Introduction
The genus Alchemilla L. (Rosaceae) is represented by 82 species in Türkiye, and among them 36 species are considered to be endemic. The endemism rate of the genus is 33.8% (Pawlowski and Walters 1972, Ozhatay et al. 2011).
Alchemilla species are very rich in tannin, salicylic acid, essential oil, phytosterol and vitamin C. The genus is medically important because of its active substances. It is consumed both as a medicinal plant and as an herbal tea. Alchemilla species are used as a wound healing agent, sedatives, diuretics, and cough suppressants (Baytop 1999, Shrivastava 2011, Polat et al. 2015). Some Alchemilla species have antioxidant, anti-inflammatory, antiproliferative, weakening and anti-aging effects (Benaiges et al. 1998, Oktyabrsky et al. 2009, Said et al. 2011). Alchemilla caucasica Buser has significant antiulcer activity. This effect is due to flavonoids (Shrivastava et al. 2007, Falchero et al. 2010, Karaoglan et al. 2020). Alchemilla orduensis Pawl. is a narrow endemic in Ordu, Giresun and Trabzon provinces in the eastern Black Sea region of Türkiye. It is listed as an Endangered (EN) species in the Red Book of Plants of Türkiye (Ekim et al. 2000).
Alchemilla orduensis is known as "Ordukeltatı" in Anatolia (Ayaz 2012). The species has an erect and dense patent and erecto-patent stem and petioles. Leaves are green and reniform. The leaves have 5-9 subtriangular lobes and 5-14 teeth. Flowers are 3-5 mm wide. Sepals and epicalyx lobes sparsely hairy and sparsely ciliate. Sepals are ovate and the epicalyx is thinner than the sepals. Alchemilla orduensis is distributed at altitudes between 1400-1600 m a.s.l. It grows in an area including lake shores, wetlands, marshes, and rocky habitats (Kalheber 1994, Ozbucak et al. 2022). The species shows morphological similarities to closely related species such as A. erzincanensis Pawl. These morphological similarities cause confusion regarding the taxonomic rank.
Chemotaxonomic characteristics can be used to classify genera and species when morphological and anatomical data are limited. Many studies have shown that essential oils can be used for chemotaxonomic purposes (Hegnauer 1986, Setyawan 2002, Tundis et al. 2014). Several different compounds have been used as taxonomic markers in the Rosaceae family, such as cyanogenic glycosides, flavonoids, tannins, sorbitol, and essential oils (Wallaart 1980, Okuda et al. 1992, Morgan et al. 1994). Essential oils are important compounds found in such plant structures as roots, leaves, bark, flowers, fruits, and seeds. Essential oils generally contain compounds such as terpenes (monoterpenes and sesquiterpenes), aldehydes, alcohols, phenols, and terpenoids (Mohamed et al. 2010, Tongnuanchan and Benjakul 2014). Alchemilla species are known to be rich in phenolic compounds such as flavonoids and tannins (Shrivastava et al. 2007, Falchero et al. 2010, Kaya et al. 2012, Ilgun et al. 2014) but there are not many studies on the essential oils. The presence of phenolic compounds has been determined in A. orduensis species (Kaya et al. 2012)
Ozbucak et al. (2022) investigated the micromorphology and some ecological characteristics of the flowers and fruits of the A. orduensis species, but investigations of the vegetative organs are lacking. Therefore, the aim of this study was to examine the anatomical and micromorphological characteristics as well as the essential oil composition of the vegetative organs of this endangered endemic species.
Material and methods
The Alchemilla orduensis species was collected from Ordu Province (A6: Aybastı, Perşembe plateau, meander) in Türkiye in 2017 (40. 416583 N, 37. 233919 E, sensu WGS84) (Fig. 1).
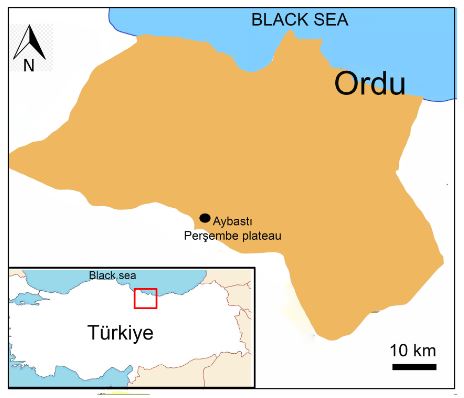
Fig. 1. Geographic distribution area of Alchemilla orduensis Pawł. in Ordu province in Türkiye. The plants were collected from Perşembe Plateau.
The plant samples were determined according to the Flora of Turkey (Pawlowski and Walters 1972). Plant materials are kept at the Faculty of Arts and Sciences of Ordu University, Türkiye. Cross and surface sections of the root, stem, leaves, rhizome, and petiole were excised by hand, and then covered with glycerin-gelatin (Vardar 1987) and stained with a safranin/fast green (1/9) mixture (Bozdağ et al. 2016). Photographs were taken using a Nikon FDX-35 microscope and all measurements and observations were made using imaging software (NIS-Elements, Version 3.00 SP5). For each parameter, measurements were made on twenty plant individuals. Stoma index and stoma ratio were calculated on the leaf surface of the plant (Meidner and Mansfield 1968). For scanning electron microscopy (SEM), the leaf samples were coated with 12.5-15 nm gold, and electron microscopy (Hitachi-SU 1510) shots were taken with a voltage of 10-15 kilovolts. Surface shapes were determined according to Stearn (1985).
The essential oil composition of both the aboveground parts (leaves, flowers, and stems) and underground parts (rhizomes and roots) of A. orduensis was determined during the flowering period, which is more suitable for essential oil production. The analysis was conducted using headspace solid phase microextraction (SPME, Supelco, Germany) followed by gas chromatography mass spectrometry (GC-MS). The above and below ground parts of 3-4 fresh plants were cut and homogenized. Half grams of the sample (approximately one third of the vial volume) was weighed into a 15 mL vial closed with a PTFE /Silicone septa cap. The sample was placed on a heating block at 60 °C under magnetic stirring. After equilibration for 15 min, a Carboxen/polydimethylsiloxane manual SPME fibre was inserted into the vial and maintained in the headspace for 30 min at 60 °C to extract volatile compounds from the sample. This analysis was performed using a Restek Rxi-5ms (30 m, 0.25 mm ID, 0.25 µm) column integrated into the Shimadzu QP 2010 Ultra GC-MS instrument. Helium was used as the carrier gas at a flow rate of 1.44 mL min-1; the column temperature program of GC was initially set at 40 °C for 2 min and gradually increased to 250 °C at 4 °C per min, then kept there for 5 min.
The essential components of the samples were determined by comparing data concerning their mass spectral libraries (NIST11-FFNSC) and LRI (Linear Retention Indices) generated using alkane standards. Relative quantitation of these compounds was also accomplished by evaluating the relative percentage for each peak (peak area/total ion chromatogram (TIC) area) (Tab. 2) (Mazı et al. 2019).
Results
Anatomical and micromorphological properties
The anatomical structures of the leaves and petiole of the above-ground part of the species were studied (Tab. 1, Fig. 2).
Tab. 1. Results of anatomical measurements (N = 20) of the aboveground (stem, petiole and leaf), and underground (root and rhizome) parts of the endangered endemic species Alchemilla orduensis. Mean ± standard deviation is shown.
The leaves of the species are of the palmate type. The leaves have 5-9 lobes, and each lobe has 5-14 teeth. The number of lobes increases with the size of the leaves. The leaf of the species is bifacial. The upper epidermis cells of the leaf are larger than the lower epidermis cells. The mesophyll layer consists of two-layered palisade parenchyma and three-layered spongy parenchyma. There are many middle vascular bundles and small vascular bundles between them. There are evident bundle sheath cells around the small vascular bundles. Collenchyma cells are located under the epidermis layer in the middle vascular bundles (Tab. 1., Fig. 2A-2H and Fig. 3A-3E).
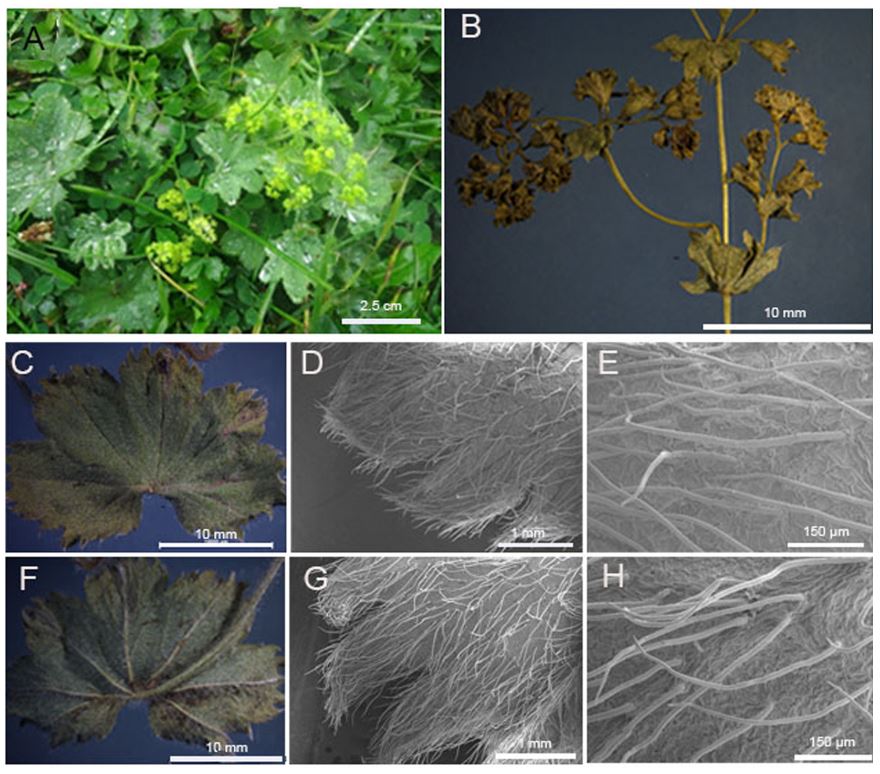
Fig. 2. General appearance of Alchemilla orduensis and light and scanning electron microscope (SEM) micrographs of the upper and lower leaf surfaces. A – general appearance of the species in its habitat. B – aboveground part of the plant. C – detailed view of upper surface of the leaf. D, E – SEM micrographs of the upper surface of the leaf and detail of the eglandular hairs. F – detailed view of the lower surface of the leaf. G, H – SEM micrographs of the lower surface of the leaf and detail of eglandular hairs. Abbreviation: vb – vascular bundle (photo: H. U. Uzunömeroglu).
The upper epidermal cells are rectangular with smooth and curved anticlinal cell walls, while the lower epidermal cells have undulated and curved anticlinal cell walls. The epidermal cell walls are prominent and raised, and stomata cells of the anomocytic type are present on both the upper and lower surfaces of the leaf. The sizes of stomata on the upper surface measure 22.969 ± 1.841 × 25.192 ± 2.860 μm, while those on the lower surface measure 23.597 ± 1.875 × 29.290 ± 2.680 μm. The lower surface of the leaves has more stomata cells. The stomatal index for the upper surface of the leaves is 0.04, while that for the lower surface is 0.17. The leaves have both eglandular and glandular hairs, with the eglandular hairs being usually very long. The density of hairs is greater on the margins of the leaf. Glandular hairs typically consist of a base cell and a secretory cell. The stomata and epidermal cells are nearly level with each other on the upper surface, while on the lower surface, the stomata are situated deeper than the epidermal cells (Tab. 1, Fig. 3F-3I).
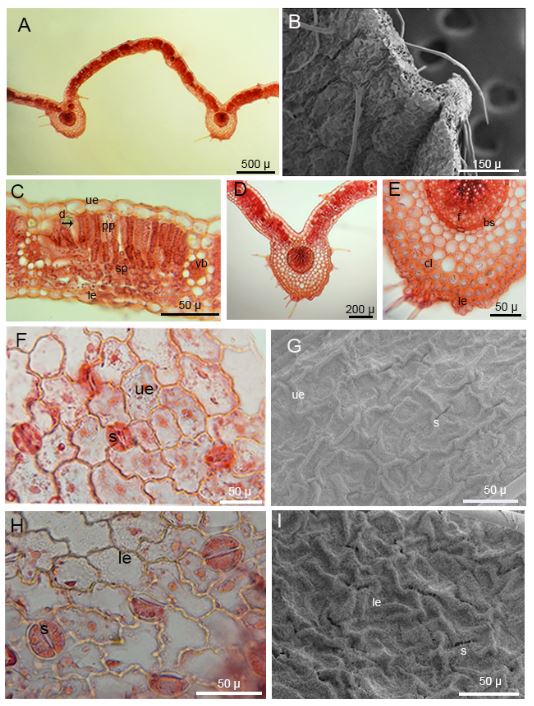
Fig. 3. Light microscope and SEM micrographs of the leaf of Alchemilla orduensis. A – cross section of leaf. B – SEM micrographs of cross section of leaf and view of eglandular hairs. C – detailed view of cross section of bifacial leaf lamina and vascular bundles, black arrow indicates druse crystal. D, E – middle vascular bundle region of leaf and detailed view of collenchyma cells and vascular bundle. F – surface section of upper surface of leaf and view of anomocytic type stomata and epidermis cells. G – SEM micrograph of stomata and epidermis cells on the upper leaf surface H – surface section of lower surface of leaf and view of anomocytic type stomata and epidermis cells. I – SEM micrograph of stomata and epidermis cells on the lower leaf surface. Abbreviations: d – druse crystal, le – lower epidermis, s – stomata, sp – spongy parenchyma, pp – palisade parenchyma, ue – upper epidermis, vb – vascular bundle.
Petiole is triangular. There are single-layered epidermis cells in the outermost part of the petiole. Numerous glandular and eglandular hairs were found on the petiole. There are 1-2 layers of collenchyma cells under the epidermis layer. Multilayered parenchyma cells follow the collenchyma layer. There are three vascular bundles in the petioles. One of these vascular bundles is large and two are smaller. Vascular bundles are of the concentric type (Tab. 1, Fig. 4A-4E).
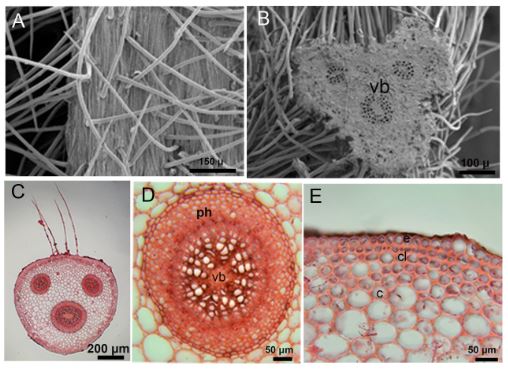
Fig. 4. Light microscope and SEM micrographs of the petiole of Alchemilla orduensis. A – SEM micrograph of hairs on the surface of the petiole. B – SEM micrograph of three vascular bundles in petiole cross-section. C – images of three vascular bundles, one large and two small, in petiole cross-section. D – detailed view of the large concentric vascular bundle. E – appearance of single-row epidermis, 1-2 row collenchyma and multi-row parenchyma cells Abbreviations: c – cortex, cl – collenchyma, e – epidermis, ph – phloem, vb – vascular bundle.
The underground parts of the species have roots and rhizomes. The outermost part of the root of species has an epiderma layer. In some areas, a periderma formation is observed. (Fig. 5A-5D). There are oval or round shaped parenchymatic cells in the cortex layer. The endoderma layer consists of multicellular and rectangular meristematic cells. There is a one layered pericycle layer under the endoderma. The cambium layer is clearly visible. Phloem is 5-6 layered. Secondary xylem covers a large area in the root. The pith rays contain 1-2 rows of ray parenchyma cells. The center of the root is filled with tetrarch shaped primary xylem elements.
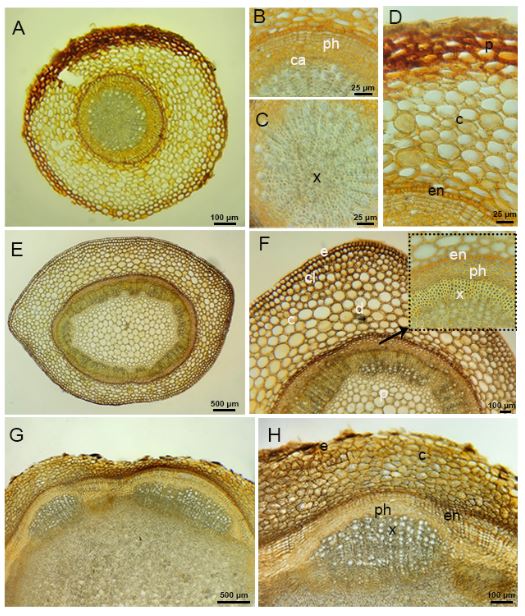
Fig. 5. Micrographs of cross section of root, stem and rhizome of Alchemilla orduensis. A – micrograph of cross section of root. B – detailed view of phloem and cambium in the root. C – xylem cells filling most of the central cylinder of the root. D – view of multilayered endodermis in the root. E – micrograph of cross section of stem. F – detailed view of the epidermis, cortex and central cylinder in the stem, arrow points to the endodermis, phloem and xylem. G, H – micrographs of cross section of rhizome. H – detailed view of epidermis, parenchyma cells and open colleteral vascular bundle. Abbreviations: c – cortex, ca – cambiyum, cl – collenchyma, e – epidermis, en – endodermis, d – druse, p – pith, ph – phloem, x – xylem.
There is a single layer of epidermis in the outermost part of the aboveground stem of the species (Fig. 5E-5F). There are 1-2 layered collenchyma cells under the epidermis. There are 9-10 rows of parenchyma cells in the cortex. The starch sheath is arranged in a single layer and is distinguishable. Vascular bundles are numerous and arranged in a ring. The vascular bundles have an evident cambium layer located between the phloem and the xylem. The pith region of the stem is filled with parenchyma cells. Druse crystals were found in groups or individually in both the cortex and pith regions of the stem (Fig. 5E-5F).
The underground stem of the species is a rhizome. Although there are epidermis cells in the outermost part, periderma formation is also observed in places. In the rhizome structure, a multi-layered meristematic endodermis is seen. Open collateral vascular bundles are present. The pith region of the rhizome is filled with parenchymatic cells. The parenchymatic cells contain a large number of starch grains and druse crystals (Fig. 5G-5H).
Essential oil composition
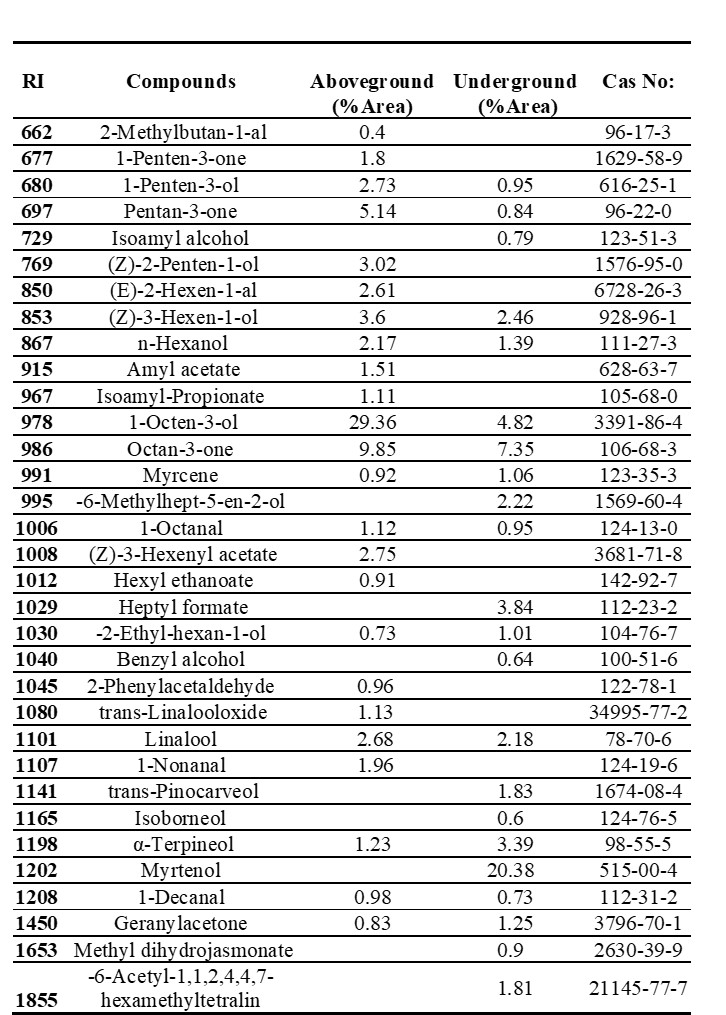
The HS-SPME/GC-MS analysis revealed the presence of 33 essential components, in total, in A. orduensis. The aboveground and underground parts contained 24 and 22 components, respectively. The most common compounds in the aboveground part were octen-3-ol (29.36%), octan-3-one (9.85%), borane-methyl sulfide complex (6%), and penton-3-one (5.14%). High amounts of myrtenol (20.38%), octan-3-one (7.35%), and methane nitroso- (7.17%) were detected in the underground parts of the plant (Tab. 2).
Tab. 2. Essential oil composition of aboveground (stem, petiole and leaf) and underground (root and rhizome) parts of Alchemilla orduensis species identified by GC-MS. Abbreviations: GC-MS – gas chromatography-mass spectrometry, RI – retention index, CAS number – chemical abstracts service number.
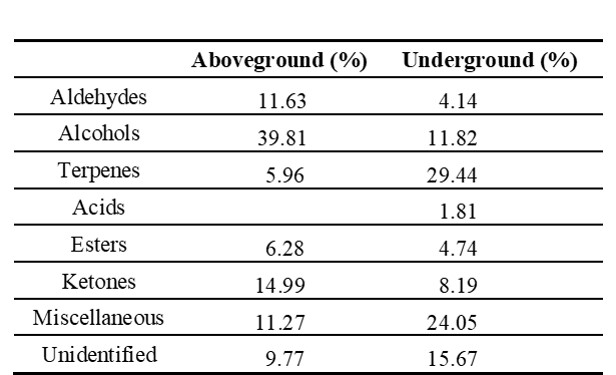
The aboveground part contained mainly alcohols (39.81%) and ketones (14.99%), while the underground part contained terpenes (23.44%) and alcohols (11.82%) (Tab. 3).
Tab. 3. Chemical groups to which the essential oils of aboveground (stem, petiole and leaf) and underground (root and rhizome) parts of Alchemilla orduensis belong, along with their respective percentages.
Discussion
In this study, the anatomical and micromorphological characteristics and the essential oil composition of the local and endangered endemic species A. orduensis were determined. The leaves of the species were found to have 5-9 lobes, with each lobe having 5-14 teeth. The number of lobes was observed to increase with the size and position of the leaves. It is worth noting that Renda et al. (2017) reported differences in the number of leaf lobes among Alchemilla species.
The roots of A. orduensis species exhibit a single-layered epidermis and exodermis layer. In the cortex, there is a multilayered endodermis composed of prominent, rectangular cells. According to Zhu et al. (2015), the formation of a cork layer and a multilayered endodermis was also observed in A. japonica Nakai et Hara. The multilayered endodermis is formed through the meristematic characterization and division of endodermis cells. The Cyperus papyrus L. (Cyperaecae) also exhibits a multilinear endodermis structure. These structures have been referred to as meristematic endodermis-derived structures by researchers (Menezes et al. 2005). The root's pith region is composed of tetrarch primary xylem elements. In A. japonica, the pith region is filled with primary xylem elements.
The rhizome-shaped underground stem of the species is composed of an outermost layer of single-layered epidermis cells, multilayered parenchyma cells, and an endodermis layer. Boruz (2010) reported that the rhizome of A. connives Buser and A. crinita Buser species have a multilayered endodermis layer. In A. orduensis, the endodermis was determined to have 8-12 layers. In A. connives, the endodermis layer has 8-10 layers, and in A. crinita, it has 7-8 layers. The rhizome of A. orduensis contains parenchymatic cells with abundant starch and druse crystals, either individually or in groups. According to Ilgun et al. (2016), the presence and arrangement of these crystals are crucial in distinguishing the species. In the present case, the stem pith is filled with parenchymatic cells and lacks ventilation cavities. According to Zhu et al. (2015), A. japonica and A. connives species have ventilation cavities, while A. glaucescens Wallr. does not have any ventilation cavities in the pith region of the stem (Boruz 2011).
The petiole of A. orduensis contains three vascular bundles, one large and two small. According to Grytsyk et al. (2019), the presence of vascular bundles in the petiole of Alchemilla species in Ukraine varies among species. Faghır et al. (2016) stated that petiole and leaf anatomical features have limited taxonomic value in Alchemilla species. The leaves of A. orduensis are bifacial, which is a common characteristic of leaves in the Rosaceae family (Watson and Dallwitz 1991). Studies on various Alchemilla species have shown that the leaves are of the bifacial type, although differences in the number of palisade and spongy parenchyma layers have been observed (Zhu et al. 2015, Ilgun et al. 2016, Jimenez-Noriega et al. 2017). The stomata in the studied species are anomocytic. Our results are consistent with previous studies on the stomata of Alchemilla species (Zhu et al. 2015, Ilgun et al. 2016).
Alchemilla orduensis has eglandular and glandular hairs on the petiole, stem, and leaves. According to Zhu et al. (2015), A. japonica has both simple and branched eglandular hairs and multicellular glandular hairs. Faghır et al. (2016) conducted a study on the petioles of 24 Alchemilla species and concluded that eglandular hairs are of taxonomic significance for these species. Alchemilla orduensis has a palisade parenchyma consisting of two layers and a spongy parenchyma consisting of two to three layers, as well as single druse crystals. In A. procumbens, it has been reported that the palisade parenchyma consists of one to two layers and druse crystals are present in the mesophyll (Jimenez-Noriega et al. 2017). The presence of druse crystals, either in clusters or individually, on leaves and stems has been shown in A. mollis (Buser) Rothm (Ilgun et al. 2016). According to Zhu et al. (2015), the presence of clustered calcium oxalate crystals in A. japonica is a distinctive character.
In this study, 33 different compounds were identified in the aboveground and underground parts of the species A. orduensis. The most abundant groups vary in the aboveground and underground parts. The major groups were found to be alcohols (39.81%) and ketones (14.99%) in the aboveground and terpenes (23.44%) and alcohols (11.82%) in the underground. Alcohol and aldehydes were reported as the most abundant essential oil groups in aboveground parts of the species A. alpina and A. xanthochlora Rothm (Falchero et al. 2008, 2009). In A. persica, the main classes were alkanes and diterpenes (Afshar et al. 2015). Alchemilla faeroensis (Lange) Buser., A. alpina L., and A. vulgaris have been reported to contain triterpenes such as oleanolic acid, ursolic acid and euscophic acid (Olafsdottir et al. 2001, Fai and Tao 2009). The major constituents were (Z)-3-hexen-1-ol (11.20%), linalool (10.36%) and 1-octen-3-ol (8.98%) in aboveground parts of A. xanthochlora species and α-terpineol (12.55%), linalool (11.03%) and (Z)-3-hexen-1-ol (10.23%) in the aboveground parts of A. alpina (Falchero et al. 2008, 2009). These components were not found in A. persica Rothm. (Afshar et al. 2015). Essential oils were analyzed in the flowers and leaves of A. flabellata Bus., A. phegophila Juz. and A. subrenata Bus. The highest essential oil content was found in the flowers of A. flabellata (16884.6 mg kg-1) and the lowest essential oil content was found in the leaves of A. phegophila (4895.5 mg kg-1) (Dubel et al. 2022). 1-Octen-3-ol (29.36%), and octan-3-one (9.85%) are the most common compounds in the aboveground of A. orduensis. According to Falchero et al. (2008, 2009) and Dubel et al. (2022), αterpinol, linalool, (Z)-3-hexen-1-ol, 1-nonanal and 1-octen-3-ol compounds, which were found in other Alchemilla species, these were also determined in our study. The ratios of essential oil components in plants belonging to the same genus are thought to be affected by the localities at which the plants were collected and by climatic factors. Myrtenol (20.38%) is the major constituent in the underground pars of A. orduensis. It is a monoterpene compound with various therapeutic properties (Clarke 2008). This substance has a membrane stabilizing effect. Thus, it helps the formation and protection of cell and organelle membranes (Dragomanova et al. 2018). Myrtenol was found in the aboveground parts of the species A. xanthochlora and A. persica (Falchero et al. 2009, Afshar et al. 2015). It was reported that this compound was not found in A. alpina species (Falchero et al. 2008). Octan-3-one is a compound found in both above and below ground parts of the A. orduensis species. It is used as an olfactory and gustatory component and has insect attractant properties (Muto et al. 2022, Reshna et al. 2022). Linalool and α-terpineol are monoterpene tertiary alcohols commonly found in A. orduensis and other Alchemilla species. They are the main floral fragrances in nature and are widely used in perfumery (Falchero et al. 2008, 2009, Dubel et al. 2022). α-terpineol has various biological applications, including use as an antioxidant, anticancer agent, and antiulcer agent. It is also of interest for its insecticidal properties (Khaleel et al. 2018).
Conclusion
This study determined the anatomical and micromorphological characteristics as well as the essential oil content of the Turkish narrow endemic species A. orduensis. Anatomical and micromorphological features are crucial in distinguishing Alchemilla species. Anatomically, important characters include the presence and arrangement of crystals, the shape of vascular bundles, and the presence of hairs in leaves and petiole. Micromorphologically, the epidermis and stomatal characteristics were also considered.
The A. orduensis species has been found to have an important medicinal potential, with 33 different compounds present in its underground and above-ground parts combined. The most common compounds are 1-Octen-3-ol in the above-ground parts and myrtenolis in the underground parts. Volatile compounds in plants vary depending on the species or ecological factors. Therefore, it is important to determine the essential oil profile of the endemic and endangered species A. orduensis collected from a special location.
