Introduction
Probiotic microorganisms are defined as living microorganisms which are consumed together or separately with foods that regulate mucosal and systemic immunity and provide nutritional and microbial balance in the intestines, and that positively affect the health of the host (Sanders et al., 2018; Zendeboodi et al., 2020). Prebiotics are defined as food components that affect the host positively by selectively stimulating the development and/or activity of probiotic microorganisms and improve their health (Davani-Davari et al., 2019). The combination of probiotics and prebiotics is called synbiotic, and studies on this subject report that this combination has many positive effects on health. (Villalva et al., 2017). Microencapsulation technique is defined as a physical method of encasing liquid or gas droplets of small solid particles with the help of thin-film layers or polymer capsules. The vitality of microorganisms is preserved by microencapsulation method, so they are not affected by the substances in the composition of foods and the reactions that occur during ripening. (Abd El Kader and Abu Hashish, 2020). Kavas et al. (2022) investigated the changes in the numbers of Lactobacillus paracasei and Bifidobacterium longum in white goat cheese produced by adding free probiotic, prebiotic and synbiotic microcapsules. They concluded that the viability of L. paracasei and B. longum was preserved by the microencapsulation technique, while the viability of probiotic bacteria was preserved at a higher level in cheese samples obtained by adding prebiotics to the microencapsules. The importance given to goat milk, which is kept technologically separate from cow and sheep milk and which is economically more valuable with its different flavour, aroma, and quality, and its products are increasing today. Goat's milk, which is mostly used in cheese production among dairy products, is a very common raw material in cheese production in many countries, especially in France (Lucatto et al., 2020). During the ripening of cheeses, highly complex biochemical events take place. Free fatty acids, which play a role in the taste and aroma of cheese, are revealed by lipolysis, which is one of the biochemical events that occur during ripening. Proteolysis influences the development of cheese structure during cheese ripening. It has an effect on the improvement of the taste of cheese through amino acids and peptides that directly affect the taste of cheese. Free amino acids are revealed by proteolysis of the proteins (McSweeney, 2017).
There are studies on the microencapsulation of different probiotic microorganisms in Feta, Cheddar, and fresh cheeses and their effects on the properties of cheeses (Heidebach et al., 2012; Ahmed et.al., 2021). However, there has not been a detailed and sufficient study on cheese produced from goat's milk. The aim of this study was to determine the effects of microcapsules containing probiotics, probiotics and prebiotics (synbiotic) on amino acid and fatty acid composition of white goat cheese during the storage period. In addition, the objective was also to determine the effects of probiotics and probiotic + prebiotics added in free form during the production of white goat cheese on amino acid and fatty acid values and to compare the effects of microencapsulation on these values.
Materials and methods
Materials
Goat milk used in the research was provided by Şemsi İgi Food Products Limited Company in Pınarbaşı/İZMİR and the production was carried out in the same facility. In the production of white cheese from goat milk, a lyophilized culture from Mayasan-Sacco with the content of Lactococcus lactis subsp. lactis + Lactococcus lactis ssp. cremoris mixture was used. It consists of mixtures of Lc. cremoris strains. Lafti B22 Bifidobacterium longum and Lafti L26 Lactobacillus casei (Lacticaseibacillus casei) (Zheng et al., 2020) were used as probiotic microorganisms (ratio in the mixture: 50 %).
Microencapsulation of probiotic microorganisms
A probiotic cell suspension was prepared by centrifuging 80 mL of 24 h old culture at 5,000 ×g. The cells were washed twice with saline solution (20 mL). Alginate was used as supporting material. Sodium alginate concentration was chosen at 1.0 % after preliminary experiments. Microcapsules were prepared using 3 different extrusion methods. To obtain the first type of microcapsule, 1 % of the probiotic culture mixture ( Lacticaseibacillus casei + Bifidobacterium longum) concentrate was mixed with 1 % sodium alginate. For the second type of microcapsule, 2 % fructooligosaccharide was added to the Na-alginate + culture mixture. For the third type of microcapsule, 2 % inulin was added to the Na-alginate + culture mixture. Additionally, autoclaved 1 % digestible pancreatic casein was added to stimulate the growth of probiotics. Microcapsules were obtained by slowly dropping the mixture into a sterile 0.1 M CaCl 2 solution and subsequently holding for 1 hour for gelation. After the capsules have been formed, those were stored in sterile 0.1 % peptone solution at +4 °C (Chen et al., 2005; Chen et al., 2006).
White goat cheese production
For cheese production, goat's milk was pasteurized at 75 °C for 15 seconds and then separated into 7 equal portions. CaCl 2 (0.01 %) (w/v) and 0.5 % (w/v) cheese culture were added. Later, for cheeses that contain free probiotic cultures, cheese with the code SHPK was obtained by adding Lacticaseibacillus casei and Bifidobacterium longum at 35-37 °C were added into 1 % cheese milk; cheese with the code SHPK+F was obtained by adding fructooligosaccharide into the milk in addition to probiotic cultures; cheese with the code SHPK+I was obtained by adding inulin into the milk in addition to probiotic cultures. Another cheese sample, MKP coded cheese, was added microcapsules containing 1 % probiotic culture microencapsulated only with alginate. By adding microcapsules containing fructooligosaccharide (FOS), MKP+F coded cheese, by adding microcapsules containing inulin, MKP+I coded cheese was obtained, respectively. K coded cheese was produced in the traditional way without any probiotic culture additives (Table 1). Then, rennet was added and left to coagulate, the curd was broken into pieces of approximately 1 cm 3 in volume and put under pressure. Cheeses were cut by removing from the press and kept in 15 % (w/v) pasteurized brine. The cheese samples were left to be pre-matured in crates until their pH was about 5.0-5.1 and packed under vacuum. The pots were left to mature at 4±1 °C for 180 days and analyses were made on the 1 st, 45 th, 90 th, and 180 th days of the maturation period.
Table1. Experimental design of goat cheese production
Acid value
The acid value, which shows the amount of free fatty acid, was determined according to Renner (1986) and the result was expressed as the equivalent of spent KOH. Each cheese sample was weighed to obtain 8-10 g of fat, and kiselguhr was added to it. Oil extraction was achieved in the samples washed three times with the help of pure diethyl ether, and the ether-oil mixture was obtained by filtering the extract through filter paper. Obtaining the pure oil from the ether-oil mixture was obtained by removing the ether at 45 °C in the rotary evaporator. 40 mL of alcohol-ether mixture (1: 1) was added to 4 g oil sample, and titrated with 0.1 N KOH prepared in alcohol and 1 % phenolphthalein. The titration value obtained was calculated by substituting it in the formula below.
Acid value (mg KOH/g fat) = V (mL) x N x 56.11 / M
V: Amount of KOH spent in titration (mL)
N: Actual normality of KOH used in titration
M: Sample (oil) amount (g)
Fatty acid analysis
Oil was extracted from cheese samples according to Renner (1993). According to this method, approximately 50 g of cheese samples were grated and thoroughly crushed with 6-8 g of kieselguhr. 200 mL of diethyl ether was added and mixed well. The mixture was filtered through filter paper. Then, the diethyl ether phase from the diethylether-oil mixture was evaporated under vacuum in a rotary evaporator (Heidolphy, Bibbysterilin Ltd. Staffordshine, England) at 45 °C and removed from the oil phase. The oil sample was kept under nitrogen gas for a while to ensure that no ether residue was left in the obtained oil. The milk fat obtained was converted into methyl esters according to AOCS (1997) and injected into gas chromatography. Model: Hewlett-Packard (6890, Avondale, PA, USA; Column: Supelco SP-2380 silica capillary (100 m x 0.25 mm i.d., 0.2 µm film thickness, Supelco INC., Bellefonte, USA); Detector: Flame ionization detector; Injection: 2 μL; Temperature Program: 100 °C to 220 °C 4 °C/minute; Injection and Detector Temperature: 300 °C; Carrier Gas: Nitrogen 1 mL/min; Split: 100:1.
Amino acid analysis
Portions of 20 g of cheese slurries were mixed with 40 mL of deionized water and WSN was extracted (Lacroix et al. 2010). After centrifugation (5,000 × g for 15 min, 4 °C), the supernatants were pipetted into cryovials and kept frozen until further analysis. Derivatization of these samples was made with the EZ-Faast kit (Phenomenex, Torrance, CA) following the manufacturer's recommendations. The derivatized samples were determined by the ThermoQuest Trace GC (FID) gas chromatography system at TÜBİTAK Marmara Research Center Food Institute (Gebze, Kocaeli). Thermoquest Trace GC/ İtaly; Kit: EZ:faast easy fast amino acid sample testing kit ; Column: Zebron Amino Acid GC Column, ZB-AAA 10m x 0.25mm; Injector: 250 °C; Oven: Increase from 110 °C to 320 °C in 26 °C increments Wait 1 minute; Detector: FID - 320 °C; Carrier (Helium): 1.5 mL/min; Sample Injection: 1 µL.
Results and discussion
Composition of fatty acids in triglyceride form
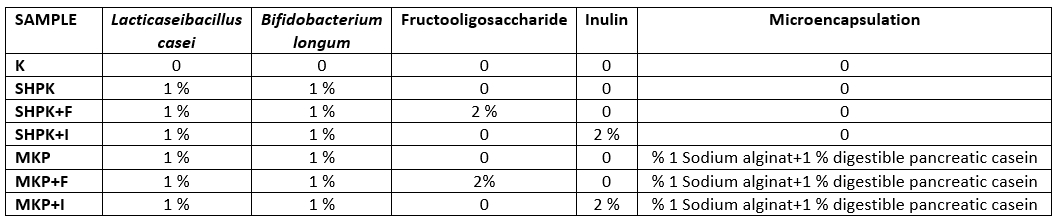
The changes in the amount of free fatty acids in goat cheeses, produced using probiotic culture in the free state, prebiotic, microencapsulated probiotic culture, and synbiotic microcapsules, to determine the lipolytic activity level, during the 180-day storage period are given in Table 2.
Table 2. Total free fatty acid value of cheese
± SD Mean; n=3
a;b;c;d;. the differences between the values in the same line are statistically significant (p<0.05)
A.B.C.D: The differences between the values in the same column are statistically significant (p<0.05)
SHPK: L. casei+B. longum were added into 1 % cheese milk; SHPK+F: L. casei +B. longum+FOS;
SHPK+I: L. casei+B. longum+inulin; MKP: microcapsules containing 1 % probiotic culture +alginate;
MKP+F: microcapsules containing FOS+alginat; MKP+I: microcapsules containing Inulin+alginate
Three basic biochemical events such as glycolysis, proteolysis, and lipolysis take place during the ripening of cheese. During ripening, especially microorganism-derived intracellular and extracellular enzymes degrade the nutrients in raw cheese in various ways (glycolysis, proteolysis, and lipolysis) and at different levels, and these products of different types and levels, form the unique flavour and texture of the cheese (Blaya et al., 2018). Short and medium-chain free fatty acids produced by lipolysis in cheese contribute directly to cheese flavour and one of the methods used to determine the degree of lipolysis is the total free fatty acids value.
As can be seen from the of Table 2, the value of total free fatty acids increases during maturation due to lipolysis because of the bacterial activity. At the end of 180 days, the highest free fatty acid ratio was determined in cheese sample (SHPK+F) with free probiotic bacteria and prebiotic added fructooligosaccharide (FOS) and in cheese containing microencapsulated probiotic bacteria and FOS (MKP+F) (p<0.05). It was concluded that the addition of FOS was effective in the formation of free fatty acids. Difference between fatty acids during storage is significant(p<0.05). In the cheeses used in our study, the highest total fatty acid value at the end of 180 days was determined in cheese produced with synbiotic microcapsules containing fructooligosaccharides (MKP+F). It was determined in the studies conducted by Mallatou et al., (2003) and Georgala et al., (2005) that total free fatty acidity values in different cheeses increased during ripening.
Saturated fatty acids
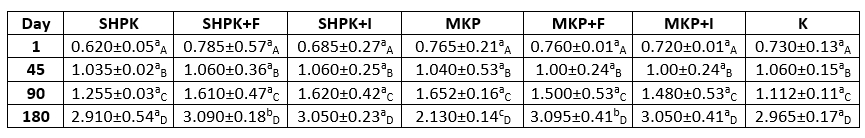
The changes in the saturated fatty acid composition of goat cheeses produced different ways in our study during storage and according to the sample type are given in Table 3. When the saturated fatty acids of trial cheeses were examined, C16 (Palmitic acid) had the highest value and C22 the lowest (Table 3) and this trend continued throughout the storage period. The saturated fatty acid ratios in all samples, with very small changes, were as C16> C18> C14> C10> C12> C4> C6> C8> C20> C22, respectively.
The saturated fatty acid ratios of the trial cheeses varied between 69.64 % and 77.21 % on the first day of storage; and at the end of the 180-day storage period, the saturated fatty acid ratios were found to change at very limited levels in the cheese samples with the codes of SHPK, SHPK+F, and MKP. This situation was not found as statistically significant (p>0.05). It was determined that microencapsulation application caused some changes in the form of increasing and decreasing effects on saturated fatty acids, but this situation was not found to be statistically significant (p>0.05). Although the addition of FOS slightly increased the amount of saturated fatty acids, in general, the addition of free or encapsulated prebiotic did not have an effect on saturated fatty acids. At the end of the 180th day, the highest amounts of saturated fatty acids were determined in the control sample.
Table 3. Saturated fatty acid composition of goat cheeses during storage
± SD Mean; n=3
SHPK: L. casei +B. longum were added into 1% cheese milk; SHPK+F: L. casei +B. longum+FOS;
SHPK+I: L. casei +B. longum+Inulin; MKP: microcapsules containing 1 % probiotic culture +alginate;
MKP+F: microcapsules containing FOS+alginat; MKP+I: microcapsules containing Inulin+alginate
Mederios et al. (2014) examined the composition of fatty acids in goat cheeses produced from goat's milk, of which different vegetable oils were added to the diets of the goats. They determined the highest ratio of unsaturated fatty acid as oleic acid (C18: 1) in goat cheeses and the highest saturated fatty acid as palmitic acid (16:0). It was determined that the diet of goats affects the fatty acid composition.
Goat milk contains a higher proportion of short and medium-chain fatty acids (C4:0-C14:0) compared to human and cow milk. Goat milk contains more caproic (C6:0), caprylic (C8:0), and capric (C10:0) acids compared to cow's milk (Djordjevic et al., 2019). Although similar studies like the one performed by Seçkin et al. (2005) reported that the short-chain fatty acids of white cheeses produced from cow's milk varied between 3-4 %, results obtained in our study were higher. These differences might be related to the use of goat's milk which has a much higher C10 ratio in goat's than cow milk. Therefore, it is considered normal for the short-chain fatty acid ratio to be low in our study.
Unsaturated fatty acids
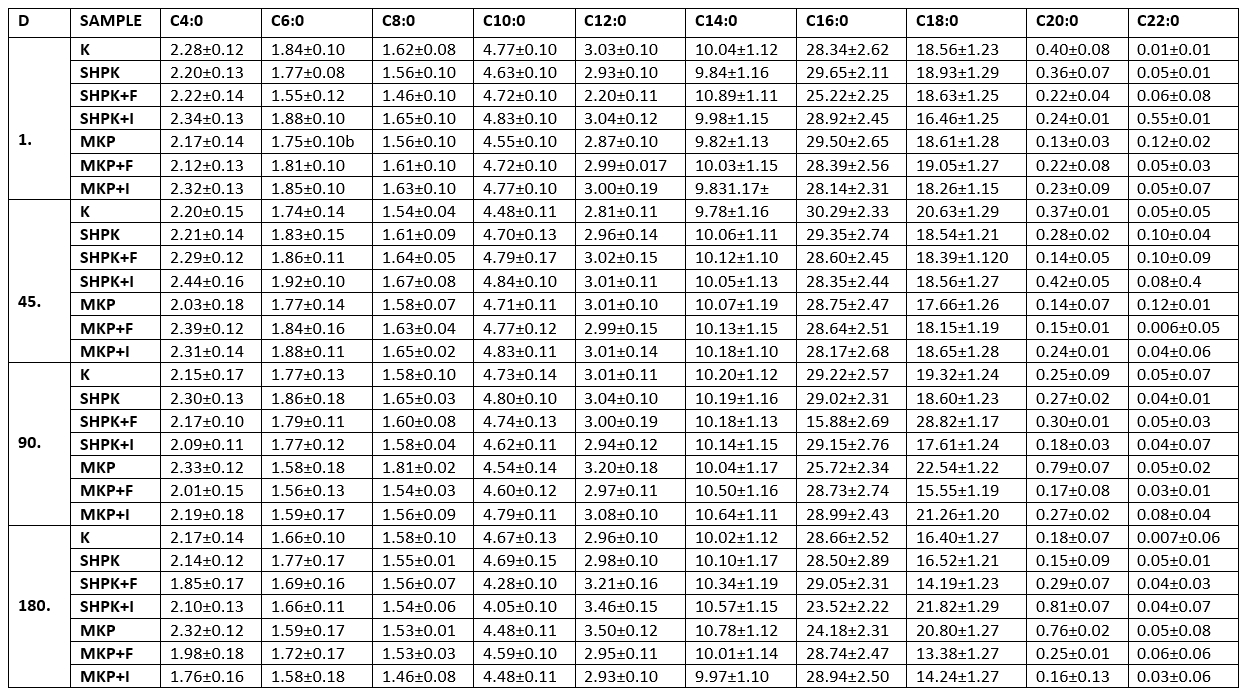
The total unsaturated fatty acid ratios of the samples varied between 23.49 % (control group) and 28.16 % (SHPK+I) on the first day of storage. Overall, the unsaturated fatty acid with the highest ratio was C 18:1, and the unsaturated fatty acid with the lowest ratio was C 18:3 (Table 4).
Table 4. Average unsaturated fatty acid composition of cheese samples
± SD Mean; n=3
SHPK: L .casei+B. longum were added into 1 % cheese milk; SHPK+F: L. casei+B. longum+FOS;
SHPK+I: L. casei+B. longum+inulin; MKP: microcapsules containing 1 % probiotic culture +alginate;
MKP+F: microcapsules containing FOS+alginat; MKP+I: microcapsules containing Inulin+alginate
The ratio of total unsaturated fatty acids ranged from the lowest 26.56 % to the highest 28.76 % in this study. According to the results, significant increases occurred in the polyunsaturated fatty acid ratios of the samples. When the trial cheeses were compared among themselves, the differences observed in the composition of polyunsaturated fatty acids were related to the supplement cultures used, the way of use, and the prebiotics (Inulin-FOS) made use of in the encapsulation. The addition of prebiotics positively affected the amount of unsaturated fatty acids during cheese ripening, which was found to be higher in prebiotic added cheese (p<0.05). It could also be observed that the addition of FOS from two different prebiotics used in the study was more effective than inulin. Microencapsulation had a positive effect on the amount of unsaturated fatty acids, which was statistically significant (p<0.05). In many studies on the same subject, the ripening of cheeses occurs as a result of the combination of physical, chemical, and biochemical events, as well as the formation of aroma substances in the ripening of cheeses. Lipolysis is one of the most important factors affecting the formation of aroma substances. In lipolysis, fatty acids are broken down and many compounds and free radicals that are contributing to aroma formation are formed and unsaturated fatty acids play a leading role in lipolysis. However, different strains of lactic acid bacteria isolated from milk and dairy products could have lipolytic activity as well as the starter cultures used, albeit to a limited extent, also could show lipolytic activity (Rathore and Sharma 2018, Guan et al., 2020), In addition, prebiotics that promote the development of starter cultures may indirectly influence lipolysis.
Overall, it could be observed that the fatty acid with the highest ratio is C 18:1 and the lowest unsaturated fatty acid is C 18:3 in the unsaturated fatty acid composition order of the trial samples. Popovic et al. (2017) investigated the organic goat cheeses and determined that the highest level of saturated fatty acids in organic cheeses was myristic, palmitic, and stearic acids; and oleic acid had the highest level among unsaturated fatty acids.
Table 5. Free amino acid contents in cheese samples without microcapsules (mg/100 g)
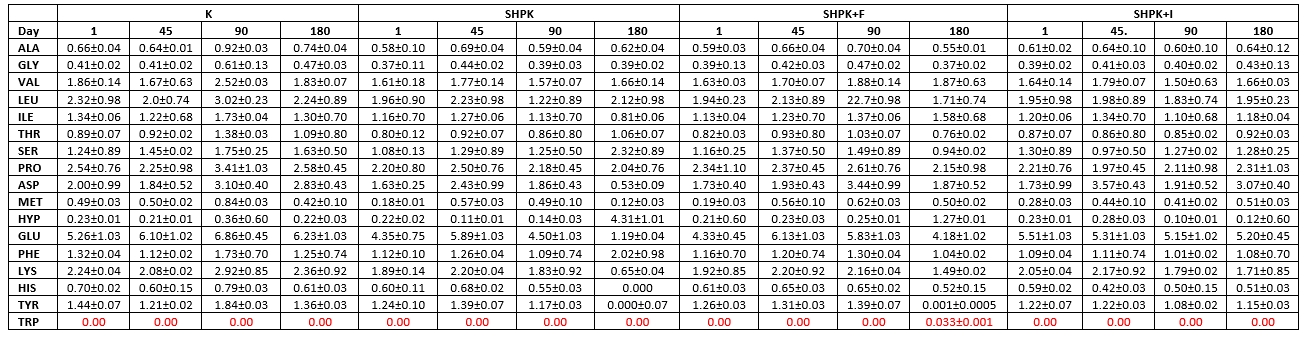
Ala: Alanine. His: Histidine .Tyr: Tyrozine. Arg: Arginine. Hyp: Hydroxyproline. Trp: Tryptophan. Asn: Asparagine. Leu: Leucine. Val: Valine. Asp: Aspartic acid. Lys: Lysine. Gly: Glycine. C-C: Cysteine. Met: Methionine. Cys-Cys: Cysteine. Thr: threonine. Phe: Phenylalanine. Glu: Glutamic Acid. Pro: Proline. Gln: Glutamine. Ser: Serine
Free amino acid values and changes during storage
Tables 5 and 6 present the total and essential amino acid compositions and changes obtained on the 1 st, 45 th, 90 th, and 180 th days of storage. Increases in essential amino acid levels of the samples were observed in the first 90 days of storage, while on the 180 th day of storage, an irregular increase in most amino acids and reductions have been recorded.
When the samples were evaluated in terms of the amount of total amino acids obtained in our study, it was found that the highest increase was in the SHPK+I cheese sample, and this determination showed that the most effective proteolysis that occurred during maturation was the sample of SHPK+I cheese. When the cheese groups were evaluated during ripening, the inoculation type of the supplement cultures used in cheese milk (microencapsulation), the prebiotic type used in the preparation of microcapsules, and the use of protein-based additives in the preparation of microencapsulation capsules were found to be effective on total amino acid levels (p<0.05).
Table 6. Amino acid amounts in microcapsule added cheese samples and their changes during storage (mg/100 g)
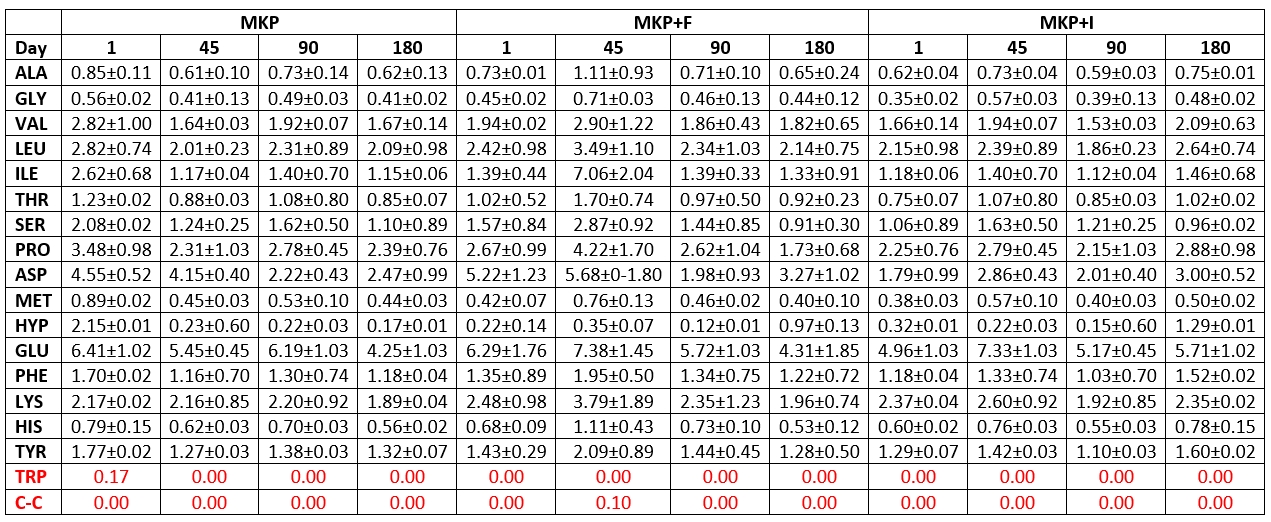
Ala: Alanine. His: Histidine .Tyr: Tyrozine. Arg: Arginine. Hyp: Hydroxyproline. Trp: Tryptophan. Asn: Asparagine. Leu: Leucine. Val: Valine. Asp: Aspartic acid. Lys: Lysine. Gly: Glycine. C-C: Cysteine. Met: Methionine. Cys-Cys: Cysteine. Thr: threonine. Phe: Phenylalanine. Glu: Glutamic Acid. Pro: Proline. Gln: Glutamine. Ser: Serine
On the 1 st day of storage, the hydroxy proline amounts in all cheese samples except MKP cheese sample had the lowest value, and on the 1 st day of storage, while the amounts of asparagine, glutamic acid, valine, leucine, proline, isoleucine were found to be at very high levels (Tables 5 and 6). At the end of ripening, glutamic acid was found in the highest amounts among all amino acids in the examined cheese samples, followed by leucine, proline, aspartic acid and lysine, respectively. This shows that phenylalanine has the highest value among the amino acids released at the end of the ripening of cheeses. Tryptophan was not detected in any of the trial cheeses during 180 days of storage, while the cysteine amino acid was detected only on the 90 th storage day in the SHPK cheese sample and on the 45 th storage day in the MKP+F cheese sample.
It is stated that the total amount of amino acids in goat cheese samples differs in ripening and storage periods. Popović et al. (2017) observed that the contents of threonine, tyrosine, valine, and isoleucine decreased while the amino acids of methionine, phenylalanine, and lysine tend to increase. Furthermore, L. casei was studied for proteolysis in milk and has shown proteolytic effect during cheese ripening (Ahtesh et al., 2016).
Conclusion
Changes in amino acids and fatty acids in goat cheeses were investigated via the addition of the free form and microencapsulated probiotics. When the cheese groups were evaluated during ripening, it is concluded that the inoculation type of the supplement cultures used in cheese milk (microencapsulation), the prebiotic type used in the preparation of microencapsules, and the use of protein-based additives in the preparation of microencapsulation capsules were found to be effective on total amino acid levels. In goat cheeses produced with the addition of probiotics and prebiotics in free form, the amount of amino acids increased during the storage period and the highest amino acid value was determined in the control sample. It was determined that the addition of inulin along with probiotics on the 180th day of storage was effective in amino acid formation compared to cheeses with free FOS added. The amino acid values of goat cheese samples containing probiotic microcapsule and synbiotic microcapsule were higher than the control group cheese sample. At the end of 180 days, the amino acid values were found to be higher in cheeses produced with microcapsules containing Inulin + probiotic. As a result, it was determined that microencapsulation had a positive effect on the amino acid values in cheese ripening. It was concluded that inulin, which is added in free form or in microcapsule production, is more effective in amino acid formation.
When the saturated fatty acids of goat cheeses produced in different ways in our study were examined, it was determined that C 16 had the highest value while C 22 had the lowest value during the storage period. However, it was determined that the unsaturated fatty acid with the highest ratio in cheese samples was C 18:1 and the unsaturated fatty acid with the lowest ratio was C 18:3. Caproic caprylic and capric lauric fatty acids acid levels, which give the characteristic aroma of cheeses, were found to be higher than cow's milk. The addition of FOS slightly increased the amount of saturated fatty acids, in general, the addition of free or encapsulated prebitoc did not have an effect on saturated fatty acids. At the end of the 180 th day, the highest amounts of saturated fatty acids were determined in the control sample. It was determined that the amount of unsaturated fatty acids was found to be higher in prebiotic added cheese (p<0.05). It was concluded that the addition of FOS from two different prebiotics used in the study was more effective than inulin. Microencapsulation application had a positive effect on the amount of unsaturated fatty acids, which was statistically significant (p<0.05).
This research article deals with improving the of a synbiotic microcaencaptulation techniques produced Turkish type white pickled cheese using fructooligosaccaride and inulin (as prebiotics) and probiotic culture. In this investigation, an optimized synbiotic cheese was produced and compared with fatty acid composition and free amino acid composition. The count of probiotic bacteria ( Lactobacillus casei and Bifidobacterium longum) in both probiotic and synbiotic microcapsulated cheeses throughout the storage period was more than standard (>10 7). Therefore, our findings may likely to be of great interest to the vision of scientists, dairy technologists, and cheese-making industries. Also further and more detailed studies should be done on microcapsulation method applications in the production of probiotic goat cheese and it should be included in the market.
Acknowledgements
This work was supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK), Grant No: TÜBİTAK TOVAG 108O039.
Sastav masnih kiselina i slobodnih aminokiselina sinbiotičkog kozjeg sira sa slobodnim i inkapsuliranim probioticima
Sažetak
Cilj ovog istraživanja bio je utvrditi promjene u slobodnim masnim kiselinama i aminokiselinama tijekom skladištenja kozjih sireva s dodatkom mikroinkapsuliranih sinbiotika te utvrditi učinak mikroinkapsulacije na te promjene tijekom skladištenja. Također, cilj je bio utvrditi utjecaj probiotika i sinbiotika (probiotika + prebiotika) dodanih u slobodnom obliku tijekom proizvodnje kozjeg sira tipa feta na sastav aminokiselina i masnih kiselina. U istraživanju su pripremljene tri vrste mikrokapsula uključujući probiotičke bakterije ( Lacticaseibacillus casei i Bifidobacterium longum), probiotik + fruktooligosaharid (FOS) i mikrokapsule koje sadrže probiotik + inulin, a koje su dodane tijekom proizvodnje sireva. Uzorci sireva čuvani su na +4 °C tijekom 180 dana tijekom kojih je određivan udjel aminokiselina i slobodnih masnih kiselina u sirevima. Od zasićenih masnih kiselina najzastupljenija je bila palmitinska kiselina (C16), dok je od nezasićenih masnih kiselina najzastupljenija bila oleinska kiselina (C18:1). Na kraju zrenja utvrđeno je da su svi uzorci sadržavali najviše glutaminske kiseline, a zatim su po udjelima slijedili leucin, prolin, asparaginska kiselina i lizin. Dodatak inulina zajedno s probioticima 180. dana čuvanja pozitivno je djelovao u formiranju aminokiselina u usporedni sa sirevima u kojima FOS nisu dodani. Osim toga, dodatak slobodnog ili mikrokapsuliranog FOS pozitivno je utjecao na povećanje udjela slobodnih masnih kiselina. Zaključno, dodatak inulina u slobodnom ili u obliku mikrokapsula, bio je učinkovitiji u povećanju udjela aminokiselina.
Ključne riječi: mikrokapsulacija; sinbiotik; kozji sir; masna kiselina; aminokiselina
