad, the weight of the internal organs, the weight of the fish without viscera and gonads as follows.
(3)
(4)
(5)
where Wg is the weight of the gonads; Wv is the weight of the internal organs, i.e., the viscera (without gonads); Wt is the weight of the fish without viscera and gonads, and CIg is the index of the gonad condition; CIv is the index of viscera condition, CIo is the index of the body condition without viscera and gonads.
The examination of the differences of the analysed parameters between the experimental groups was performed using the statistical programme TIBCO Statistica (v14.0.0,15). ANOVA and non-parametric Kruskal-Wallis tests were used to process the data obtained. The relationship between gonadosmatic index and the Fulton condition index was estimated using linear regression analysis. Prior to the least square regression was calculated, the significance (p<0.05) of the Pearson correlation was tested. The proportion of the accounted variations was estimated by the coefficient of determination (R2), and interpretation of the strength of correlations between the mentioned variables was determined according to Petz et al. [17].
3. RESULTS AND DISCUSSION / Rezultati i rasprava
3.1. Bogues somatic indices / Somatski indeksi bugve
Out of the total number of samples (N=133), 33 bogues were caught at location A (in the immediate vicinity of cages), 56 from floating cages (location C), and 44 individuals belonged to the wild bogue population and were caught far away from the cages (location W). The biometric data of the male and female bogues are listed in Table 1. A minimum total length of 19 cm was recorded for the specimens at location C and a maximum total length of 36.4 cm at location A (Figure 2). The minimum body weight was 63.45 g, while the maximum weight was 566.57 g and both values were recorded in bogues from location C. A total length of 641 specimens ranged from 10.8 to 24.9 cm and total weight from 12.99 to 141.11 g in bogue from the Gulf of Antalya (Turkey) [9]. However, these smaller bogues from Turkey were from wild population, while in this study maximum weight was found in specimen sampled from floating cage. Furthermore, when comparing fish weight by the three different locations in our research, bogues collected from location W had significantly lower weight than those collected from locations C and A (Kruskal-Wallis test; p<0.05). However, the total length was significantly higher for the samples from location A, than for those from locations C and W (Kruskal-Wallis test; p<0.05). In addition, similar condition index (CI) values were found between specimens from locations A and W (Table 2). The fish from location C had a significantly greater condition index (CI) and viscerosomatic index (VSI) than the specimens from locations A and W, although gonadosomatic index (GSI) values were similar in bogues from locations C and W.
Table 1 Biometry of male and female bogues from the Eastern Adriatic Sea (total length LT, total weight TW; average value ± SD) (Location: immediate vicinity of the cages (A), floating cage (C), and far from the cages (W).
Tablica 1. Biometrija mužjaka i ženki iz istočnog Jadranskog mora (ukupna duljina LT, ukupna težina TW; prosječna vrijednost ± SD) (Lokacija: neposredna blizina kaveza (A), plutajući kavez (C) i daleko od kaveza (W).
| Location/sex | A ♂ | A ♀ | C ♂ | C ♀ | W ♂ | W ♀ |
|---|---|---|---|---|---|---|
| TL (cm) | 31.66±1.50ab | 32.28±2.47a | 28.76±4.08bc | 29.10±4.25bc | 28.74±1.61c | 29.65±2.01abc |
| TW (g) | 323.3±50.3a | 348.4±77.3a | 299.0±116.2ab | 323.1±148.9a | 244.2±46.0b | 269.2±48.4ab |
*Different letter in superscript represents statistical differences between measured variables
Table 2 Fulton’s condition index (CI), gonadosomatic index (GSI) and viscerosomatic index (VSI) (mean ± SD) of male and female bogues from the Eastern Adriatic Sea (Location: immediate vicinity of the cages (A), floating cage (C), and far from the cages (W).
Tablica 2. Fultonov indeks kondicije (CI), gonadosomatski indeks (GSI) i viscerosomatski indeks (VSI) (srednja vrijednost ± SD) mužjaka i ženki bugve iz istočnog Jadranskog mora (Lokacija: neposredna blizina kaveza (A), plutajući kavez ( C), i daleko od kaveza (W).
*Different letter in superscript represents statistical differences between measured variables
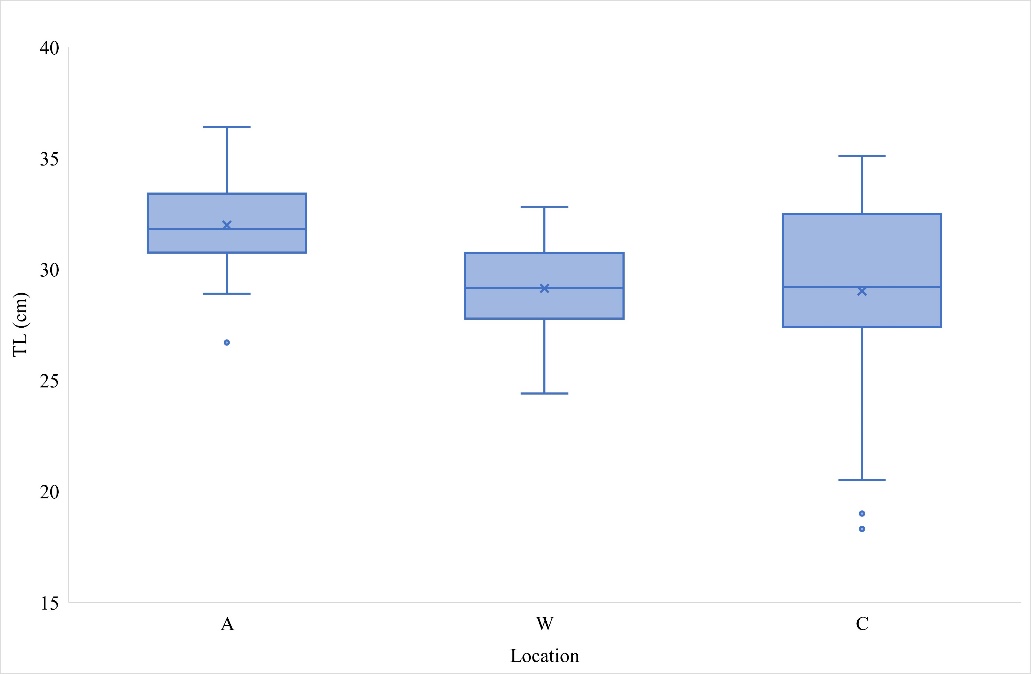
Figure 2 Box-whisker plots of total length (TL) frequency distribution of bogues (Boops boops), from the Eastern Adriatic Sea (Location A- immediate vicinity of the cages; Location W- far from the cages; Location C- floating cage). The boundary of the box closest to zero (lower part) indicates the 25th percentile, a line within the box marks the median, x represents average value, and the boundary of the box farthest from zero (upper part) indicates the 75th percentile. Whiskers (error bars) above and below the box indicate upper and lower extremes,
while single data points mark the outliers.
Slika 2. Dijagrami pravokutnika raspodjele učestalosti ukupne duljine (TL) bugve (Boops boops), iz istočnog Jadranskog mora (Lokacija A – neposredna blizina kaveza; Lokacija W – daleko od kaveza; Lokacija C – pluta jući kavez). Granica okvira najbliža nuli (donji dio) označava 25. percentil, linija unutar okvira označava medijan, x predstavlja prosječnu vrijednost, a granica okvira najudaljenija od nule (gornji dio) označava 75. percentil. Brkovi (trake pogrešaka) iznad i ispod okvira označavaju gornje i donje ekstreme, dok pojedinačne podatkovne točke označavaju netipične vrijednosti.
The linear regression between CI and GSI in bogues from all three locations is shown in Figure 3. Corelation between GSI and CI (location A) was positive (r=0.197) and not significant (p>0.05), for location C it was positive (r=0.479) and significant (p<0.05), and for location W it was negative (r= - 0.059) and not significant (p>0.05). Furthermore, the relationship between CI and GSI in bogues from location A (R2=0.039) and W (R2=0.004) was weak, while moderate relationship was found in location C (R2=0.229).
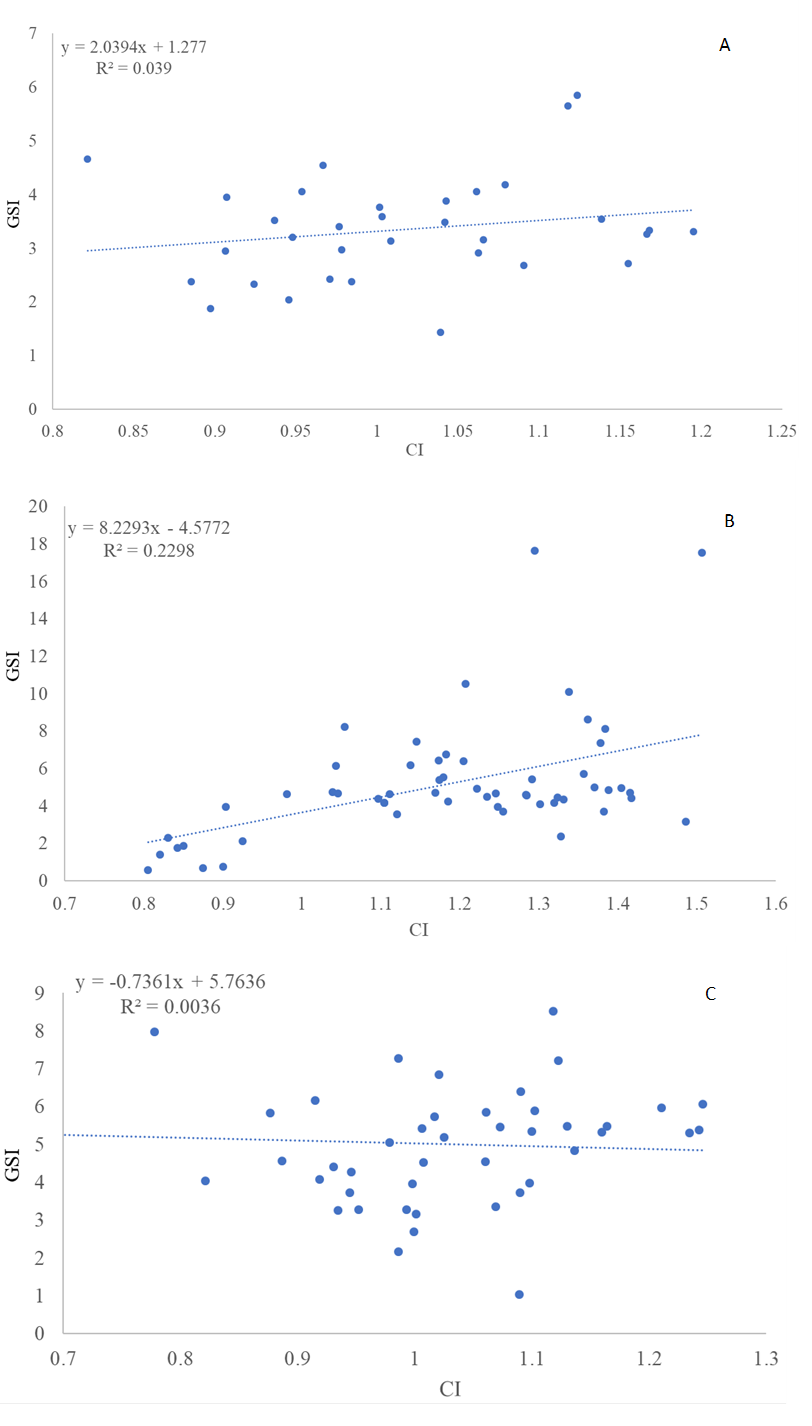
Figure 3 Condition index (CI) and gonadosomatic index (GSI) relationship in bogue (Boops boops), from the (A) immediate vicinity of the cages - location A, from the (B) floating cage - location C, and specimens caught far from the cages, i.e., from the (C) wild populations - location W, Eastern Adriatic Sea
Slika 3. Odnos indeksa kondicije (CI) i gonadosomatskog indeksa (GSI) bugve (Boops boops) iz (A) neposredne blizine kaveza – lokacija A, iz (B) plutajućeg kaveza – lokacija C i jedinke ulovljene daleko iz kaveza, tj. iz (C) populacija divljih riba – lokacija W, istočni Jadran
In all three locations GSI values were high and ranged from 3.33 % to 5.27% in May, indicating the spawning period of bogue in the middle part of the Eastern Adriatic Sea, which is consistent with a previous study on the maturation of this species, where the peak of the spawning season was in May [16]. However, the results for the same species from the southern part of the Adriatic showed maximum values of GSI in February (females 5.97%; males 4.21%) [15]. In the other parts of the Mediterranean, spawning period of bogue differ, although the reported peaks are mostly during spring months, GSI values are higher from January to July, which seems to be related to the temperature, but also to other ecological parameters [8,15,18,19,20,21,22]. In this study, the CI of bogue also showed high values in May, which varied depending on the location, and averaged from 1.01 to 1.19. The highest values of the condition index of bogue from the southern Adriatic Sea were observed in September (1.025 for females and 1.029 for males) while the lowest values were recorded in April (0.87 for females and 0.85 for males) [15]. For bogue from the Southern Tyrrhenian Sea, the CI fluctuated between 0.84 to 1.04 over the course of the year [18]. The correlation between CI and GSI in bogue samples collected from three different locations in this study was mostly weak. Reproduction also correlated poorly or not at all with the condition of bogues from the Italian and Portuguese coast [18,23]. However, when comparing bogues from wild populations with those from floating cages, a significantly higher CI was found for specimens from the cages. Fish farms also had a direct effect on the condition index, including muscle and total fat content, occurring in bogue inside or around the sea cages in Spain, and this effect disappeared completely at 3 km from the cages [6].
3.2. Decomposition of condition index (CI) / Dekompozicija indeksa kondicije (CI)
Since the gonadosomatic index (GSI) was similar between the samples from location C (floating cage) and location W (bogue from the wild population), a decomposition of the condition index (CI) was performed. Considering that GSI (%) presents a ratio of gonad weight and body weight, it can be affected by changes gonad weight or body weight. This problem has been previously observed and discussed in different ways, depending on fish species, maturity stage, etc. [24,25,26,27]. In the research estimating the maturity ogive in Chilean hake (Merluccius gayi gayi), GSI was computed as the ratio between the gonad weight and the gutted weight [24]. Another study emphasised that the correlation coefficients between ovary weight and standard length or cube of standard length are generally higher than the correlation coefficients between ovarian weight and body weight in fluffy sculpin (Oligocottus snyderi) and inland silverside (Menidia beryllina) [25]. In a previous work, the study of teleost fish species from the Pacific coast of North America also showed that gonad weight is in some cases better correlated with other expressions of body size than body weight, and the use of the relative gonad index (RGI) was proposed [26].
Thus, if the weight of each body part is separated, the condition index (CI) can be decomposed to better understand the change in the weight of each body part that leads to a change in the condition index of these individual body parts. To facilitate the interpretation of the results, the decomposition of CI was performed according to the principle of specific growth rate (SGR) factorization [27].
The results showed that the condition index of the gonads (CIg) and the condition index of the body without internal organs (CIo) were similar between bogues from the two mentioned locations, i.e., bogues from the floating cage and those from the wild population (Table 3). The condition index of viscera (CIv) was significantly different in bogues (both sexes) caught in the floating cages (location C), compared to those from the immediate vicinity of the cages (A), and those caught far from the cages (W). Possible reason for our findings could be different food availability around fish fam and far from the cages.
Table 3 Somatic indices (average value ± SD) of male and female bogues Boops boops from the Eastern Adriatic Sea (CIg- condition index of gonads; CIv- condition index of viscera, CIo condition index of body without internal organs), locations: immediate vicinity of the cages (A), floating cage (C), and far from the cages (W).
Tablica 3. Somatski indeksi (prosječna vrijednost ± SD) mužjaka i ženke bugve Boops boops iz istočnog Jadrana (Cig – Indeks kondicije spolnih žlijezda; CIv – Indeks kondicije unutarnjih organa, CIo – indeks kondicije tijela bez unutarnjih organa), lokacije: neposredno u blizini kaveza (A), plutajući kavez (C) i daleko od kaveza (W).
*Different letter in superscript represents statistical differences between measured variables
Corelation between CIg and CI of all bogues was positive (r=0.479) and significant (p<0.05). The linear regression between CIg and CI of bogues from all three locations (A, C, W) is shown in Figure 4. The coefficient of determination is 0.3635, indicating a moderate connection between those two parameters.
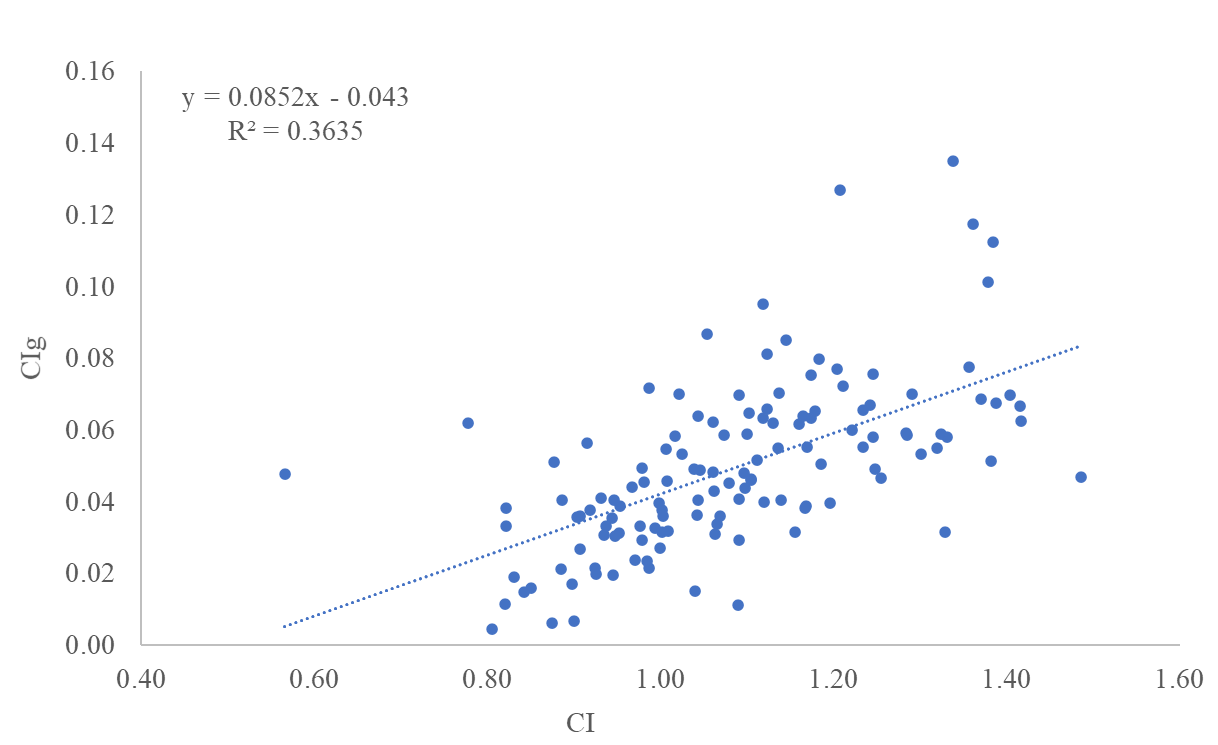
Figure 4 Condition index (CI) and condition index of gonads (CIg) relationship in bogue (Boops boops), Eastern Adriatic Sea
Slika 4. Odnos indeksa kondicije (CI) i indeksa kondicije gonada (CIg) bugve (Boops boops), istočno Jadransko more
4. CONCLUSION / Zaključak
To conclude, in most published data as mentioned above, the observed biological parameters differ significantly between wild fish populations and fish aggregating around floating cages or living in them. In this study, of all biometric parameters as well as all somatic indices, only the total weight of fish was significantly different (lower) in bogues from wild populations (location W) compared to samples from floating cage (location C) and those from the immediate vicinity of cages (location A). For most of our results (Table 1, 2 and 3), the values were, as expected, higher for bogues from the floating cages (location C) than for samples caught far from the cages (location W). Unexpectedly, however, bogues collected in floating cages differed significantly from specimens caught in the immediate vicinity of the cages in most of the studied parameters (TL, CI, GSI, VSI, CIg, CIv, CIo). Therefore, it is crucial to increase knowledge about the interaction between aquaculture and the entire marine ecosystem in order to better understand its temporal and spatial impact.
Author Contributions: Conceptualization, B.M., S.Č. and B.P.; Methodology, L.B.; Software, L.B. and T.G.; Validation, B.M., S.Č., L.B. and B.P.; Formal Analysis, T.G. and S.Č.; Investigation, B.B., M.M., Š.U. and T.G.; Resources, Š.F. and Š.U.; Writing – Original Draft Preparation, B.M., S.Č. and L.B.; Writing – Review & Editing, B.P., B.B., M.M., Š.U. and T.G.
Funding: The research presented in the manuscript did not receive any external funding.
Conflict of interest: None.
Acknowledgments: The authors would like to express their gratitude to Cromaris d.d. for helpful cooperation during this research.
